Abstract
The articular disc is a dense collagenous tissue containing disc cells that are phenotypically described as chondrocyte-like cells or fibrochondrocytes. Despite the possible existence of these phenotypes in systemic joints, little is known about the detailed classification of the articular disc cells in the temporomandibular joint. In this immunocytochemical study we examined the localization and distribution patterns of nestin and glial fibrillary acidic protein (GFAP) in the articular disc of the rat temporomandibular joint at postnatal day 1, and weeks 1, 2, 4 and 8, based on the status of tooth eruption and occlusion. Nestin and GFAP are intermediate filament proteins whose expression patterns are closely related to cell differentiation and cell migration. Both types of immunopositive cell greatly increased postnatally to a stable level after postnatal week 4, but they showed different distribution patterns and cell morphologies. Nestin-reactive disc cells, which were characterized by a meagre cytoplasm and thin cytoplasmic processes, were scattered in the articular disc, whereas GFAP-positive cells, characterized by broader processes, existed exclusively in the deeper area. In mature discs, the major proportion of articular disc cells exhibited GFAP immunoreactivity. Furthermore, a double-immunostaining demonstrated that the nestin-negative cells, consisting of GFAP-positive and -negative cells, exhibited immunoreactions for heat shock protein 25. These findings indicate that the articular disc cells comprise at least three types in the rat temporomandibular joint and suggest that their expressions closely relate to mechanical loading forces within the joint, including occlusal force, as observed through postnatal development.
Keywords: articular disc, glial fibrillary acidic protein, intermediate filament, nestin, phenotype, temporomandibular joint
Introduction
The articular disc, a component of the temporomandibular joint, divides the upper and lower articular cavities. From a biomechanical point of view, this structure is considered to function in shock absorption, joint stability, load bearing and joint lubrication in jaw movements. Histologically, the articular disc is a dense collagenous tissue that includes the articular disc cells, which have been regarded as chondrocyte-like cells or fibrochondrocytes (Berkovitz & Pacy, 2000, 2002). Compared with numerous morphological studies on the structure, biological properties and developmental aspects of the temporomandibular joint, little information is available regarding the histological and developmental characterizations of articular disc cells (Bae et al. 1998; Nozawa-Inoue et al. 1999; Detamore et al. 2006; Johns & Athanasiou, 2007). Although morphological and immunological studies have shown two or three phenotypes for the articular disc cells in systemic joints (cf. Ghadially et al. 1983; Nakata et al. 2001; Verdonk et al. 2005), to our knowledge, no report has presented a distinct clarification of the articular disc cells in the temporomandibular joint.
Intermediate filaments, called 10-nm filaments, are intracellular skeletal elements that serve to maintain cell shape. The remodeling of the proteins composing the intermediate filaments has been believed to relate to morphological changes in cell differentiation and cell migration; the proteins are expressed in cell-type-specific patterns following and demarcating pathways of embryonic development and cellular differentiation (Herrmann & Aebi, 2000; Omary et al. 2004). By structure, they fall into six major subtypes: type I, acidic keratin in epithelial cells; type II, basic keratin in epithelial cells; type III, glial fibrillary acidic protein (GFAP) in astrocytes/Schwann cells, desmin in muscle/myoblasts and vimentin in mesenchymal tissues; type IV, neurofilaments in neurons; type V, lamin in nucleus; and type VI, nestin in undifferentiated cells. Single cells frequently express more than two intermediate filaments (Niki et al. 1999; Buniatian et al. 2002; Zou et al. 2006; Kishaba et al. 2010), and over 45 varieties are expressed in many different combinations depending on species, tissue, cell and age (Fliegner & Liem, 1991; Herrmann & Aebi, 2000), suggesting that the expression of intermediate filaments plays a critical role in morphological changes or functional status.
Nestin was first identified in neuroepithelial stem cells of the rat (Hockfield & Mckay, 1985; Lendahl et al. 1990), and has been regarded as a marker of neural and non-neural stem/progenitor cells (Cattaneo & McKay, 1990; Lendahl et al. 1990; Wiese et al. 2004). However, numerous studies have confirmed the expression of this protein in several non-neuronal tissues not only during their developmental processes but also in conditions of injury or repair (Wiese et al. 2004), indicating that this protein is a useful marker for undifferentiated cells. On the other hand, GFAP is a type III intermediate filament that was first identified in astrocytes (Eng, 1985), but its wide distribution in other cell types has been reported under normal and pathological conditions (Fields & Yan, 1985; Jessen & Mirsky, 1985; Georgiou et al. 1994; Hainfellner et al. 2001; Nolte et al. 2001; Byers et al. 2004). Interestingly, most of the GFAP-positive cells can be derived from the cranial neural crest (Chai et al. 2000), indicating the possible expression of articular disc cells, which also originate from cranial neural crest cells. Furthermore, GFAP has been considered to be involved in calcification and chondrocyte differentiation because of its expression in elastic cartilage and cartilaginous differentiating tumors (Kepes & Perentes, 1988; Notohara et al. 1990; Santos et al. 2009), suggesting a possible role for GFAP in the articular disc cells.
