Abstract
Classical fiber dissection of post mortem human brains enables us to isolate a fiber tract by removing the cortex and overlying white matter. In the current work, a modification of the dissection methodology is presented that preserves the cortex and the relationships within the brain during all stages of dissection, i.e. ‘cortex-sparing fiber dissection’. Thirty post mortem human hemispheres (15 right side and 15 left side) were dissected using cortex-sparing fiber dissection. Magnetic resonance imaging study of a healthy brain was analyzed using diffusion tensor imaging (DTI)-based tractography software. DTI fiber tract reconstructions were compared with cortex-sparing fiber dissection results. The fibers of the superior longitudinal fasciculus (SLF), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF) and uncinate fasciculus (UF) were isolated so as to enable identification of their cortical terminations. Two segments of the SLF were identified: first, an indirect and superficial component composed of a horizontal and vertical segment; and second, a direct and deep component or arcuate fasciculus. The IFOF runs within the insula, temporal stem and sagittal stratum, and connects the frontal operculum with the occipital, parietal and temporo-basal cortex. The UF crosses the limen insulae and connects the orbito-frontal gyri with the anterior temporal lobe. Finally, a portion of the ILF was isolated connecting the fusiform gyrus with the occipital gyri. These results indicate that cortex-sparing fiber dissection facilitates study of the 3D anatomy of human brain tracts, enabling the tracing of fibers to their terminations in the cortex. Consequently, it is an important tool for neurosurgical training and neuroanatomical research.
Keywords: fiber dissection, white matter tracts, diffusion tensor imaging tractography, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, uncinate fasciculus
Introduction
The white matter of the cerebrum underlies the outer cortex of gray matter, and is composed of densely packed axons that are organized in fascicles or fiber tracts. These tracts form a complex 3D architecture within the hemispheres, the brainstem and the spinal cord. A detailed knowledge of the architectural anatomy of the white matter tracts is paramount, for surgical decision-making and strategical planning for surgical management of parenchymal brain lesions, such as gliomas and arteriovenous malformations. Specific knowledge of the trajectories and cortical terminations of the white matter bundles is also of great value for neuroscience research, as it opens a new door to understanding brain functioning by anatomical connectivity.
Magnetic resonance imaging may be sensitized to diffusion of water molecules in the direction of the field gradient. Diffusion tensor is a mathematic model of diffusion of water molecules in 3D space. The tensor is a matrix of numbers derived from diffusion measurements in several different directions, from which one can estimate the diffusivity in any arbitrary direction or determine the direction of maximum diffusivity. Within white matter, diffusion is anisotropic (directionally dependent) as axonal membranes and myelin sheaths present barriers to the motion of water molecules in directions not parallel to their own orientation (Jellison et al. 2004). Based on these principles, diffusion tensor imaging (DTI) tractography is able to map white matter fiber direction, providing a unique opportunity to study white matter architecture in vivo (Jones et al. 1999; Basser et al. 2000; Le Bihan et al. 2001; Catani et al. 2002, 2003, 2005; Mori & van Zijl, 2002; Hagmann et al. 2003). Consequently, DTI tractography has renewed interest and opened new avenues for in vivo analysis of white matter anatomy in the human brain. However, there are two inherent shortcomings to DTI studies. First, the inability to follow the terminal branches of the white matter bundles, therefore their final cortical terminations can only be inferred from the location and orientation of the average tract end points (Catani et al. 2003). Second, the inability to validate the DTI findings with a detailed anatomical exposition of the white matter fibers studied, therefore some of the white matter pathways observed with the DTI technique may not correspond to actual anatomical connections (Pierpaoli et al. 2001; Catani et al. 2002, 2003; Schmahmann et al. 2007). This is especially relevant in areas where knowledge of white matter neuroanatomy is limited or there are conflicting results. This is illustrated by the fact that the recent literature revealed many inconsistencies regarding the definitions, course and cortical terminations of the major associative brain pathways, such as the superior longitudinal fasciculus (SLF), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF) and uncinate fasciculus (UF; Ture et al. 1997, 1999, 2000; Henry et al. 2004; Catani et al. 2005; Makris et al. 2005; Barrick et al. 2007; Catani & Thiebaut de Schotten, 2008; Lawes et al. 2008; Rilling et al. 2008; Fernández-Miranda et al. 2008b; Bernal & Ardila, 2009; Gharabaghi et al. 2009).
Autoradiography is generally considered the anatomical gold standard to validate DTI findings; however, these studies are of course impossible in humans (Pierpaoli et al. 2001; Catani et al. 2002, 2003; Schmahmann et al. 2007). The ‘Brianbow’ technique is a new and promising method to analyze neural network architecture. It is based on staining individual neurons with transgenic strategies for combinatorial expression of fluorescent proteins in the cells (Livet et al. 2007). This technique, however, has been applied for small neural volumes, and has to prove its utility to analysis neural circuitry in large-scale networks in humans. Alternatively, the technique of fiber dissection in post mortem human brains, first described by Klingler (Klingler, 1935; Ludwig & Klingler, 1956; Klingler & Gloor, 1960), and based on freezing of the brains during the fixation process can provide isolation and tracking in the post mortem human brain. However, fiber dissection requires removal of the cortex and overlying white matter in order to reveal the deeper tracts (Klingler, 1935; Ludwig & Klingler, 1956; Klingler & Gloor, 1960; Ture et al. 1997, 1999, 2000; Peuskens et al. 2004; Sincoff et al. 2004; Choi et al. 2006; Fernández-Miranda et al. 2008a,b; Glasser & Rilling, 2008; Lawes et al. 2008). Our group initially performed classical fiber dissection, but then we realized that the extensive removal of surrounding brain tissue precludes the orientation of tracts in relation to cortical landmarks, which hampers analysis of cortical terminations.
