Abstract
Peripheral nerve axotomy in adult mice elicits a complex response that includes increased glucose uptake in regenerating nerve cells. This work analyses the expression of the neuronal glucose transporters GLUT3, GLUT4 and GLUT8 in the facial nucleus of adult mice during the first days after facial nerve axotomy. Our results show that whereas GLUT3 levels do not vary, GLUT4 and GLUT8 immunoreactivity increases in the cell body of the injured motoneurons after the lesion. A sharp increase in GLUT4 immunoreactivity was detected 3 days after the nerve injury and levels remained high on Day 8, but to a lesser extent. GLUT8 also increased the levels but later than GLUT4, as they only rose on Day 8 post-lesion. These results indicate that glucose transport is activated in regenerating motoneurons and that GLUT4 plays a main role in this function. These results also suggest that metabolic defects involving impairment of glucose transporters may be principal components of the neurotoxic mechanisms leading to motoneuron death.
Keywords: glucose transport, GLUT, motoneuron, nerve lesion, neuron
Introduction
Neuronal responses after peripheral nerve injuries are one of the best characterized models of neuronal regeneration. Facial nerve axotomy in rodents has been established as a classical experimental paradigm to study nerve regeneration and degeneration. Facial nerve axotomy does not produce a direct CNS trauma and most rodent facial motoneurons survive in the adult after the lesion, providing a useful model in which to study the mechanisms of nerve regeneration (Terrado et al. 2000a; Moran & Graeber, 2004). A motor nerve injury causes structural, electrophysiological, molecular and metabolic changes both in the nerve cell and in the surrounding glia. During the first day after the lesion injured cells reorganise their metabolic priorities and activate several metabolic processes, including glucose metabolism (Moran & Graeber, 2004). Classic studies have shown that motor nerve injury increases glucose uptake and utilisation in the lesioned motor nuclei (Kreutzberg & Emmert, 1980; Singer & Mehler, 1980; Smith et al. 1984; Ito et al. 1999), indicating that glucose metabolism is important in the regenerating motoneurons after axotomy.
Glucose uptake in the mammalian nervous system is mediated by the family of facilitative glucose transporter proteins, GLUT (Wood & Trayhurn, 2003; Uldry & Thorens, 2004). Of the 13 GLUT family members, several are present in neurons: GLUT3 is located in the neuropil and is considered the main neuronal transporter (McEwen & Reagan, 2004; Simpson et al. 2008), while GLUT4 and GLUT8 are both widely distributed in neurons and mainly located in the cell bodies (El Messari et al. 1998; Choeiri et al. 2002; Sankar et al. 2002; Gomez et al. 2010). Only certain aspects are known about the role that GLUT molecules play in the recovery of neurons after injury, but given their role as fuel transporters, some particular GLUTs are probably needed to provide an energy support to neurons when they have high metabolic requirements. In this sense, GLUT3 levels increase after different hypoxic–ischemic experimental paradigms and after diffuse traumatic brain injury (Urabe et al. 1996; Devaskar et al. 1999; Hamlin et al. 2001). Nevertheless, the regulation of glucose transporters after a peripheral nerve lesion has not been studied.
Materials and methods
C57/BL6 mice from Harlan (Barcelona, Spain) were housed, bred and killed according to European Council legislation (86/609/EEC) on experimental animal protection. All the experimental protocols were approved by the Ethics Committee of the Cardenal Herrera CEU University, and met the local guidelines (Spanish Law 32/2007) and European regulations (EU Directive 86/609). Experimenters hold the official accreditation for animal work (Spanish Law 32/2007). The animals were maintained in a 12-h day/night cycle at constant room temperature (22 ºC), with free access to water and standard mouse fodder. Adult mice (8–10 weeks old) were anesthetised intraperitoneally with 1 μL g−1 ketamine chlorhydrate (Imalgene1000; Merial, Barcelona, Spain) and 0.45 μL g−1 xylacine (Rompun 2%; Bayer, Barcelona, Spain), and the left facial nerve was cut after its exit from the stylomastoid foramen, as previously described (Terrado et al. 2000a,b;). A simple transection was performed, and the proximal and distal stumps were left one in front of the other. On Days 1, 3 and 8 post-lesion (n = 3 each time point), the mice were killed by an overdose of pentobarbital (100 mg kg−1 intraperitoneally).