Through our analysis on periodontal nerve terminals using nestin (Saito et al. 2009) and GFAP immunohistochemistry (Ajima et al. 2001), we have noticed the presence of nestin and GFAP-positive cells in the rat temporomandibular joint. However, there has been no report noting the expression of nestin and GFAP-positive cells in the articular disc of the rat temporomandibular joint in spite of a study on GFAP-reactive cells in the intervertebral disc of human fetal and newborn (Kasper & Stosiek, 1990). Thus, the present investigation was undertaken to examine the expression of nestin and GFAP in the articular disc of the rat temporomandibular joint by immunohistochemical techniques. It focuses on their developmental aspects and ultrastructural configurations. Keeping in mind the close relationship between the function of the temporomandibular joint and occlusion, we set five stages for the observation period (postnatal day 1, and weeks 1, 2, 4 and 8) whose classification is based on the status of tooth eruption and occlusion (Nakakura-Ohshima et al. 1993, 1995). In this study, we further attempted to clarify the phenotypes of the articular disc by employing a double-immunostaining using an antibody to heat shock protein 25 (Hsp25), as its strong expression has been reported in the articular disc cells of the rat temporomandibular joint (Nozawa-Inoue et al. 1999).
Materials and methods
All experiments were approved and performed based on the guidelines of the Niigata University Intramural Animal Use and Care Committee (approval number #68 in 2009). The animals were housed in a temperature-controlled room under 12 h light/dark laboratory conditions with free access to chow and water.
Animals and tissue preparations
A total of 30 male Wistar rats were used in this study. At the appointed time [postnatal day 1 (7.0–8.0 g), and week 1 (16–18 g), 2 (24–27 g), 4 (65–72 g) and 8 (265–280 g); n = 6 each at period], the animals were transcardially perfused with 4% paraformaldehyde in a 0.1 m phosphate buffer, pH 7.4, under deep anesthesia with an intraperitoneal injection of chloral hydrate (400 mg kg−1). After perfusion fixation, the heads were removed en bloc, cut sagittally into two halves, and immersed in the same fixative for an additional 12 h. They were demineralized with a 10% EDTA-2Na solution at 4 °C with slight agitation. Half of the head obtained from each animal was immersed in a 30% sucrose solution at 4 °C overnight for cryoprotection and then frozen in liquid nitrogen. Sagittal sections of the temporomandibular joint were prepared at a thickness of either 10 μm or 20 μm in a cryostat (C3050S; Leica, Nussloch, Germany) and mounted onto silanized glass slides. The other half was conventionally embedded in paraffin and cut at a thickness of 5 μm using a Retratome (REM-700; Yamato Koki Industrial, Asaka, Japan).
Immunohistochemistry of nestin and GFAP
For immunohistochemical procedures, both cryostat and paraffin sections were reacted with primarily monoclonal antibodies to either GFAP (1 : 2000, clone GA5; Millipore, Temecula, CA, USA) or nestin (1 : 2500, clone Rat-401; Chemicon International, Temecula, CA, USA) overnight at room temperature. They were incubated with a biotinylated horse anti-mouse IgG (1 : 100; Vector Lab., Burlingame, CA, USA), followed with horseradish peroxidase-conjugated avidin (ABC kit, Vector Lab.). Final visualization used 3, 3′-diaminobenzidine (0.04%) and hydrogen peroxide (0.003%) in a 0.05 m Tris-HCl buffer at pH 7.6. The immunostained sections were counterstained with 0.03% methylene blue.
For immunocytochemistry at the electron microscopic level, we used a nanogold-conjugated mouse IgG (Nanoprobes, Yaphank, NY, USA) – instead of a biotinylated one – and a silver enhancement technique for cryostat sections. These immunoreacted sections were osmificated, dehydrated through ascending ethanols, and finally embedded in an epoxy resin (Epon 812; TAAB, Berkshire, UK). Ultrathin sections, 70 nm thick, were prepared with an ultramicrotome (Ultracut R, Leica) and briefly double-stained with uranyl acetate and lead citrate. They were examined in an H-7650 transmission electron microscope at an accelerating voltage of 75 kv (Hitachi, Tokyo, Japan).
Double-fluorescent immunostaining
A double-immunolabeling was performed in cryostat sections for the identification of nestin- or GFAP-immunoreactive cells. In addition, a co-localization of Hsp25, a marker for the articular disc cells, and either nestin or GFAP was also checked by a double-immunofluorescent method. Information on the primary and secondary antibodies used in this study is shown in Table 1. The nucleus was stained with DAPI (Vector Lab.). The immunostained sections were observed with a fluorescent microscope (AxioImazer M; Carl Zeiss, Jena, Germany).
Table 1.
