Abstract
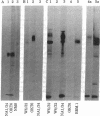
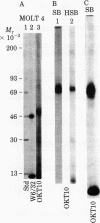
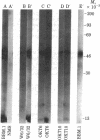
Three cell surface antigens that are structurally related to the human major histocompatibility antigens (called HLA antigens) have been characterized from the leukemic T cell line MOLT-4. One antigen is a glycoprotein of Mr 49,000 recognized by two monoclonal antibodies. OKT6 and NA1/34, and is associated with a Mr 12,000 subunit that crossreacts serologically with beta 2-microglobulin but can be distinguished from it by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. A second antigen, defined by the monoclonal antibody OKT10, is a Mr 46,000 protein associated with a small subunit distinct from beta 2-microglobulin. The OKT10 antigen is not restricted to T cells and is found on all T and B lymphoblastoid cell lines tested. The third protein is a beta 2-microglobulin-associated glycoprotein of Mr 43,000 that is serologically distinct from the OKT6 (NA1/34), OKT10, and HLA antigens. It is found on some, but not all, T cell lines but is absent from any other hematopoietic cell lines tested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Fithian E., Kung P., Goldstein G., Rubenfeld M., Fenoglio C., Edelson R. Reactivity of Langerhans cells with hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2541–2544. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. H., Cullen S. E., Sachs D. H. Immunochemical evidence for an additional H-2 region closely linked to H-2D. J Exp Med. 1977 Feb 1;145(2):438–442. doi: 10.1084/jem.145.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Hämmerling U., Flaherty L. Qat-4 and Qat-5, new murine T-cell antigens governed by the Tla region and identified by monoclonal antibodies. J Exp Med. 1979 Jul 1;150(1):108–116. doi: 10.1084/jem.150.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Selby W. S., Thomas J. A., Granger S., Kung P. C., Goldstein G. Human T lymphocytes of inducer and suppressor type occupy different microenvironments. Nature. 1980 Nov 6;288(5786):81–84. doi: 10.1038/288081a0. [DOI] [PubMed] [Google Scholar]
- Jongsma A., van Someren H., Westerveld A., Hagemeijer A., Pearson P. Localization of genes on human chromosomes by studies of human-Chinese hamster somatic cell hybrids. Assignment of PGM3 to chromosome C6 and regional mapping of the PGD, PGM1 and pep-C genes on chromosome A1. Humangenetik. 1973 Dec 10;20(3):195–202. doi: 10.1007/BF00385730. [DOI] [PubMed] [Google Scholar]
- Judd W., Poodry C. A., Broder S., Friedman S. M., Chess L., Strominger J. L. High molecular weight antigens present on human T cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6805–6809. doi: 10.1073/pnas.77.11.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Flaherty L., Lee G., Watanabe T., Michaelson J. Qa antigen expression on functional lymphoid, myeloid, and stem cells in adult mice. J Immunol. 1980 Jun;124(6):2879–2885. [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Michaelson J., Flaherty L., Vitetta E., Poulik M. D. Molecular similarities between the Qa-2 alloantigen and other gene products of the 17th chromosome of the mouse. J Exp Med. 1977 Apr 1;145(4):1066–1070. doi: 10.1084/jem.145.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson J., Rothenberg E., Boyse E. A. Genetic polymorphism of murine beta 2-microglobulin detected biochemically. Immunogenetics. 1980 Jul;11(1):93–95. doi: 10.1007/BF01567773. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Rothenberg E., Boyse E. A. Synthesis and processing of molecules bearing thymus leukemia antigen. J Exp Med. 1979 Oct 1;150(4):777–791. doi: 10.1084/jem.150.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- Stanton T. H., Hood L. Biochemical identification of the Qa-1 alloantigen. Immunogenetics. 1980;11(3):309–314. doi: 10.1007/BF01567797. [DOI] [PubMed] [Google Scholar]
- Tada N., Tanigaki N., Pressman D. Human cell membrane components bound to beta2-microglobulin in T cell-type cell lines. J Immunol. 1978 Feb;120(2):513–519. [PubMed] [Google Scholar]
- Tanigaki N., Tokuyama H., Fukunishi T., Minowada J., Pressman D. Human cell membrane components dominant in T cell lineage: identification and characterization of human TL-like antigens. J Immunol. 1979 Dec;123(6):2906–2914. [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W., Boyse E. A. Association of a beta2-microglobulin-like subunit with H-2 and TL alloantigens on murine thymocytes. J Immunol. 1975 Jan;114(1 Pt 1):252–254. [PubMed] [Google Scholar]
- Ziegler A., Milstein C. A small polypeptide different from beta2-microglobin associated with a human cell surface antigen. Nature. 1979 May 17;279(5710):243–244. doi: 10.1038/279243a0. [DOI] [PubMed] [Google Scholar]