Therefore, we developed a modification of the classical fiber dissection methodology in which removal of brain tissue was kept to a minimum in order to preserve the cortex and relationships within the brain until the end of the dissection. Consequently, the trajectory and the orientation of white matter tracts can now be identified reliably and reproducibly. Moreover, the cortical connectivity of the tracts can also be analyzed in more detail than when using DTI. We postulate this dissection technique as ‘cortex-sparing fiber dissection’, referring to the fact that the cortex is preserved until the end of the dissection. In the present work, 30 human post mortem hemispheres were dissected using cortex-sparing fiber dissection, and the anatomy of the SLF, IFOF, ILF and UF was analyzed.
Materials and methods
Cortex-sparing fiber dissection
Thirty human cerebral hemispheres (15 right side and 15 left side) were removed from bodies embalmed with 10% formalin solution for at least 40 days. The age range of subjects of whom brains were obtained was 65–84 years old. The pia mater, arachnoid membrane and vascular structures were carefully removed, and the hemispheres were frozen at −15 °C for 15 days (Fig. 1). The water crystallization induced by the freezing process disrupts the structure of the gray matter (with high water content), enabling us to peel off the cortex from the brain surface. The freezing process also spreads along the white matter fibers, facilitating the dissection of the fiber tracts. The specimens were washed under running water for several hours before performing the dissection.
Fig. 1.
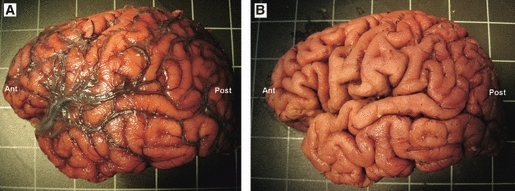
Brain preparation for fiber dissection. (A) Lateral surface of a left hemisphere after it has been removed from the cranium. The pia mater and arachnoid membranes overlie the cortex. (B) Lateral surface of the hemisphere following the removal of the pia mater and arachnoid membranes. Ant, anterior; Post, posterior.
Before the dissection started, the specific surface anatomy of the sulci and gyri was studied in detail. The specimens were dissected in a stepwise systematic manner, from lateral to medial. In the first step of dissection a wooden spatula is used to remove the cortex within the depth of the sulci only, with preservation of the cortex of the convexity surface of the gyri (Figs 2 and 3A). This is a crucial step in the dissection, as it skeletonizes the stems of the gyri, providing space to dissect the white matter, but at the same time preserves the cortical anatomical landmarks until the end of the dissection. Then, the stem of superior, middle and inferior temporal gyri were cut in a plane perpendicular to the convexity surface of the hemisphere, and the anatomic planes of cleavage between the fibers were opened enabling us to follow single tracts (Figs 4 and 5). Care was taken to avoid transecting axons, by utilizing blunt, rather than sharp, dissection, thus minimizing the risk of spurious tracts. The only fibers cut during the dissection were the U-shaped fibers (also known as intergyral or arcuate fibers), that interconnect adjacent gyri as short association fibers. Artifacts and damage to the structures of interest can occur because of insufficient removal of the cortex within the sulci, or by forcing the dissection in an orientation that is not following the natural axonal planes, or dissection at areas with densely packed crossing fibers. Therefore, experience, patience and a systematic procedure are essential in obtaining reliable and reproducible results. In the present study, we demonstrated the isolation of the fibers of the SLF, IFOF, ILF and UF. It was also possible to identify the cortical terminations of these fascicles. The Atlas of the Cerebral Sulci of Ono et al. (1990) was useful to identify and define the specific cortical connections.
Fig. 2.
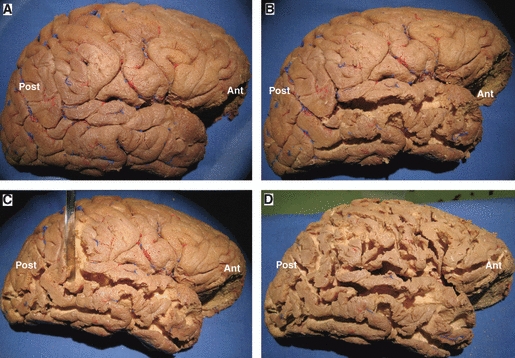
Cortex-sparing fiber dissection steps: removal of the cortex. (A) Lateral surface of a right hemisphere following the removal of the pia mater and arachnoidal membranes. (B–D) Sequential removal of the cortex within the depth of the sulci. It is worth noting that the cortex of the convexity surface of the gyri was not removed in order to preserve the anatomical landmarks until the end of the dissection. Ant, anterior; Post, posterior.
Fig. 3.
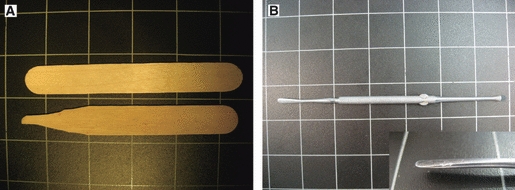
Instruments used for cortex-sparing fiber dissection. (A) Handmade wooden spatula that is used in the initial steps of the dissection, to remove the brain cortex within the depth of the sulci. (B) Curved metallic dissector that was used for further steps in the dissection, to isolate the fibers of a specific tract.