Materials
All the generic reagents were obtained from Sigma (St Louis, MO, USA) or Roche Diagnostics (Barcelona, Spain). A rabbit polyclonal antibody against a synthetic peptide corresponding to the 11 C-terminal residues (466–477) of mouse GLUT8 was prepared by Q-Biogene (Illkrich, France), as previously described (Gomez et al. 2006). Rabbit antiGLUT3 antibody was obtained from Calbiochem (San Diego, CA, USA). Rabbit antiGLUT4 antibodies from Calbiochem and Abcam (Cambridge, UK) were used, obtaining similar results.
Immunohistochemistry
The immunohistochemical methods were performed as previously described (Gomez et al. 2010). The brains were extracted and placed overnight in 4% paraformaldehyde. After fixation, tissues were dehydrated in increasing concentrations of ethanol, embedded in paraffin, serially sectioned (3 μm) in an HM 310 Microm microtome and collected on polylysine-coated slides. Sections were deparaffined and rehydrated. Antigen retrieval was performed by heating the sections at 100 ºC in a water-bath for 15 min in citrate buffer (10 mm pH 8). Then, the sections were washed three times in 0.1 m phosphate buffer with 0.2% Triton X-100 (PBST), incubated with 3% H2O2 in methanol for 20 min to quench endogenous peroxidase activity, and processed for the immunohistochemical analysis with the corresponding primary antibody. Immunohistochemistry for GLUT8 was performed following the immunoperoxidase procedure corresponding to the Vectastain Elite ABC kit from Vector Laboratories (Burlingame, CA, USA). Sections were incubated overnight with the rabbit anti-GLUT8 polyclonal antiserum (dilution 1 : 500) at 4 ºC. The non-specific signal was blocked with 10% normal goat serum. The GLUT3 and GLUT4 immunohistochemistry procedures (1 : 300 dilution for both) were similar to GLUT8, but incubation with the primary antibody was performed for 2 h at room temperature followed by overnight incubation at 4 ºC.
Image quantification
Images of the facial nuclei (coordinates: interaural: −1.88 to −2.68 mm; bregma: −5.68 to −6.48 mm) were captured and analysed with the NIS-elements BR 2.30 program from Nikon (Tokyo, Japan), and the mean density (MD), indicative of the immunoreactivity (IR) levels in the facial nuclei, was calculated. For the MD calculation of GLUT4-IR and GLUT8-IR, the area of the motoneurons was defined and the MD was calculated for each motoneuron. The mean of the MDs of the injured cells in a particular section was compared with the MD of the contralateral non-lesioned cells analysed on the same slide, which underwent the same immunohistochemical protocol (which was confirmed by analyzing the background staining on both sides). The MD of the control sections was taken as one and was compared with the MD of the contralateral, lesioned nucleus. To calculate the MD of GLUT3-IR, the facial nucleus area was selected and the MD was calculated on the total nucleus section surface. The results obtained are expressed as the mean ± SD. Statistical significances were determined by a Student's t-test.
Results
GLUT3-IR in the facial nucleus was detected, as expected, in the neuropil, while the cell bodies were devoid of GLUT3-IR. Facial nerve axotomy did not alter GLUT3-IR localisation, and the levels of GLUT3-IR in the nucleus of the lesioned nerve did not change on any of the three analyzed post-lesion times (Days 1, 3 and 8) if compared with the non-injured contralateral nucleus (Figs 1 and 4).
Fig. 1.
