Abstract
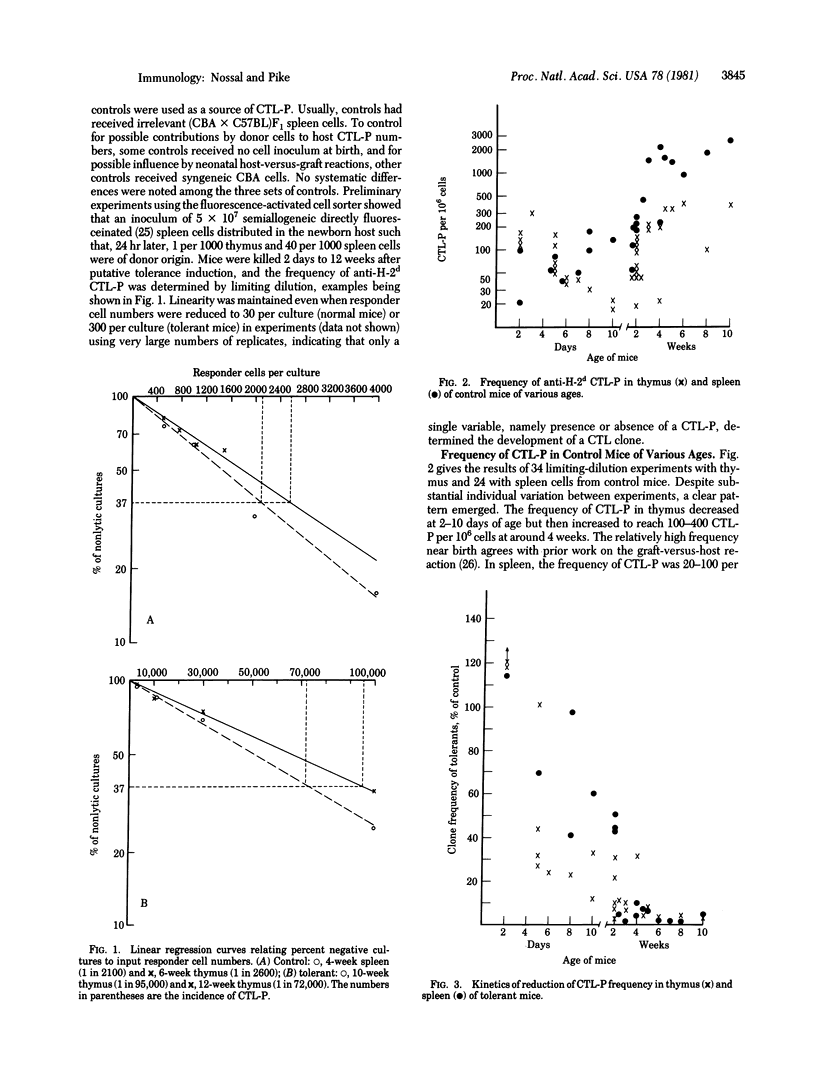
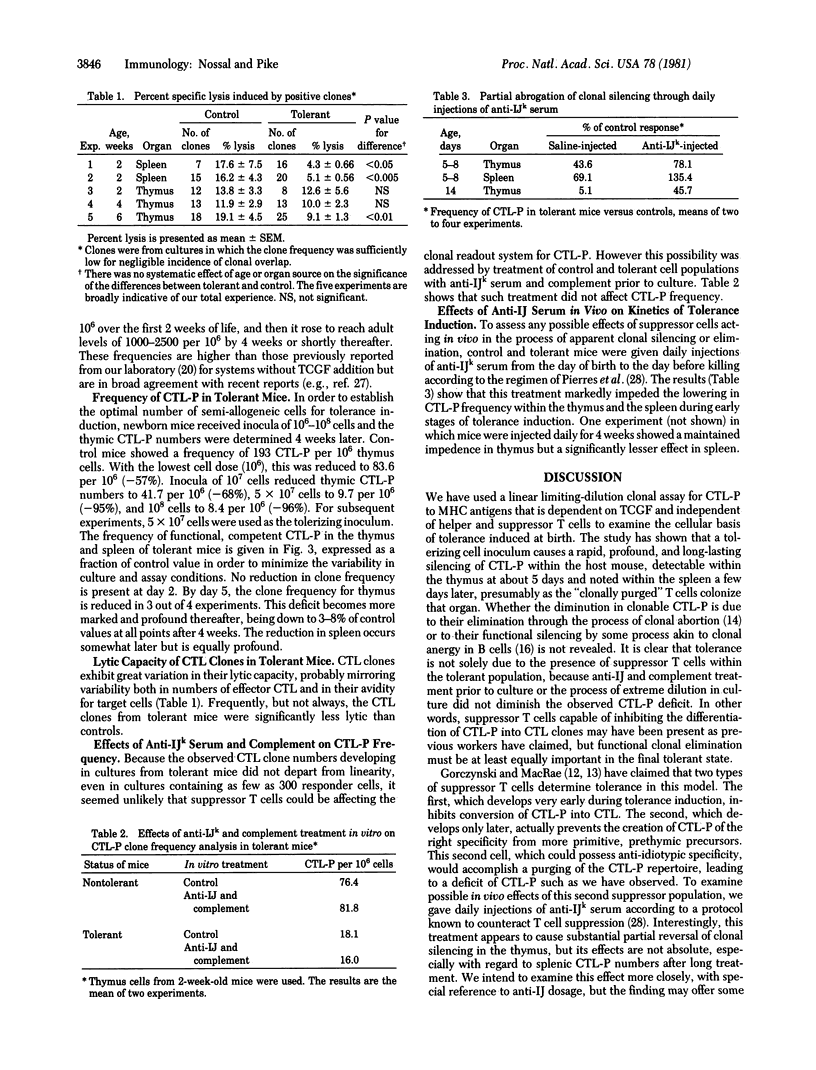
CBA (H-2k) mice were rendered tolerant to H-2d antigens by injection of (CBA X BALB/c)F1 spleen cells at birth. At intervals of 2 days to 12 weeks, the frequencies of anti-H-2d cytotoxic T lymphocyte precursor cells (CTL-P) in thymus and spleen were determined by using a limiting-dilution microculture assay system for CTL-P. This assay, utilizing irradiated H-2d stimulator cells and concanavalin A-induced spleen cell conditioned medium, was shown to be linear over the range 30 to 100,000 responder cells and uninfluenced by IJ-positive cells. A profound and long-lasting deficit in activatable CTL-P, first demonstrable by day 5 of life in the thymus and day 8-10 in the spleen, developed in mice rendered tolerant, reaching a greater than 95% reduction by 6 weeks. Functional clonal deletion thus seems to be at least as important in the tolerant state as suppressor T cells. Repeated in vivo administration of anti-IJk serum partially inhibited clonal deletion, suggesting either that suppressor T cells are actively involved in producing clonal deletion or that IJk-bearing cells in the donor inoculum or the host represent an important factor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Boehmer H., Sprent J., Nabholz M. Tolerance to histocompatibility determinants in tetraparental bone marrow chimeras. J Exp Med. 1975 Feb 1;141(2):322–334. doi: 10.1084/jem.141.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent L., Brooks C., Lubling N., Thomas A. V. Attempts to demonstrate an in vivo role for serum blocking factors in tolerant mice. Transplantation. 1972 Sep;14(3):382–387. doi: 10.1097/00007890-197209000-00016. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Direct fluorescent labeling of cells with fluorescein or rhodamine isothiocyanate. II. Potential application to studies of lymphocyte migration and maturation. J Immunol Methods. 1980;37(2):109–121. doi: 10.1016/0022-1759(80)90196-9. [DOI] [PubMed] [Google Scholar]
- COHEN M. W., THORBECKE G. J., HOCHWALD G. M., JACOBSON E. B. INDUCTION OF GRAFT-VERSUS-HOST REACTION IN NEWBORN MICE BY INJECTION OF NEWBORN OR ADULT HOMOLOGOUS THYMUS CELLS. Proc Soc Exp Biol Med. 1963 Oct;114:242–244. doi: 10.3181/00379727-114-28640. [DOI] [PubMed] [Google Scholar]
- Ceredig R. Frequency of alloreactive cytotoxic T cell precursors in the mouse thymus and spleen during ontogeny. Transplantation. 1979 Nov;28(5):377–381. doi: 10.1097/00007890-197911000-00006. [DOI] [PubMed] [Google Scholar]