Fig. 4.
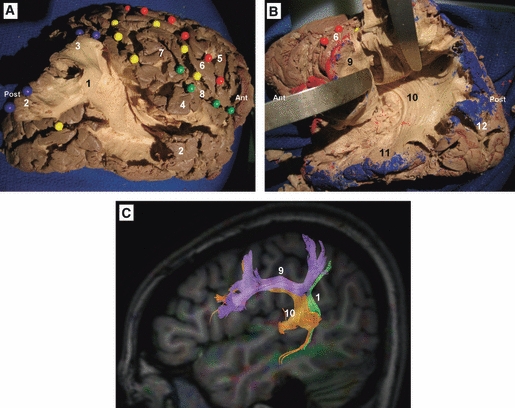
Cortex-sparing fiber dissection of the SLF. (A) Fiber dissection of the vertical segment of the SLF in a right hemisphere. The tract has been tilted and separated from the deeply located AF. Red spheres mark the central sulcus, yellow spheres mark the intraparietal sulcus and the intermediate sulcus of Jensen, green spheres mark the Sylvian fissure, and blue spheres mark the cortical connections of the vertical segment of the SLF. (B) In this left hemisphere the horizontal segment of the SLF has been tilted and separated from the deeply located AF. (A and B) The isolated tracts with preservation of brain structure. (C) DTI tractography reconstruction of the three components of the SLF in a left hemisphere. 1, vertical segment of the SLF; 2, middle temporal gyrus; 3, angular gyrus; 4, superior temporal gyrus; 5, precentral gyrus; 6, postcentral gyrus; 7, supramarginal gyrus; 8, sylvian fissure; 9, horizontal segment of the SLF; 10, AF; 11, inferior temporal gyrus; 12, occipital lobe. Ant, anterior; Post, posterior.
Fig. 5.
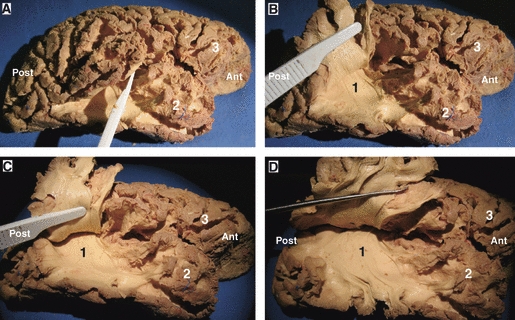
(A–D) Cortex-sparing fiber dissection of the AF in a right hemisphere. This figure shows how cortex-sparing fiber dissection enabled us to isolate this deeper bundle without destroying the superficial components of the fascicle. The fibers of this bundle have a postero-superior orientation, they curve around the caudal end of the Sylvian fissure to take an anterior orientation, then they run within the white matter of the parietal and frontal operculum and terminate at the frontal operculum. 1, AF; 2, temporal lobe; 3, frontal lobe. Ant, anterior; Post, posterior.
The dissections were performed under loupe magnification (× 3.2). Handmade wooden spatulas were used in the initial steps of the dissection (Fig. 3A); once the fibers of a specific tract were identified, the dissection was continued using curved metallic dissectors with various blunt tip sizes (Fig. 3B,C). Sequential digital pictures were taken during the dissection. In between the dissection sessions, brains were stored at room temperature in 4% formalin solution or, alternatively, if there were weeks between sessions, brains were refrozen in water and afterwards rinsed again with room temperature tap water until completely thawed. This freezing and thawing cycle was repeated a maximum of six–seven times, as the quality of the brain tissue deteriorates after some cycles.
DTI tractography study
One healthy volunteer of 52 years old was studied with brain magnetic resonance imaging (MRI) performed on a whole-body 3.0-T scanner (Achieva 3.0T; Philips Healthcare, Best, the Netherlands) with an eight-channel head coil. DTI was performed using a single-shot multislice spin echo-echo planar sequence with the following attributes: diffusion sensitization, 1300 s mm−2; TR, 9577 ms; TE, 77 ms; voxel size 2 mm; no gap between slices; matrix, 224 × 224. Sixty-four diffusion gradient directions were obtained. The DTI data sets and anatomic MRI scans were analyzed with Fiber Trak software for diffusion tensor analysis and fiber tracking from MR Workspace (Philips Healthcare).
We applied a knowledge-based multiple region-of-interest approach in which the tracking algorithm was initiated from user-defined seed regions. Axonal projections were traced both in anterograde and retrograde directions according to the direction of the principal eigenvector in each voxel of the region of interest. Tracking terminated when the fractional anisotropy value was lower than 0.18.
Results
A detailed analysis of the surface anatomy of each hemisphere was performed, and variations in the gyral and sulcal pattern were noted. The anatomy of the following fiber pathways was analyzed.
SLF
The dissection started at the posterior portion of the superior and middle temporal gyri. At this level the fibers of the superficial portion of the SLF were easily separated from the deep portion or arcuate fasciculus (AF). Two superficial components of the SLF were identified: first, horizontal segment, connecting the inferior parietal lobe and the posterior portion of the superior temporal gyrus with the frontal operculum (Figs 4B,C and 11); second, vertical segment, the fibers of this tract had a postero-superior orientation, run lateral to the AF, and connect the posterior portion of the middle temporal gyrus with the posterior portion of the inferior parietal lobe, i.e. the angular gyrus (Figs 4A,C and 11).