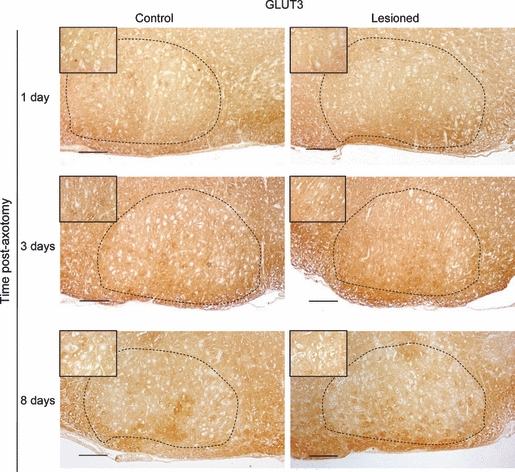
GLUT3 immunoreactivity in the facial nucleus on Days 1, 3 and 8 post-axotomy and in the contralateral non-injured nucleus. Insets show a magnification of some representative facial motoneurons. The dotted line delineates the facial nucleus area. Scale bar: 100 μm.
Fig. 4.
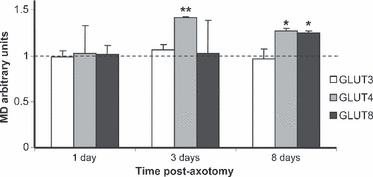
Quantification of the mean density (MD) of GLUT3, GLUT4 and GLUT8 immunoreactivity in the facial nucleus. The MD of the control nucleus was taken as 1, and the density of the immunoreactivity in the lesioned nuclei represents the variation in relation to the contralateral non-injured nuclei. *P< 0.05; **P< 0.01.
GLUT4-IR in the facial nucleus was located in the neuronal cell bodies of the motoneurons. GLUT4-IR localisation was not modified after the facial nerve lesion, but GLUT4 levels increased. One day after nerve injury, the intensity of labeling in the lesioned cells remained unchanged. However, on Day 3 post-axotomy the intensity of GLUT4-IR markedly increased in the injured cells, where levels were 42% higher than those of the control nucleus (P< 0.01). On Day 8 post-lesion, levels remained high, although the differences with the control motoneurons were not as high as they were 5 days before (27%, P< 0.05; Figs 2 and 4).
Fig. 2.
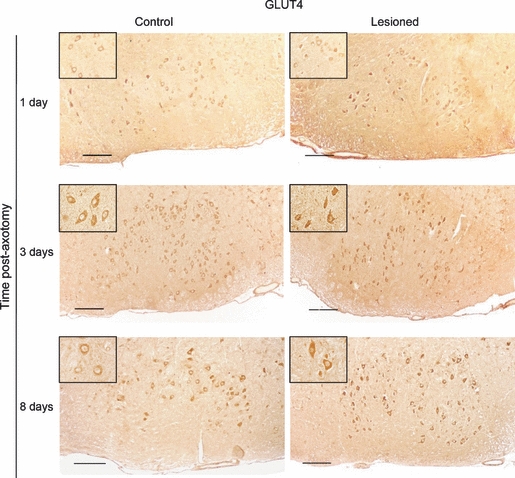
GLUT4 immunoreactivity in the facial nucleus on Days 1, 3 and 8 post-axotomy and in the contralateral non-injured nucleus. Insets show a magnification of some representative facial motoneurons. Scale bar: 100 μm.
GLUT8-IR was located in the motoneurons’ cell bodies of the facial nucleus similarly to GLUT4, and the localization of the immunoreactivity was not modified after the lesion. Conversely, facial nerve injury induced an increase in GLUT8-IR, although the levels of this transporter did not reach those of GLUT4. GLUT8-IR variations were not significant 1 and 3 days post-lesion; however, after 8 days the GLUT8 levels were 24% (P< 0.05) higher in the lesioned nucleus when compared with the contralateral non-injured motoneurons (Figs 3 and 4).