- Czitrom A. A., Sunshine G. H., Mitchison N. A. Suppression of the proliferative response to H-2D by I-J subregion gene products. Immunogenetics. 1980 Jul;11(1):97–102. doi: 10.1007/BF01567774. [DOI] [PubMed] [Google Scholar]
- Dorsch S., Roser R. Recirculating, suppressor T cells in transplantation tolerance. J Exp Med. 1977 May 1;145(5):1144–1157. doi: 10.1084/jem.145.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch F. W., Engers H. D., Cerottini J. C., Bruner K. T. Generation of cytotoxic T lymphocytes in vitro. VII. Suppressive effect of irradiated MLC cells on CTL response. J Immunol. 1976 Mar;116(3):716–723. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Tolerance to sheep red cells: breakage with thymocytes and horse red cells. Science. 1972 Mar 3;175(4025):996–997. doi: 10.1126/science.175.4025.996. [DOI] [PubMed] [Google Scholar]
- Gorczynski R. M., MacRae S. Suppression of cytotoxic response to histoincompatible cells. I. Evidence for two types of T lymphocyte-derived suppressors acting at different stages in the induction of a cytotoxic response. J Immunol. 1979 Mar;122(3):737–746. [PubMed] [Google Scholar]
- Gorczynski R. M., MacRae S. Suppression of cytotoxic response to histoincompatible cells. II. Analysis of the role of two independent T suppressor pools in maintenance of neonatally induced allograft tolerance in mice. J Immunol. 1979 Mar;122(3):747–752. [PubMed] [Google Scholar]
- Hellström I., Hellström K. E., Allison A. C. Neonatally induced allograft tolerance may be mediated by serum-borne factors. Nature. 1971 Mar 5;230(5288):49–50. doi: 10.1038/230049a0. [DOI] [PubMed] [Google Scholar]
- Holán V., Hilgert I., Chutná J., Hasek M. The regulatory role of I-J subregion in neonatal tolerance induction to H-2D alloantigens. J Immunogenet. 1980 Jun;7(3):221–228. doi: 10.1111/j.1744-313x.1980.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Kay T. W., Pike B. L., Nossal G. J. Mechanisms of clonal abortion tolerogenesis. IV. Verification of the hypothesis in living mice. J Immunol. 1980 Apr;124(4):1579–1584. [PubMed] [Google Scholar]
- Kilshaw P. J., Brent L., Pinto M. Suppressor T cells in mice made unresponsive to skin allografts. Nature. 1975 Jun 5;255(5508):489–491. doi: 10.1038/255489a0. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Wilson D. B. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med. 1977 Mar 1;145(3):508–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Sy M. S., Claman H. N. The induction of hapten-specific T cell tolerance using hapten-modified lymphoid membranes. II. Relative roles of suppressor T cells and clone inhibition in the tolerant state. Eur J Immunol. 1977 Mar;7(3):165–170. doi: 10.1002/eji.1830070310. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Clonal anergy: persistence in tolerant mice of antigen-binding B lymphocytes incapable of responding to antigen or mitogen. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1602–1606. doi: 10.1073/pnas.77.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Mechanisms of clonal abortion tolerogenesis. I. Response of immature hapten-specific B lymphocytes. J Exp Med. 1978 Nov 1;148(5):1161–1170. doi: 10.1084/jem.148.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Single cell studies on the antibody-forming potential of fractionated, hapten-specific B lymphocytes. Immunology. 1976 Feb;30(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Pierres M., Germain R. N., Dorf M. E., Benacerraf B. In vivo effects of anti-Ia alloantisera. I. Elimination of specific suppression by in vivo administration of antisera specific for I-J controlled determinants. J Exp Med. 1978 Mar 1;147(3):656–666. doi: 10.1084/jem.147.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Kay T. W., Nossal G. J. Relative sensitivity of fetal and newborn mice to induction of hapten-specific B cell tolerance. J Exp Med. 1980 Nov 1;152(5):1407–1412. doi: 10.1084/jem.152.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Warner N. L. The role of suppressor cells in avian allogeneic tolerance: implications for the pathogenesis of Marek's disease. J Immunol. 1974 Sep;113(3):904–909. [PubMed] [Google Scholar]
- Ryser J. E., MacDonald H. R. Limiting dilution analysis of alloantigen-reactive T lymphocytes. I. Comparison of precursor frequencies for proliferative and cytolytic responses. J Immunol. 1979 May;122(5):1691–1696. [PubMed] [Google Scholar]
- Skinner M. A., Marbrook J. An estimation of the frequency of precursor cells which generate cytotoxic lymphocytes. J Exp Med. 1976 Jun 1;143(6):1562–1567. doi: 10.1084/jem.143.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Harley E., Phillips R. A., Miller R. G. Quantitative studies on the precursors of cytotoxic lymphocytes. I. Characterization of a clonal assay and determination of the size of clones derived from single precursors. J Immunol. 1977 Mar;118(3):1049–1056. [PubMed] [Google Scholar]
- Wagner H., Räollinghoff M., Pfizenmaier K., Hardt C., Johnscher G. T-T cell interactions during in vitro cytotoxic T lymphocyte (CTL) responses. II. Helper factor from activated Lyt 1+ T cells is rate limiting i) in T cell responses to nonimmunogenic alloantigen, ii) in thymocyte responses to allogeneic stimulator cells, and III) recruits allo- or H-2-restricted CTL precursors from the Lyt 123+ T subset. J Immunol. 1980 Mar;124(3):1058–1067. [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]