Fig. 11.

DTI tractography reconstruction of the association bundles of a left hemisphere. 1, IFOF; 2, ILF; 3, UF; 4, AF; 5, horizontal segment of the SLF; 6, vertical segment of the SLF. Ant, anterior; Post, posterior.
The temporo-frontal segment of the SLF corresponds to the classical AF, which is a long white matter tract deeply located to the vertical and horizontal segments of the superficial SLF (Figs 4B,C, 5 and 11). At this point it is important to remark that the dissection technique used here enabled us to isolate this deeper bundle without destroying the superficial components of the fascicle (Fig. 5). This dissection started by opening the stem of the middle and inferior temporal gyrus. Then, the deep surface of the bundle was carefully separated from the deeper structures. At the temporal lobe, a clear plane of cleavage was identified between the fibers of the AF, that have a superior-inferior orientation, and the fibers of the sagittal stratum (i.e. densely packed fibers located at the lateral surface of the atrium and composed by the optic radiations, IFOF and tapetum) with an anterior-posterior orientation. At the caudal limit of the insula the direction of the AF changes to take a horizontal and anterior orientation, then it runs within the white matter of the parietal and frontal operculum to terminate at the posterior portion of the frontal operculum at which point it is difficult to isolate the individual tracts using this modified technique.
UF
Once the lateral surface of the temporal, occipital, parietal and frontal lobes had been dissected, the opercula can be easily lifted, allowing the removal of the hidden cortex of the inner surface of the operculum, exposing the insular cortex (Fig. 6A). The surface anatomy of the insula was studied. The insula has a triangular shape. The insular apex, the most prominent laterally projecting area of the insular convexity, is directed anteriorly and inferiorly toward the limen insulae. The antero-inferior portion of the insular cortex was removed, exposing the extreme capsule (Fig. 6B). At the antero-inferior portion of the extreme and external capsule, the UF and the IFOF were exposed (Fig. 6C). In this region both fasciculi narrow in section, and the IFOF is located dorsal and posterior to the UF (Figs 6D, 8 and 11). From this region the UF fans out into the frontal and temporal lobe (Figs 8 and 11). At the temporal lobe, the UF crosses the anterior third of the temporal stem, passing through the region of the limen insulae, and a few millimeters of the inferior limiting sulcus of the insula (Figs 6D and 8). Cortical connections of the tract with the temporal pole and the anterior portion of the superior and middle temporal gyri were identified. At the frontal lobe, the tract crosses the anterior portion of the frontal isthmus to terminate at the medial and lateral orbito-frontal cortex.
Fig. 6.
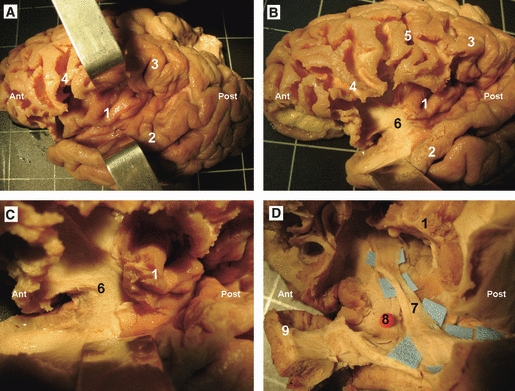
Stepwise dissection of the UF and the IFOF in a left hemisphere. (A) The lateral surface of the temporal, occipital, parietal and frontal lobes has been dissected, enabling us to lift the opercula, allowing the removal of the hidden cortex of the inner surface of the operculum, exposing the insular cortex. (B) The antero-inferior portion of the insular cortex has been removed, exposing the extreme and external capsule. (C) Enlarged view of the previous picture, where the curving fibers of the UF are observed. (D) Further dissection of the antero-inferior portion of the insular lobe, at this region the UF and the IFOF narrow in section and the IFOF is located dorsal and posterior to the UF. 1, insular lobe; 2, superior temporal gyrus; 3, inferior parietal lobe; 4, frontal operculum; 5, central sulcus; 6, antero-inferior portion of the extreme and external capsule; 7, IFOF; 8, UF; 9, temporal pole. Ant, anterior; Post, posterior.
Fig. 8.
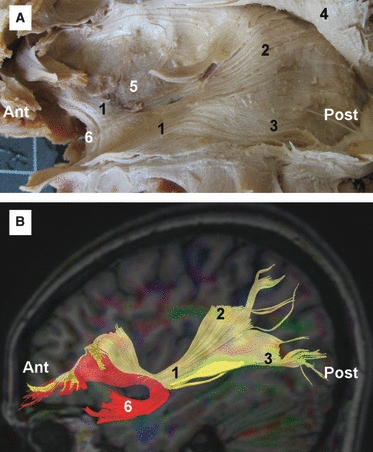
(A) Fiber dissection of the IFOF in a left hemisphere. (B) DTI tractography reconstruction of the IFOF and UF in a left hemisphere. 1, IFOF; 2, fibers of the IFOF connecting with the superior parietal lobe; 3, fibers of the IFOF connecting with the occipital and temporal lobe; 4, AC tilted superiorly; 5, claustrum; 6, UF. Ant, anterior; Post, posterior.