Antibodies used for double immunostaining
| Monoclonal | Fluorescent dye | Polyclonal | Fluorescent dye |
|---|---|---|---|
| Nestin (clone: Rat-401) 1 : 2500, Chemicon International, Temecula, CA, USA | Cy3 1 : 500, Jackson ImmunoResearch Lab., West Grove, PA, USA | GFAP 1 : 4000, Dako, Glostrup, Denmark | FITC 1 : 200, Vector Lab., Burlingame, CA, USA |
| GFAP (clone:GA5) 1 : 2000, Millipore, Temecula, CA, USA | Hsp 25 (poly) 1 : 1000, Stressgen Biotechnologies, Victoria, Canada |
Results
Postnatal day 1
At this stage, the lower articular cavity had not yet completely formed, although the upper articular cavity had expanded in the anterior–posterior direction. As we reported previously (Suzuki et al. 2005), the articular disc was not separated from the condylar surface where several blood capillaries lay at the central portion of the lower articular cavity.
A very few cells immunoreacting with nestin existed at the anterior and posterior portions of the articular disc (Fig. 1A). Flat in profile, they were located near the articular disc surface but never in the deep area of the articular disc. The nestin-positive cells sometimes extended their thin cytoplasmic extensions towards the articular cavity (arrow in Fig. 1A). The immunoreaction for nestin was localized in the cytoplasm devoid of the nucleus. In contrast, our careful observations could find no nestin-positive cells at the central portion of the articular disc (Fig. 1B). The endothelial cells between the articular disc and condylar surface also expressed nestin immunopositivity (Fig. 1A,B).
Fig. 1.
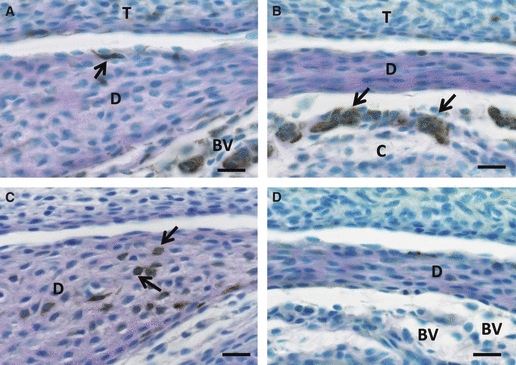
Immunoreactions for nestin (A, B) and GFAP (C, D) at postnatal day 1. (A, B) A few slender cells with nestin expression (arrow in A) are observed along the anterior portion of the articular disc (D) surface. The central portion of the disc never contains any distinct positive cells (B). However, the invading blood capillaries into the future lower articular cavity area show intense nestin immunoreaction (arrows in B). (C, D) Some cells with strong GFAP immunoreaction exist in the deeper area of the anterior articular disc (arrows), although immunoreaction for GFAP exists neither in the central area of the disc (D) nor blood capillaries (BV) between the surface of the mandibular condyle and the disc. Scale bars: 20 μm. T, temporal bone.
On the other hand, an expression of GFAP immunoreaction was recognizable in a few cells with poor cytoplasm situated in the deep areas of the anterior and posterior portions (Fig. 1C), but not in the central portions (Fig. 1D). However, the endothelial cells lacked any GFAP immunoreactivity.
Postnatal week 1
At postnatal week 1, formation of articular cavity had completely finished and the area of the articular disc was clearly divided into three portions: a thick anterior band, a thin intermediate zone, and a thick posterior band – all of which were observed in mature animals.
Compared with the previous stage, the nestin- or GFAP-immunoreactive cells had increased in number (Fig. 2). The location and morphology of the nestin-positive cells were the same as those of the reactive cells at the previous stage: at the anterior and posterior bands they existed near the disc surface and possessed thin cytoplasmic extensions (Fig. 2A). At the intermediate zone, slender cells with nestin immunopositivity came to arrange themselves along the disc surface (Fig. 2B). Some cells with poor cytoplasm also exhibited nestin immunoreaction in deep areas (Fig. 2A).
Fig. 2.
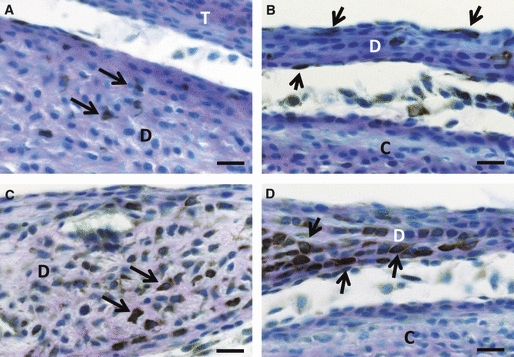
Immunoreactions for nestin (A, B) and GFAP (C, D) in the anterior (A, C) and central portions (B, D) of the developing articular disc at postnatal week 1. (A) Some nestin-positive cells with thin cytoplasmic projections (arrows) appear near the surface at the anterior portion. (B) The center of the articular disc (D) comes to contain some slender cells expressing nestin immunoreaction at the surface of the disc (arrows). (C, D) Almost all cells in the articular disc have an immunopositive reaction for GFAP. Some GFAP-positive cells exhibit a strong GFAP immunoreaction (arrows). Scale bars: 20 μm. C, mandibular condyle; T, temporal bone.