Fig. 3.

GLUT8 immunoreactivity in the facial nucleus on Days 1, 3 and 8 post-axotomy and in the contralateral non-injured nucleus. Insets show a magnification of some representative facial motoneurons. Scale bar: 100 μm.
Discussion
Peripheral nerve axotomy induces a complex response in the CNS that activates several metabolic processes, including glucose metabolism. Glucose uptake increases in regenerating nerve cells as a response to nerve lesion (Kreutzberg & Emmert, 1980; Smith et al. 1984). More precisely, a 23% increase in glucose uptake was noted 7 days after the facial nerve lesion in adult rats (Ito et al. 1999). Our results reveal that the levels of GLUT4 and GLUT8 increase over a similar period, which indicates that probably these transporters are responsible for the glucose transport that occurs after peripheral nerve injury. Whereas the levels of GLUT3, considered the ideal choice for neuronal transporters (Simpson et al. 2008), do not increase their levels, GLUT4 and GLUT8 strikingly rise after axotomy. The increase in GLUT4 is higher and takes place earlier, suggesting that this transporter may play a main role. GLUT4 levels could be regulated by insulin, which is produced in the brain (Schechter et al. 1992) and stimulates the translocation of GLUT4 to plasma membranes in SH-SY5Y human neuroblastoma cells (Benomar et al. 2005), the hippocampus (Grillo et al. 2009) and cultured cerebellar neurons (Bakirtzi et al. 2009). However, the levels of insulin in the adult brain are low (Schechter et al. 1992) and, to our knowledge, insulin upregulation after motoneuron lesion has not been reported. On the other hand, GLUT4 is also regulated by the insulin-like growth factor 1 (IGF1) through IGF1-induced Akt phosphorylation (Cheng et al. 2000). The Akt signaling pathway is activated in sciatic motoneurons after nerve axotomy (Murashov et al. 2001), and the expression of IGF1 and its receptor IGFR increases 4–7 days after facial nerve transection (Gehrmann et al. 1994). This suggests that GLUT4 overexpression in lesioned facial neurons may be regulated by IGF1. GLUT8 is thought to be an intracellular transporter that carries glucose between intracellular components (Piroli et al. 2002). Nevertheless, GLUT8 levels are high in cell clones with high levels of glucose consumption, which would suggest that their levels are related to glucose metabolism (Romero et al. 2007). The correlative increase of GLUT4 and GLUT8 suggests that GLUT4 is a transporter for an urgent situation when considerable glucose uptake is needed, and that GLUT8 is regulated later to intracellularly manage sugar.
Interestingly, glucose uptake or utilisation lowers when the motoneurons degenerate. This has been observed in axotomised neonatal rats, in which the injured motoneurons do not survive injury (Ito et al. 1999), and also in a mouse model of motoneuron disease, where glucose uptake is reduced in brain motor regions and in the spinal cord (Browne et al. 2006). Moreover, levels of glucose transport in cerebral cortex synaptic terminals are markedly decreased in Cu/Zn-SOD mutant mice, which are models of motoneuron disease (Guo et al. 2000). These findings suggest that the metabolic defects involving impairment of glucose transporters may be principal components of the neurotoxic mechanisms leading to motoneuron death. The levels of glucose transporters increase postnatally in the brain, (Vannucci et al. 1998), which indicates a more important role for GLUT transporters in the adult than in the younger brain (Rafiki et al. 2003; Gomez et al. 2010). It is likely that the increase of GLUTs expression and, by extension, of glucose uptake, can be protective for motoneurons in the adult brain, compared with the neonatal brain. We cannot rule out that, in addition to GLUT4 and GLUT8, other non-neuronal transporters, like GLUT1, could also be upregulated after a nerve lesion in order to transport glucose from the brain vessels to the parenchyma. Moreover, other metabolic mechanisms can also be involved. In this sense, we cannot exclude that astrocytes, which are metabolic regulators in the brain (Allaman et al. 2010), may also play a role in the lesion recovery. In fact, astrocytes become reactive and undergo important changes after motoneuron injury (Moran & Graeber, 2004), and the condition of the astrocytes has a role in the disease progression in a mouse model of motoneuron disease (see Allaman et al. 2010 for review). Nevertheless, the increase in glucose uptake observed in previous studies, together with the increase in glucose transporters that we show here, suggest that glucose metabolism and glucose transporters expression in the neurons is necessary for nerve recovery.