IFOF
The IFOF is first identified at the antero-inferior portion of the extreme and external capsule, at this level the IFOF has a posterior and inferior orientation. Then, the deep surface of the IFOF was carefully separated from the deeper structures, isolating the tract from the rest of the components of the temporal stem and sagittal stratum (Fig. 7A–C). As is shown in Fig. 7, a thin and blunt metal dissector was used for this stage of the dissection. Care was taken to avoid transecting axons, by gently separating the axons with the metallic dissector, rather than cutting, thus minimizing the risk of spurious tracts. At the temporal stem the tract crosses the posterior two-thirds of the temporal stem, in the region between the posterior limit of the UF and the lateral geniculate body. At the lateral portion of the temporal stem, the fibers of the IFOF turn in a posterior direction coursing above the roof of the temporal horn, superior and medial to the optic radiations (Figs 7D, 8 and 11). At the posterior portion of the temporal stem the fibers of the IFOF turn medially to join the sagittal stratum. At the lateral surface of the atrium of the ventricle, the IFOF is located medial to the fibers of the AF and lateral to the optic radiations. Posteriorly, the IFOF is mainly connected to the posterior portion of the convexity surface of the occipital lobe; however, connections with the superior parietal lobe and the temporo-basal area were also identified (Fig. 8).
Fig. 7.
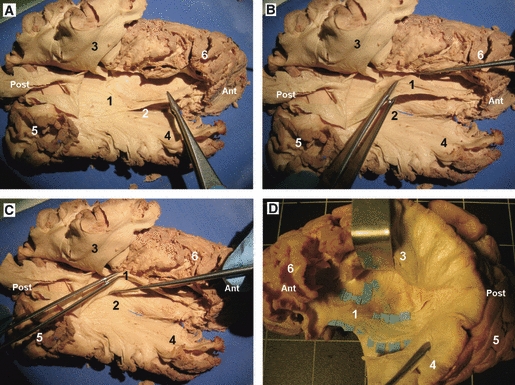
(A–C) Cortex-sparing fiber dissection of the IFOF in a right hemisphere. The deep surface of the IFOF was separated from the deeply located optic radiations. (D) In this left hemisphere, the insula and AF have been tilted superiorly and the temporal lobe inferiorly, exposing the IFOF while it crosses the insular lobe and the temporal stem. 1, IFOF; 2, optic radiations; 3, AF; 4, temporal lobe; 5, occipital lobe; 6, frontal lobe. Ant, anterior; Post, posterior.
Temporal and occipital base and ILF
The occipitotemporal or fusiform gyrus is located at the temporal and occipital base. Medially, the fusiform gyrus is separated from the parahippocampal gyrus and the lingula by the collateral sulcus; laterally, it is separated from the basal surface of the inferior temporal and occipital gyri by the occipitotemporal sulcus. The dissection of this area revealed that the collateral sulcus is deep and the cortex is firmly attached to its surface, as it is in the central and calcarine sulcus. Using a thin metal dissector it was possible to remove the cortex within this sulcus and skeletonize the stem of the fusiform gyrus. As is shown in Fig. 9A,B, the stem of this gyrus has a horizontal orientation, parallel to the basal surface of the temporal lobe. Then, the stem of the gyrus was cut in a plane perpendicular to the direction of the gyrus stem, and the anatomic planes of cleavage between the fibers were opened enabling us to follow the fibers (Figs 9C and 10). The dissections revealed that the fibers originating at the fusiform gyrus have a posterior and lateral orientation, running inferiorly to the occipital horn of the lateral ventricle, finally they terminate at the posterior portion of the inferior occipital gyrus. These fibers that connect the fusiform gyrus with the occipital lobe are part of the occipito-temporal projection system of the ILF. The ILF also connects the temporal pole with the occipital lobe (Figs 10 and 11).
Fig. 9.
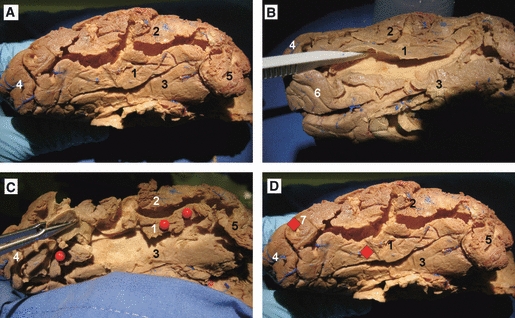
Cortex-sparing fiber dissection of the ILF and temporal base in a right hemisphere. (A) In this hemisphere the cortex within the depth of the collateral sulcus and the occipitotemporal sulcus have been removed. The occipitotemporal or fusiform gyrus is located at the temporal and occipital base. (B) The stem of the occipitotemporal sulcus has been tilted with a dissector. (C) The stem of the occipitotemporal gyrus has been cut in a plane perpendicular to the direction of the gyrus stem, and the anatomic planes of cleavage between the fibers has been opened enabling us to follow the fibers until the cortical termination. (D) The dissections revealed that these fibers connected the fusiform gyrus with the posterior portion of the inferior occipital gyrus. 1, fusiform gyrus; 2, inferior temporal gyrus; 3, parahippocampal gyrus; 4, occipital pole; 5, temporal pole; 6, calcarine fissure; 7, inferior occipital gyrus. Ant, anterior; Post, posterior.
Fig. 10.
