Abstract
In continuation to our studies on radioresistance in meningioma, here we show that radiation treatment (7Gy) induces G2/M cell cycle arrest in meningioma cells. Phosphorylation of Chk2, Cdc25c and Cdc2 were found to be key events since interference with Chk2 activation and cyclin B1/Cdc2 interaction led to permanent arrest followed by apoptosis. Irradiated cells showed recovery and formed aggressive intracranial tumors with rapid spread and morbidity. Nevertheless, knock down of uPAR with or without radiation induced permanent arrest in G2/M phase and subsequent apoptosis in vitro and in vivo. In conclusion, our data suggest that combination treatment with radiation and uPAR knockdown or other inhibitors resulting in non-reversible G2/M arrest may be beneficial in the management of meningiomas.
Keywords: meningioma, radiotherapy, G2/M cell cycle arrest, Chk2
1. Introduction
External beam radiotherapy (RT) has proven beneficial for patients with incompletely resected meningiomas [1]. Additionally, RT has been used for high surgical risk patients, patients with meningiomas situated at surgically inaccessible areas and within soft tissues, and patients of advanced age [2]. Stereotactic radiosurgery has also been used for meningiomas that are of irregular shape and size [3;4]. Further, intensity-modulated radiotherapy (IMRT) has proven to be successful in patients with complex-shaped meningioma of the skull base with overall local control of 93.6% [5]. Nonetheless, the benefit of radiation treatment has been questioned in the management of meningiomas [6]. Although the median recurrence-free survival after radiation treatment is higher for patients with benign meningiomas as compared to patients with higher-grade meningiomas (WHO grade II/III), recurrence has been reported [7]. Oncocytic meningioma, an uncommon variant of meningioma, also suggests that radiation therapy might worsen the course of disease [8]. To a different end, higher doses of radiation increase the risk of radiation-induced meningioma (RIM) formation with a sharp decrease in the latency period between exposure and tumor development. Even exposure to low radiation doses has been shown to significantly increase the risk of meningioma [9;10]. Adding to the increasing concerns of radiation therapy for meningioma, radiation has been shown to induce de-differentiation of meningioma into osteosarcoma in two independent studies [11;12]. All of these studies document the phenomenon of radiation resistance with meningioma but the precise mechanism of action (e.g., repair, cell cycle control or some other responsible process) is yet to be elucidated.
A complicated network of redundant and superimposed checkpoint controls are in charge of the regulatory mechanisms and maintenance of the order of key cell cycle phase transitions [13]. Ionizing radiation causes damage to DNA that is apparently proportional to the absorbed dose. The cellular response to DNA damage results in a pleiotropic activation of numerous signaling pathways, cell cycle checkpoints, DNA repair and transcription [14]. However, the extent of DNA damage determines whether to extend cell cycle arrest or abrogate the check points, leaving options for cellular machinery to repair and subsist or to give up and culminate in apoptotic death [15;16]. During the first half of the 20th century, human neural tissue was generally considered relatively resistant to the carcinogenic and other ill effects of ionizing radiation. As a result, exposure to relatively high doses of x-rays from diagnostic examinations and therapeutic treatment was common.
In tumour cells, uPAR is aberrantly expressed through activation of signalling pathways by genetic alterations and microenvironmental influences. Coordination of extracellular matrix (ECM) proteolysis and cell signalling by uPAR underlies its important function in cell proliferation and survival and makes it an attractive therapeutic target in cancer. Many studies show that uPAR mediates survival cell signalling through the MAP kinases pathways, FAK, Src and Rac[17–20]. Other pathways, such as JAK–STAT and PI3K–Akt, have also been implicated in uPAR signalling[21–23]. Although anti-uPAR therapeutic agents are yet to enter clinical trials, uPAR presents multiple opportunities for targeted therapies that might be beneficial in cancer and, potentially, other human diseases. For these reasons and as an extension of our previous studies on meningioma that investigated potential benefits or drawbacks and associated changes with ionizing radiation in meningioma treatment regimens, the present study analyzed the mechanism and outcome of radiotherapy in combination with uPAR gene silencing in vitro and in vivo.