Many articular disc cells enlarged to have GFAP immunoreaction, though GFAP immuno-intensity varied among the positive cells (Fig. 2C,D). However, there was no GFAP-positive cell that lined the articular cavity, unlike the nestin-positive cells.
Postnatal week 2
The articular disc at this stage had become obviously thicker in anterior and posterior bands than at the previous stage.
In addition to the articular surface, nestin-positive cells appeared in the deep area of the articular disc (Fig. 3A,B). They extended thin cytoplasmic processes. The endothelial cells in the deep area were also positive in nestin immunoreaction (Fig. 3C).
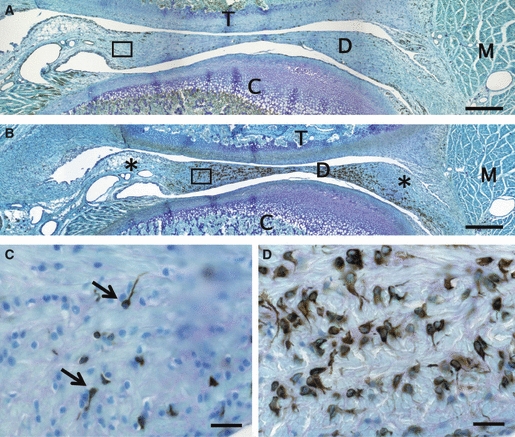
Fig. 3.
Immunoexpression of nestin (A–C) and GFAP (D–F) in the articular disc (D) at postnatal week 2. (A–C) The nestin-positive cells extend their thin cytoplasmic processes (arrows) in the anterior band (A) and intermediate zone (B) of the disc. An arrowhead indicates a nestin-positive cell at the surface (B). In the posterior band (C), endothelial cells (arrowheads) are positive in nestin immunoreaction. (D–F) The central area of the disc contains a major population of disc cells with strong GFAP immunoreactions, while the peripheral area has weakly positive cells (D). These strong positive cells assume large round profiles with long and thick cytoplasmic projections in the anterior portion (E) or flat profiles with thick ramified processes in the intermediate zone (F). Scale bars: 20 μm in (A–C, E, F), 100 μm in (D). C, mandibular condyle; T, temporal bone.
Disc cells with strong GFAP immunoreaction were widely distributed throughout the rat articular disc (Fig. 3D). These cells were exclusively located in the deep area of the articular disc (Fig. 3E). The peripheral area of the articular disc contained fewer GFAP-immunopositive cells. The GFAP-reactive cells at the intermediate zone and the anterior and posterior bands developed several elaborated cytoplasmic extensions (Fig. 3E,F).
Postnatal weeks 4 and 8
At postnatal week 4, no remarkable morphological change was recognizable in the rat articular disc (Fig. 4A,B). The nestin- (Fig. 4A) or GFAP-reactive cells (Fig. 4B) were widely distributed throughout the articular disc in spite of fewer GFAP-positive cells in the posterior and anterior-upper areas (Fig. 4B). The deep area of the articular disc included two types of articular disc cells with nestin immunoreaction by the presence or lack of thin cytoplasmic extensions (Fig. 4C). On the other hand, a large number of GFAP-reactive cells had long, thick cytoplasmic projections (Fig. 4D).
Fig. 4.
Immunopositivities for nestin (A, C) and GFAP (B, D) in the articular disc at postnatal week 4. (A) The nestin-positive cells are widely distributed throughout the articular disc (D) in maximum numbers. (B) Numerous strongly GFAP-expressing cells are scattered all over the disc. However, the posterior and anterior-upper areas (*) contain a few cells with GFAP immunoreaction. (C) Higher magnification of the boxed area in (A). Nestin-immunoreactive cells with (arrows) or without a thin cytoplasmic projection are situated in a deeper position from the articular cavity. (D) Almost all GFAP-positive cells, round and large, have long thick cytoplasmic projections in the anterior band. Scale bars: 500 μm in (A, B), 20 μm in (C, D). C, mandibular condyle; M, muscle; T, temporal bone.
The morphology and distribution pattern of the nestin- or GFAP-immunopositive cells remained unchanged throughout the rat articular disc at postnatal week 8, being identical to those observed at postnatal week 2 (Fig. 4).
Findings of double-immunostaining at postnatal week 8
Double-fluorescent immunostaining demonstrated that the nestin-reactive cells never co-localized with GFAP (Fig. 5A) or Hsp25 immunoreactions (Fig. 5B) in the rat articular disc cells throughout the entire disc. On the other hand, almost all articular disc cells expressed Hsp25 immunoreaction (Fig. 5B). A major population of Hsp25-positive cells co-expressed GFAP immunoreaction (Fig. 5C). However, the articular disc cells near the surface lacked any GFAP immunoreaction. These GFAP-negative/Hsp25-positive cells seemed to be comparatively smaller than GFAP and Hsp25 co-localized cells.