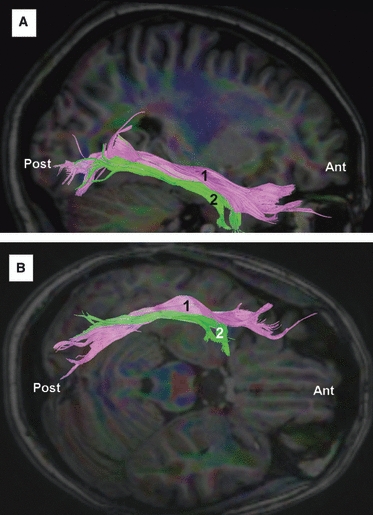
DTI tractography reconstruction of the ILF in a right hemisphere. (A) Lateral view. (B) Inferior view. The ILF (1) connects the temporal pole with the occipital lobe. There are also fibers of the ILF (2) that connect the temporal base (fusiform gyrus) with the posterior portion of the inferior occipital gyrus. Ant, anterior; Post, posterior.
Discussion
Methodological considerations
In the last two decades the rapid development of DTI tractography has enabled us to visualize the subcortical anatomy in the living human brain; in addition, intraoperative mapping of subcortical structures has enabled us to study the functional role of each tract (Duffau, 2008; Duffau et al. 2008a,b, 2009). On the bases of these new insights a renewed interest in analyzing white matter anatomy has been observed. Despite numerous reports analyzing white matter anatomy in the last two decades, the exact 3D geometry and cortical terminations of the main fiber bundles remains poorly described. This is explained by the inherent shortcomings of the available techniques to study white matter anatomy. In this sense, DTI tractography is unable to define the exact cortical termination of the bundles, and the tracts reconstructions has not been validated with a detailed anatomical exposition of the white matter fibers studied (Pierpaoli et al. 2001; Catani et al. 2002, 2003; Schmahmann et al. 2007). Several anatomical studies have recently correlated DTI tractography results with fiber dissection (Rubino et al. 2005; Fernández-Miranda et al. 2008a,b; Mahaney & Abdulrauf, 2008; Peltier et al. 2009, 2010). However, in these studies the classical fiber dissection technique was used, in which adjacent tissue is removed to demonstrate the dissected tract. Destruction of adjacent tissue eliminates important cortical landmarks, making it impossible to analyze the cortical terminations and 3D relationships of a specific fiber tract. In the present work, cortex-sparing fiber dissection is presented; this is an adaptation of the classical dissection methodology where the tracts are isolated without removing the surrounding white matter and cortical structures. Consequently, cortex-sparing fiber dissection enables us to study the 3D relational anatomy of the tract, and to follow the fibers until the cortical termination to analyze the cortical connectivity pattern.
It is important to remark that the quality of the dissections depend on the quality of the specimens, the dissection is especially difficult in brains of elderly subjects with lacunar infarcts. It is also worth mentioning that cortex-sparing fiber dissection is time consuming, and that to obtain proper dissections experience is required; the initial dissection results are frequently disappointing. However, after this initial learning curve, fiber dissection enables us to understand the complicated architecture of the white matter. In our opinion, none of the other techniques to learn white matter anatomy can substitute fiber dissection laboratory training, as it gives a true appreciation of the 3D relationships of the fiber bundles.
Analysis of the anatomy of the major brain associative bundles
Remarkable controversy exists regarding the anatomical course and cortical terminations of the SLF (Henry et al. 2004; Catani et al. 2005; Makris et al. 2005; Barrick et al. 2007; Catani & Thiebaut de Schotten, 2008; Lawes et al. 2008; Rilling et al. 2008; Fernández-Miranda et al. 2008b; Bernal & Ardila, 2009; Gharabaghi et al. 2009). Catani et al. (2005) revealed with DTI tractography that the SLF is composed of two parallel pathways connecting temporal, parietal and frontal regions: first, a direct pathway corresponding to the classical AF; and second, an indirect pathway, running parallel and lateral to the direct pathway, and consisting of an anterior or horizontal segment linking the lateral frontal cortex with the inferior parietal lobe, and a posterior or vertical segment linking the inferior parietal lobe with the temporal lobe. In the present study the two components were isolated. The vertical segment of the indirect pathway has been isolated (see Fig. 4A), and it has been demonstrated how this tract connects the posterior portion of the middle temporal gyrus with the angular gyrus. The horizontal segment of the indirect pathway connects the supramarginal gyrus and posterior portion of the superior temporal gyrus with the frontal operculum. The AF is a long association fiber tract deep and parallel to the indirect pathway. Our dissections revealed that it connects the posterior portion of the middle and inferior temporal gyrus with the frontal operculum.
The UF is a ventral associative C-shaped bundle that connects the anterior temporal lobe with the frontal cortex (Catani et al. 2002). The exact cortical connections of the UF are unknown. Connections with the temporal pole, amygdala, hippocampal formation, and superior and middle temporal gyri have been described (Ebeling & von Cramon, 1992; Sincoff et al. 2004; Schmahmann et al. 2007), as well as connections with the orbital gyri, area subcallosa, gyrus rectus, frontal pole and inferior frontal gyrus (Ebeling & von Cramon, 1992; Peuskens et al. 2004; Schmahmann et al. 2007; Wang et al. 2008). In our current work, the temporal lobe connections were identified with the temporal pole and the anterior portion of the superior and middle temporal gyri, and the frontal lobe connections with the medial and lateral orbito-frontal cortex. One finding that is of importance for surgical planning is that the tract crosses the anterior third of the temporal stem, passing through the region of the limen insulae and a few millimeters of the inferior limiting sulcus of the insula (Fig. 6D).