Acknowledgments
This research has been supported by grants from the CEU Cardenal Herrera University (PRUCH), the Copernicus-Santander Research Program, and by grant SAF 2004–00228 from the Spanish Government and the European Regional Development Fund (ERDF/FEDER). The research group is a member of the Network for Cooperative Research on Membrane Transport Proteins (REIT), co-funded by the Spanish Ministry of Education and Science and by the European Regional Development Fund (ERDF; Grant BFU2007-30688-E/BFI).
References
- Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2010;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Bakirtzi K, Belfort G, Lopez-Coviella I, et al. Cerebellar neurons possess a vesicular compartment structurally and functionally similar to Glut4-storage vesicles from peripheral insulin-sensitive tissues. J Neurosci. 2009;29:5193–5201. doi: 10.1523/JNEUROSCI.0858-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benomar Y, Roy AF, Aubourg A, et al. Cross down-regulation of leptin and insulin receptor expression and signalling in a human neuronal cell line. Biochem J. 2005;388:929–939. doi: 10.1042/BJ20041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE, Yang L, DiMauro JP, et al. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol Dis. 2006;22:599–610. doi: 10.1016/j.nbd.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Reinhardt RR, Lee WH, et al. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci USA. 2000;97:10 236–10 241. doi: 10.1073/pnas.170008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choeiri C, Staines W, Messier C. Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience. 2002;111:19–34. doi: 10.1016/s0306-4522(01)00619-4. [DOI] [PubMed] [Google Scholar]
- Devaskar SU, Rajakumar PA, Mink RB, et al. Effect of development and hypoxic-ischemia upon rabbit brain glucose transporter expression. Brain Res. 1999;823:113–128. doi: 10.1016/s0006-8993(99)01143-9. [DOI] [PubMed] [Google Scholar]
- El Messari S, Leloup C, Quignon M, et al. Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J Comp Neurol. 1998;399:492–512. doi: 10.1002/(sici)1096-9861(19981005)399:4<492::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Yao DL, Bonetti B, et al. Expression of insulin-like growth factor-I and related peptides during motoneuron regeneration. Exp Neurol. 1994;128:202–210. doi: 10.1006/exnr.1994.1128. [DOI] [PubMed] [Google Scholar]
- Gomez O, Romero A, Terrado J, et al. Differential expression of glucose transporter GLUT8 during mouse spermatogenesis. Reproduction. 2006;131:63–70. doi: 10.1530/rep.1.00750. [DOI] [PubMed] [Google Scholar]
- Gomez O, Ballester-Lurbe B, Poch E, et al. Developmental regulation of glucose transporters GLUT3, GLUT4 and GLUT8 in the mouse cerebellar cortex. J Anat. 2010;217:616–623. doi: 10.1111/j.1469-7580.2010.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Hendry RM, et al. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Kindy MS, Kruman I, et al. ALS-linked Cu/Zn-SOD mutation impairs cerebral synaptic glucose and glutamate transport and exacerbates ischemic brain injury. J Cereb Blood Flow Metab. 2000;20:463–468. doi: 10.1097/00004647-200003000-00004. [DOI] [PubMed] [Google Scholar]