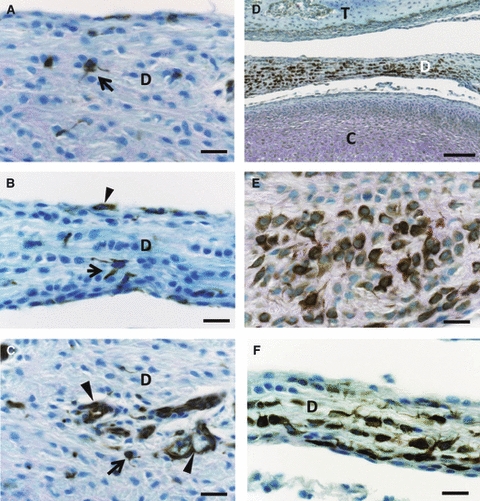
Fig. 5.
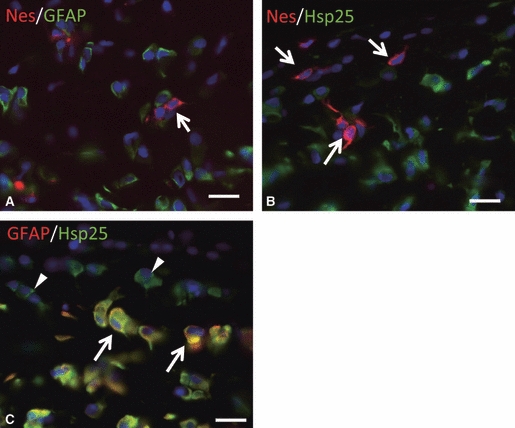
Co-localization of nestin with GFAP (A), Hsp25 (B) and GFAP with Hsp25 (C) in the anterior band of the articular disc as demonstrated by a double-immunofluorescent staining at postnatal week 8. (A, B) Some nestin-positive round cells (arrows in A, B; red) with thin cytoplasmic projections do not express GFAP (green in A) or Hsp25 (green in B). (C) Almost all GFAP-positive cells appear to have Hsp25 immunoreactions (arrows) and possess thick processes and a rich cytoplasm. However, some Hsp25-positive cells crossing to the edge of the articular disc do not have any GFAP-positive reaction (arrowheads). Scale bars: 20 μm.
Immunoelectron microscopic observation of nestin- and GFAP-reactive cells at postnatal week 8
Immunoreactions for nestin and GFAP were easily identified as electron-dense particles under the electron microscope at this stage (Fig. 6).
Fig. 6.
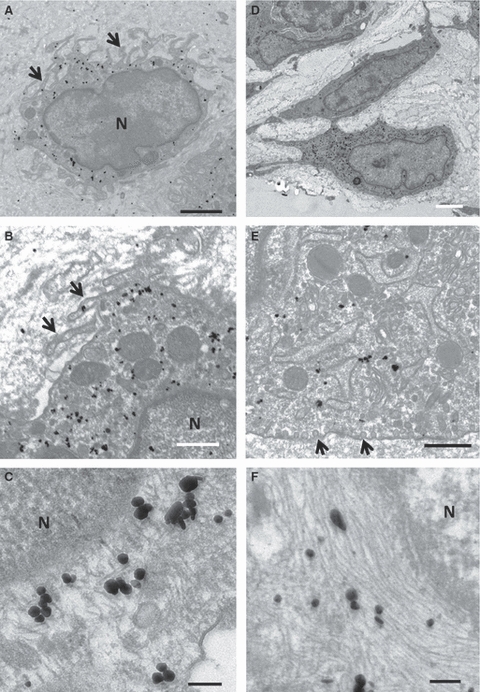
Electron micrographs of nestin- (A–C) and GFAP- (D–F) positive cells in the articular disc at postnatal week 8. (A, B) Nestin-immunopositive cells have many pseudopodia-like thick cytoplasmic projections (arrows) with some large mitochondria (A). They have not, however, developed cell organelles (B). (C) Immunoreactions for nestin, appearing as electron-dense particles, are distributed in scattered intracellular filaments that never form bundles. (D) A GFAP-immunopositive cell in the articular disc has a large, irregularly shaped nucleus. (E) This cell contains a well-developed rough endoplasmic reticulum and Golgi complexes and mitochondria in its cytoplasm. Many caveola-like invaginations are observed along the cell membranes (arrows). (F) The GFAP-positive filaments arrange themselves in parallel to form thick bundles between the cell organelles and near the nucleus. Scale bars: 2 μm in (A), 500 nm in (B, E), 100 nm in (C, F), 2.5 μm in (D). N, nucleus.
The articular disc cells with nestin immunoreaction were characterized by many short and thin cytoplasmic processes – appearing as pseudopodia-like processes – and an indented nucleus with clear chromatin (Fig. 6A). Their poor cytoplasm contained a few cell organellae, including a rough-surfaced endoplasmic reticulum and Golgi complex (Fig. 6B). Some large mitochondria were scattered in their cytoplasm. The nestin immunoreaction was recognizable on comparatively thin and short intermediate filaments, which did not form fiber bundles (Fig. 6C).