The IFOF is a ventral associative bundle that connects the frontal lobe with the occipital and parietal lobes via the temporal lobe and insula. In 1909, Curran first described this fascicle using post mortem fiber dissection. Since that time many others have used DTI tractography and white matter dissection to elucidate the anatomical course of this tract (Ture et al. 2000; Catani et al. 2002; Peuskens et al. 2004; Catani & Thiebaut de Schotten, 2008; Fernández-Miranda et al. 2008b; Wang et al. 2008; Martino et al. 2010a,b;). Our group has recently reviewed the anatomy of this bundle with fiber dissection (Martino et al. 2010a,b;). It was shown that this tract crosses the posterior two-thirds of the temporal stem, in the region between the posterior limit of the UF and the lateral geniculate body. Cortex-sparing fiber dissection enabled to isolate the IFOF at the sagittal stratum without destroying the surrounding tracts (see Fig. 7). This revealed that the IFOF is located medial to the fibers of the AF and lateral to the optic radiations in the lateral surface of the atrium of the ventricle. Finally, the fibers were followed until the posterior cortical terminations at the convexity surface of the occipital lobe, superior parietal lobe and temporo-basal area.
Recently, DTI tractography studies have demonstrated that the ILF is composed of a direct and indirect pathway (Catani et al. 2003). The indirect pathway, i.e. the occipitotemporal projection system, consists of U-shaped fibers that connect adjacent gyri at the inferior temporal and occipital convexity. The direct pathway is composed of long association fibers that are located medial to the short fibers. Connections of this tract with the anterior portion of the superior, middle and inferior temporal gyri on the lateral surface of the temporal lobe, the fusiform gyrus, parahippocampal gyrus, the amygdala and the hippocampus have been described (Catani et al. 2003; Schmahmann & Pandya, 2006; Epelbaum et al. 2008). In our current work, the temporal and occipital base were dissected revealing fibers that originate at the fusiform gyrus, and have a horizontal and lateral orientation, running inferior to the occipital horn of the lateral ventricle. Our dissections revealed that these fibers terminate at the posterior portion of the inferior occipital gyrus. These fibers connecting the fusiform gyrus with the occipital lobe represent a medial portion of the ILF. The lateral portion of this tract connects the temporal pole with the occipital lobe (Figs 10 and 11).
Conclusions
Cortex-sparing fiber dissection is presented as a modification of the classical fiber dissection methodology. This enables isolation of a tract with preservation of brain structures during all dissection steps. Consequently, cortex-sparing fiber dissection provides a better representation of the 3D relational anatomy of the tract, and it enables us to follow the fibers until the cortical termination to analyze the cortical anatomical connectivity.
Neuroanatomical laboratory training is very valuable to study and understand the anatomy of white matter fibers. In particular, cortex-sparing fiber dissection facilitates knowledge of this complex anatomy. In our opinion, none of the recently developed surgical guides such as neuronavigation, intraoperative MRI or ultrasonography can provide a similar comprehensive understanding of the 3D fiber pathways organization obtained by fiber dissection laboratory training.
Despite the important progress in white matter anatomy understanding due to DTI tractography, the precise trajectory and cortical connections of the subcortical bundles remains poorly determined. Cortex-sparing fiber dissection may help to elucidate the anatomy of these tracts as it enables us to isolate and track the terminal fibers projecting to the cortex, thus it helps to understand the cortical connectivity of specific tracts. The functional role of a tract may be inferred from its topography within the brain. Knowing the functional role of the cortical areas connected by a certain bundle, it is possible to develop new insights into the putative functional properties of these connections.
Acknowledgments
The authors are deeply grateful to Dr Stephen Metcalfe (from the Department of Neurological Surgery of Newcastle upon Tyne, UK) for English revision and correction of the manuscript. Juan Martino received specific funding from the 11/18 API grant entitled ‘Estudio de la conectividad conectividad cerebral mediante disección de fibras estructural’, ‘Fundación Marqués de Valdecilla’, IFIMAV, Santander, Cantabria, Spain, 8 October 2010.
Author contributions
J.M. designed the research and drafted the manuscript, J.M., P.C.D.W.H., F.V., C.B., E.M.L., J.A.G-P. and H.D. acquired the data, all authors contributed to data analysis/interpretation, critical revision of the manuscript and approval of the article.