- Hamlin GP, Cernak I, Wixey JA, et al. Increased expression of neuronal glucose transporter 3 but not glial glucose transporter 1 following severe diffuse traumatic brain injury in rats. J Neurotrauma. 2001;18:1011–1018. doi: 10.1089/08977150152693700. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Nagata E, et al. Uncoupling of cerebral blood flow and glucose utilization in the regenerating facial nucleus after axotomy. Neurosci Res. 1999;35:207–215. doi: 10.1016/s0168-0102(99)00088-7. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW, Emmert H. Glucose utilization of motor nuclei during regeneration: a [14C]2-deoxyglucose study. Exp Neurol. 1980;70:712–716. doi: 10.1016/0014-4886(80)90197-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res Brain Res Rev. 2004;44:154–178. doi: 10.1016/j.brainresrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Murashov AK, Haq IU, Hill C, et al. Crosstalk between p38, Hsp25 and Akt in spinal motor neurons after sciatic nerve injury. Brain Res Mol Brain Res. 2001;93:199–208. doi: 10.1016/s0169-328x(01)00212-1. [DOI] [PubMed] [Google Scholar]
- Piroli GG, Grillo CA, Hoskin EK, et al. Peripheral glucose administration stimulates the translocation of GLUT8 glucose transporter to the endoplasmic reticulum in the rat hippocampus. J Comp Neurol. 2002;452:103–114. doi: 10.1002/cne.10368. [DOI] [PubMed] [Google Scholar]
- Rafiki A, Boulland JL, Halestrap AP, et al. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience. 2003;122:677–688. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Romero A, Terrado J, Brot-Laroche E, et al. Glucose transporter GLUT8 mRNA expression in intestinal Caco-2 cells is regulated by growth and metabolism. Horm Metab Res. 2007;39:62–64. doi: 10.1055/s-2007-957351. [DOI] [PubMed] [Google Scholar]
- Sankar R, Thamotharan S, Shin D, et al. Insulin-responsive glucose transporters-GLUT8 and GLUT4 are expressed in the developing mammalian brain. Brain Res Mol Brain Res. 2002;107:157–165. doi: 10.1016/s0169-328x(02)00487-4. [DOI] [PubMed] [Google Scholar]
- Schechter R, Whitmire J, Holtzclaw L, et al. Developmental regulation of insulin in the mammalian central nervous system. Brain Res. 1992;582:27–37. doi: 10.1016/0006-8993(92)90313-x. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Dwyer D, Malide D, et al. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab. 2008;295:E242–E253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Mehler S. 2-deoxy[14C]glucose uptake in the rat hypoglossal nucleus after nerve transection. Exp Neurol. 1980;69:617–626. doi: 10.1016/0014-4886(80)90055-2. [DOI] [PubMed] [Google Scholar]
- Smith CB, Crane AM, Kadekaro M, et al. Stimulation of protein synthesis and glucose utilization in the hypoglossal nucleus induced by axotomy. J Neurosci. 1984;4:2489–2496. doi: 10.1523/JNEUROSCI.04-10-02489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrado J, Monnier D, Perrelet D, et al. NGF-induced motoneuron cell death depends on the genetic background and motoneuron sub-type. Neuroreport. 2000a;11:1473–1477. [PubMed] [Google Scholar]
- Terrado J, Monnier D, Perrelet D, et al. Soluble TNF receptors partially protect injured motoneurons in the postnatal CNS. Eur J Neurosci. 2000b;12:3443–3447. doi: 10.1046/j.1460-9568.2000.00240.x. [DOI] [PubMed] [Google Scholar]
- Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- Urabe T, Hattori N, Nagamatsu S, et al. Expression of glucose transporters in rat brain following transient focal ischemic injury. J Neurochem. 1996;67:265–271. doi: 10.1046/j.1471-4159.1996.67010265.x. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Clark RR, Koehler-Stec E, et al. Glucose transporter expression in brain: relationship to cerebral glucose utilization. Dev Neurosci. 1998;20:369–379. doi: 10.1159/000017333. [DOI] [PubMed] [Google Scholar]
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]