The GFAP-reactive cells featured a large, clear, irregular nucleus and thick and long cell process (Fig. 6D). They had also developed cell organellae such as a rough-surfaced endoplasmic reticulum and Golgi complex (Fig. 6E) in their cytoplasm and caveola-like invaginations along their cell membranes (Fig. 6E). The GFAP-reactive intermediate filaments formed thick bundles that appeared to surround the nucleus (Fig. 6F).
The phenotypes of the articular disc cells in mature rats are summarized in Table 2.
Table 2.
Immunological and morphological characterizations of the rat articular disc
| Co-localization of immunoreaction | |||
|---|---|---|---|
| Hsp25 | Nestin | GFAP | Feature |
| + | − | + | Large nucleus and rich cytoplasm |
| + | − | + | Thick and long cell processes |
| + | − | + | A major population of articular disc cells |
| + | − | − | Present near the articular surface |
| − | + | − | Small nucleus and poor cytoplasm |
| − | + | − | Thin and short cell processes |
| − | + | − | Scattered in the articular disc |
Discussion
The temporomandibular joint, which plays crucial roles in complicated jaw movements, is a bilateral symmetric synovial joint between the head of the mandibular condyle and the mandibular fossa of the temporal bone (Bernick, 1987). The articular disc, a major component of the temporomandibular joint, is usually exposed to various mechanical stresses, including intermittent occlusal force. Histological observations demonstrate that this structure is an avascular fibrous tissue consisting of a dense network of collagen fibers and disc cells called chondrocyte-like cells or fibrochondrocytes (Berkovitz & Pacy, 2000, 2002). The location and structure of the articular disc readily suggest that the articular disc cells serve in its maintenance and preservation. From the viewpoint of the expression patterns of intermediate filaments and Hsp25, the present immunohistochemical study was able to reveal at least three phenotypes in the rat articular disc: articular disc cells with Hsp25 (+)/nestin (−)/GFAP (+); with Hsp25 (+)/nestin (−)/GFAP (−); and with Hsp25 (−)/nestin (+)/GFAP (−) (Table 2). Because a previous study failed to find Hsp25-negative cells in the articular disc (Nozawa-Inoue et al. 1999), this is the first report to demonstrate another type of articular disc cell that is devoid of Hsp25 immunoreaction. In immunoelectron microscopic observations, the Hsp25 (−)/nestin (+)/GFAP (−) disc cells were characteristic of a poor cytoplasm with fewer cell organellae – a feature common to the nestin-positive disc cells, suggesting that this is a kind of immature or undifferentiating cell. There is no evidence of whether this immature type of cell can differentiate into GFAP- and/or Hsp25-positive cells with thick cell processes and a rich cytoplasm, though nestin is replaced by GFAP in nervous tissue during development (Lendahl et al. 1990).
It is notable that a smaller number of articular disc cells showed immunoreaction for nestin. This protein was first discovered in rat neuroepithelial stem cells (Hockfield & Mckay, 1985; Lendahl et al. 1990), but recent studies have confirmed its expression in many non-neuronal cells (cf. Wiese et al. 2004). Current immunohistochemical observations at light and electron microscopic levels indicate that the articular disc cells with nestin immunoreaction may have a capacity for differentiation in the mature articular disc, as shown in the human knee meniscus, which has been reported to contain mesenchymal stem cells (Segawa et al. 2009). Although our preliminary study has shown a low level of apoptosis and mitosis in the articular disc cells (unpublished data), the existence of nestin-expressing cells implies the possibility that they have an ability to differentiate into the other types of cells – including endothelial cells and pericytes – for repair of the damaged articular disc.
It is well known that temporomandibular joint plays crucial roles in jaw movement. Occlusion between upper and lower teeth is an important factor that regulates jaw movement (Matthews, 1975). Previous studies have suggested the involvement of occlusal forces in the development of the extracellular matrices in the articular disc of the temporomandibular joint (Toriya et al. 2006; Feng et al. 2010). These findings readily prompted us to hypothesize a close relationship between mechanical stress and the development of the intermediate filaments in the articular disc. In this study, we divided the observation period into five stages. This classification is based on the status of tooth eruption and occlusion (Nakakura-Ohshima et al. 1993, 1995): postnatal day 1 (incisors not erupted) and postnatal weeks 1 (upper and lower incisors erupted and the occlusion of the incisors begun), 2 (the commencement of occlusion having started between upper and lower molars), and 4 and 8 (occlusion established between upper and lower molars). Current observations of the rat temporomandibular joint showed that the immunoexpression patterns of nestin and GFAP in the articular disc cells reflected the developmental process of the articular disc: no remarkable changes in the immunoexpression pattern, and the morphology and distribution of immunoreactive articular disc cells was recognizable after the occlusion of the upper and lower molars was established. This finding indicates that the addition of mechanical stress via teeth may contribute to the maturation of the articular disc. In particular, as the GFAP-expressing cells drastically increased in number when occlusion had commenced between upper and lower molars, it is possible that the GFAP immunoexpression is a key indicator of the maturation of the articular disc.