References
- Barrick TR, Lawes IN, Mackay CE, et al. White matter pathway asymmetry underlies functional lateralization. Cereb Cortex. 2007;17:591–598. doi: 10.1093/cercor/bhk004. [DOI] [PubMed] [Google Scholar]
- Basser P, Pajevic S, Pierpaoli C, et al. In vivo fiber tracking using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bernal B, Ardila A. The role of the arcuate fasciculus in conduction aphasia. Brain. 2009;132:2309–2316. doi: 10.1093/brain/awp206. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, et al. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, et al. Occipito-temporal connections in the human brain. Brain. 2003;129:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Choi C, Rubino PA, Fernandez-Miranda JC, et al. Meyer's loop and the optic radiations in the transsylvian approach to the mediobasal temporal lobe. Neurosurgery. 2006;59:ONS228–ONS235. doi: 10.1227/01.NEU.0000223374.69144.81. [DOI] [PubMed] [Google Scholar]
- Duffau H. The anatomo-functional connectivity of language revisited. New insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46:927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Duffau H, Leroy M, Gatignol P. Cortico-subcortical organization of language networks in the right hemisphere: an electrostimulation study in left-handers. Neuropsychologia. 2008a;46:3197–3209. doi: 10.1016/j.neuropsychologia.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Duffau H, Peggy Gatignol ST, Mandonnet E, et al. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg. 2008b;109:461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Duffau H, Moritz-Gasser S, Gatignol P. Functional outcome after language mapping for insular World Health Organization Grade II gliomas in the dominant hemisphere: experience with 24 patients. Neurosurg Focus. 2009;27:E7. doi: 10.3171/2009.5.FOCUS0938. [DOI] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, et al. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44:962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Fernández-Miranda JC, Rhoton AL, Jr, Kakizawa Y, et al. The claustrum and its projection system in the human brain: a microsurgical and tractographic anatomical study. J Neurosurg. 2008a;108:764–774. doi: 10.3171/JNS/2008/108/4/0764. [DOI] [PubMed] [Google Scholar]
- Fernández-Miranda JC, Rhoton AL, Jr, Alvarez-Linera J, et al. Three-dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery. 2008b;62:989–1026. doi: 10.1227/01.neu.0000333767.05328.49. [DOI] [PubMed] [Google Scholar]
- Gharabaghi A, Kunath F, Erb M, et al. Perisylvian white matter connectivity in the human right hemisphere. BMC Neurosci. 2009;10:15. doi: 10.1186/1471-2202-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain's language pathways. Cereb Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Thiran JP, Jonasson L, et al. DTI mapping of human brain connectivity: statistical fibre tracking and virtual dissection. Neuroimage. 2003;19:545–554. doi: 10.1016/s1053-8119(03)00142-3. [DOI] [PubMed] [Google Scholar]
- Henry RG, Berman JI, Nagarajan SS, et al. Subcortical pathways serving cortical language sites: initial experience with diffusion tensor imaging fiber tracking combined with intraoperative language mapping. Neuroimage. 2004;21:616–622. doi: 10.1016/j.neuroimage.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. Am J Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Simmons A, Williams SCR, et al. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Klingler J. Erleichterung der makroskopischen Praeparation des Gehirns durch den Gefrierprozess. Schweiz Arch Neurol Psychiatr. 1935;36:247–256. [Google Scholar]
- Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. J Comp Neurol. 1960;115:333–369. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- Lawes IN, Barrick TR, Murugam AB, et al. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. Neuroimage. 2008;39:79. doi: 10.1016/j.neuroimage.2007.06.041. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Ludwig E, Klingler J. Atlas Cerebri Humani: Der innere Bau des Gehirns dargestellt auf Grund mackroskopischer Präparate. Boston: Brown; 1956. [Google Scholar]
- Mahaney KB, Abdulrauf SI. Anatomic relationship of the optic radiations to the atrium of the lateral ventricle: description of a novel entry point to the trigone. Neurosurgery. 2008;63:195–202. doi: 10.1227/01.NEU.0000313121.58694.4A. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, et al. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010a;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Martino J, Vergani F, Robles SG, et al. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery. 2010b;66:4–12. doi: 10.1227/01.NEU.0000348564.28415.FA. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: principles and strategies – a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the Cerebral Sulci. New York: Georg Thieme; 1990. [Google Scholar]
- Peltier J, Verclytte S, Delmaire C, et al. Microsurgical anatomy of the temporal stem: clinical relevance and correlations with diffusion tensor imaging fiber tracking. J Neurosurg. 2009;17:1–6. doi: 10.3171/2009.6.JNS08132. [DOI] [PubMed] [Google Scholar]
- Peltier J, Verclytte S, Delmaire C, et al. Microsurgical anatomy of the ventral callosal radiations: new destination, correlations with diffusion tensor imaging fiber-tracking, and clinical relevance. J Neurosurg. 2010;112:512–519. doi: 10.3171/2009.6.JNS081712. [DOI] [PubMed] [Google Scholar]
- Peuskens D, van Loon J, Van Calenbergh F, et al. Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery. 2004;55:1174–1184. doi: 10.1227/01.neu.0000140843.62311.24. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pjevic S, et al. Water diffusion changes in wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci. 2008;11:426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- Rubino PA, Rhoton AL, Jr, Tong X, et al. Three-dimensional relationships of the optic radiation. Neurosurgery. 2005;57:219–227. doi: 10.1227/01.neu.0000176415.83417.16. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford UP; 2006. [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Sincoff EH, Tan Y, Abdulrauf SI. White matter fiber dissection of the optic radiations of the temporal lobe and implications for surgical approaches to the temporal horn. J Neurosurg. 2004;101:739–746. doi: 10.3171/jns.2004.101.5.0739. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil MG, Pait TG. Is there a superior occipitofrontal fasciculus? A microsurgical anatomic study. Neurosurgery. 1997;40:1226–1232. doi: 10.1097/00006123-199706000-00022. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil DC, Al-Mefty O, et al. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–733. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- Ture U, Yasargil MG, Friedman AH, et al. Fiber dissection technique: lateral aspect of the brain. Neurosurgery. 2000;47:417–426. doi: 10.1097/00006123-200008000-00028. [DOI] [PubMed] [Google Scholar]
- Wang F, Sun T, Li X-G, et al. Diffusion tensor tractography of the temporal stem on the inferior limiting sulcus. J Neurosurg. 2008;108:775–781. doi: 10.3171/JNS/2008/108/4/0775. [DOI] [PubMed] [Google Scholar]