In conclusion, the articular disc cells comprise at least three types in the rat temporomandibular joint as defined by the expression patterns of intermediate filaments, including nestin and GFAP. This study was, however, carried out on intact rats through their postnatal development. Further observations on older animals and under pathological conditions including over-loading or loss of occlusal force are needed for clarifying the significance of the expression of these intermediate filaments.
Acknowledgments
We thank Mr Masaaki Hoshino for his technical assistance. This study was supported by Grants from the Japan Society for the Promotion of Science (JSPS; Nos. 20890074 to A.S. and 22592207 to K.O.) and a Grant for Promotion of Niigata University Research Projects (No. 21C160 to A.S).
Author contributions
Hitoshi Miyako, Akiko Suzuki: experiments, acquisition of data, data analysis and drafting of the manuscript. Kayoko Nozawa-Inoue: experimental design and critical revision of the manuscript. Jin Magara, Yoshiro Kawano, Kazuhiro Ono: experiments. Takeyasu Maeda: principal investigator, critical revision of the manuscript, and approval of article.
References
- Ajima H, Kawano Y, Takagi R, et al. The exact expression of glial fibrillary acidic protein (GFAP) in trigeminal ganglion and dental pulp. Arch Histol Cytol. 2001;64:503–511. doi: 10.1679/aohc.64.503. [DOI] [PubMed] [Google Scholar]
- Bae YC, Park KP, Park MJ, et al. Development of vimentin filaments in the cells of the articular disc of the rat squamosomandibular joint with age. Arch Oral Biol. 1998;43:579–583. doi: 10.1016/s0003-9969(98)00033-8. [DOI] [PubMed] [Google Scholar]
- Berkovitz BK, Pacy J. Age changes in the cells of the intra-articular disc of the temporomandibular joints of rats and marmosets. Arch Oral Biol. 2000;45:987–995. doi: 10.1016/s0003-9969(00)00067-4. [DOI] [PubMed] [Google Scholar]
- Berkovitz BK, Pacy J. Ultrastructure of the human intra-articular disc of the temporomandibular joint. Eur J Orthod. 2002;24:151–158. doi: 10.1093/ejo/24.2.151. [DOI] [PubMed] [Google Scholar]
- Bernick S. Development, structure and function of temporomandibular joint. In: Avery JK, editor. Oral Development and Histology. Baltimore: Williams & Wilkins; 1987. pp. 348–364. [Google Scholar]
- Buniatian GH, Hartmann HJ, Traub P, et al. Glial fibrillary acidic protein-positive cells of the kidney are capable of raising a protective biochemical barrier similar to astrocytes: expression of metallothionein in podocytes. Anat Rec. 2002;267:296–306. doi: 10.1002/ar.10115. [DOI] [PubMed] [Google Scholar]
- Byers MR, Maeda T, Brown AM, et al. GFAP immunoreactivity and transcription in trigeminal and dental tissues of rats and transgenic GFP/GFAP mice. Microsc Res Tech. 2004;65:295–307. doi: 10.1002/jemt.20130. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Detamore MS, Hegde JN, Wagle RR, et al. Cell type and distribution in the porcine temporomandibular joint disc. J Oral Maxillofac Surg. 2006;64:243–248. doi: 10.1016/j.joms.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985;8:203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Feng J, Gu Z, Lin X, et al. Postnatal development of type II collagen and aggrecan mRNA expression in a rabbit craniomandibular joint. Anat Rec. 2010;293:1574–1580. doi: 10.1002/ar.21200. [DOI] [PubMed] [Google Scholar]
- Fields KL, Yan SH. A subset of Schwann cells in peripheral nerves contain a 50-KD protein antigenically related to astrocyte intermediate filaments. J Neuroimmunol. 1985;8:311–330. doi: 10.1016/s0165-5728(85)80070-9. [DOI] [PubMed] [Google Scholar]
- Fliegner KH, Liem RK. Cellular and molecular biology of neuronal intermediate filaments. Int Rev Cytol. 1991;131:109–167. doi: 10.1016/s0074-7696(08)62018-5. [DOI] [PubMed] [Google Scholar]
- Georgiou J, Robitaille R, Trimble WS, et al. Synaptic regulation of glial protein expression in vivo. Neuron. 1994;12:443–455. doi: 10.1016/0896-6273(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Ghadially FN, Lalonde JM, Wedge JH. Ultrastructure of normal and torn menisci of the human knee joint. J Anat. 1983;136:773–791. [PMC free article] [PubMed] [Google Scholar]
- Hainfellner JA, Voigtländer T, Ströbel T, et al. Fibroblasts can express glial fibrillary acidic protein (GFAP) in vivo. J Neuropathol Exp Neurol. 2001;60:449–461. doi: 10.1093/jnen/60.5.449. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- Hockfield S, Mckay RDG. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Glial fibrillary acidic polypeptides in peripheral glia, molecular weight, heterogeneity and distribution. J Neuroimmunol. 1985;8:377–393. doi: 10.1016/s0165-5728(85)80074-6. [DOI] [PubMed] [Google Scholar]
- Johns DE, Athanasiou KA. Design characteristics for temporomandibular joint disc tissue engineering: learning from tendon and articular cartilage. Proc Inst Mech Eng H. 2007;221:509–526. doi: 10.1243/09544119JEIM158. [DOI] [PubMed] [Google Scholar]
- Kasper M, Stosiek P. Detection of GFAP in vertebral fibrocartilage in human fetal and newborn tissue. Acta Histochem. 1990;88:25–27. doi: 10.1016/S0065-1281(11)80242-4. [DOI] [PubMed] [Google Scholar]
- Kepes JJ, Perentes E. Glial fibrillary acidic protein in chondrocytes of elastic cartilage in th human epiglottis: An immunohistochemical study with polyvalent and monoclonal antibodies. Anat Rec. 1988;220:296–299. doi: 10.1002/ar.1092200311. [DOI] [PubMed] [Google Scholar]
- Kishaba Y, Matsubara D, Niki T. Heterogeneous expression of nestin in myofibroblasts of various human tissues. Pathol Int. 2010;60:378–385. doi: 10.1111/j.1440-1827.2010.02532.x. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, Mckay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Matthews B. Mastication. In: Lanelle CLB, editor. Applied Physiology of Mouth. Bristol: Wrights; 1975. pp. 199–242. [Google Scholar]
- Nakakura-Ohshima K, Maeda T, Sato O, et al. Postnatal development of periodontal innervation in rat incisors: an immunohistochemical study using protein gene product 9.5 antibody. Arch Histol Cytol. 1993;56:385–398. doi: 10.1679/aohc.56.385. [DOI] [PubMed] [Google Scholar]
- Nakakura-Ohshima K, Maeda T, Ohshima H, et al. Postnatal development of periodontal Ruffini endings in rat incisors: an immunoelectron microscopic study using protein gene product 9.5 (PGP 9.5) antibody. J Comp Neurol. 1995;362:551–564. doi: 10.1002/cne.903620409. [DOI] [PubMed] [Google Scholar]
- Nakata K, Shino K, Hamada M, et al. Human meniscus cell: characterization of the primary culture and use for tissue engineering. Clin Orthop Relat Res. 2001;391:S208–S218. [PubMed] [Google Scholar]
- Niki T, Pekny M, Hellemans K, et al. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520–527. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- Nolte C, Matyash M, Pivneva T, et al. GFAP promoter-controlled EGFAP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- Notohara K, Hsueh CL, Awai M. Glial fibrillary acidic protein immunoreactivity of chondrocytes in immature and mature teratomas. Acta Pathologica Japonica. 1990;40:335–342. doi: 10.1111/j.1440-1827.1990.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Nozawa-Inoue K, Ohshima H, Kawano Y, et al. Immunocytochemical demonstration of heat shock protein 25 in the rat temporomandibular joint. Arch Histol Cytol. 1999;62:483–491. doi: 10.1679/aohc.62.483. [DOI] [PubMed] [Google Scholar]
- Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- Saito S, Suzuki A, Nozawa-Inoue K, et al. Immunohistochemical detection of nestin in the periodontal Ruffini endings of the rat incisor. Neurosci Lett. 2009;449:195–200. doi: 10.1016/j.neulet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Santos GC, Carvalho KC, Falzoni R, et al. Glial fibrillary acidic protein in tumor types with cartilaginous differentiation. Mod Pathol. 2009;22:1321–1327. doi: 10.1038/modpathol.2009.99. [DOI] [PubMed] [Google Scholar]
- Segawa Y, Muneta T, Makino H, et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Nozawa-Inoue K, Ikeda N, et al. Development of the articular cavity in the rat temporomandibular joint with special reference to the behavior of endothelial cells and macrophages. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:908–916. doi: 10.1002/ar.a.20228. [DOI] [PubMed] [Google Scholar]
- Toriya N, Takuma T, Arakawa T, et al. Expression and localization of versican during postnatal development of rat temporomandibular joint disc. Histochem Cell Biol. 2006;125:205–214. doi: 10.1007/s00418-005-0020-1. [DOI] [PubMed] [Google Scholar]
- Verdonk PCM, Forsyth RG, Wang J, et al. Characterization of human knee meniscus cell phenotype. Osteoarthritis Cartilage. 2005;13:548–560. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, et al. Nestin expression – a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Yaoita E, Watanabe Y, et al. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 2006;448:485–492. doi: 10.1007/s00428-005-0134-9. [DOI] [PubMed] [Google Scholar]