2. Material and Methods
2.1 Cell culture conditions
We used the following human meningioma cell lines: IOMM-Lee [24], SF3061 [25], and CH 157 MN [26] cell lines, which were kindly provided by Dr. Ian E. McCutcheon (University of Texas M.D. Anderson Cancer Center, Houston, TX), Dr. Yancey Gillespie (University of Alabama at Birmingham), and Dr. Anitha Lal (University of California, San Francisco, CA). IOMM-Lee and CH 157 MN luciferase cell lines were provided by Dr. Randy L. Jensen (University of Utah). All the cell lines were maintained in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Pitsburg, PA) supplemented with 10% fetal bovine serum, 100 U/mL streptomycin and 100 U/mL penicillin (Invitrogen, Carlsbad, CA). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C, treated with NSC 109555 ditosylate or Nutlin or Aloisine (Santa Cruz Biotechnology, Santa Cruz, CA), and incubated for the indicated period of time in complete medium. pChk2 (Thr 68), Chk2, p53, cyclin B1, Cdc25C, pCdc25c (Ser 216), pCdc2 (Thr 14/Tyr15) and GAPDH antibodies were obtained from (Santa Cruz Biotechnology, Santa Cruz, CA). Propidium iodide was obtained from Biosure (Grass Valley, CA).
2.2 Radiation treatment
The RS 2000 Biological Irradiator (Rad Source Technologies, Inc., Boca Raton, FL) X-ray unit operated at 150 kV/50 mA and delivering 0.71 Gy/min was used for radiation treatments. Cells were given a single dose of radiation (3, 5, 7 or 10 Gy). For experiments involving the inhibitors, radiation was given after 1 hr of inhibitor treatment.
2.3 Colony formation assay
Cell survival after exposure to x-rays at various doses was quantified by plating IOMM Lee, CH 157 MN and SF3061 cells (1×104) into 150-mm plates containing the complete medium. After the indicated periods of incubation, cell colonies were rinsed with PBS, fixed with methanol, and then stained with 2% Giemsa solution.
2.4 MTT proliferation assay
IOMM Lee, CH 157 MN and SF3061 cells (2×105) were seeded in 6-well plates and irradiated as described earlier. Six hours later, cells were trypsinized, counted and seeded at 1×104 cells per well in 96-well plates (8 wells per treatment group). After the indicated hours of incubation in conditioned medium, 20 μL of MTT reagent were added to the cells, followed by another 4 hrs of incubation at 37°C. Acid-isopropanol (0.04 M HCl/isopropanol) was added to all wells and mixed vigorously so that the formazan crystals dissolved effectively. Absorbance was measured on a microtiter plate reader (Model 680, BioRad, Hercules, CA) with a test wavelength of 550 nm and a reference wavelength of 655 nm.
2.5 Cell cycle analysis
Cell cycle distribution was assayed by determining DNA content. Cells were irradiated and incubated for the indicated time periods. Cells were then fixed in 3% (w/v) paraformaldehyde for 10 min, permeabilized on ice in PBS-0.5% Triton X-100 for 15 min, washed and resuspended in 0.5 mL of PBS containing 1% FBS, 1 mg/mL RNaseA and 50 μg/mL propidium iodide. The samples were incubated for 1 hr at 37°C and then subjected to FACS analysis.
2.6 Transfection studies
Plasmids expressing siRNA were constructed as described elsewhere [27]. All transfection experiments were performed with fuGene HD transfection reagent as per the manufacturer’s protocol (Roche Applied Science). IOMM-Lee and CH 157 MN cells were transfected with plasmid constructs containing scrambled sequence (SV) or ShRNA for uPAR expressing sequences. After 6 h of transfection, complete medium was added and kept for 18h. Later the cells were irradiated at 7 Gy dose and incubated for further 24h before subjecting to FACS or Western blotting analysis.
2.7 Western blotting
After radiation or inhibitor treatment for a specified time interval, monolayer cells were collected and lysed as described previously [28]. Cell lysates were cleared by centrifugation at 14,000 rpm for 15 min. Lysates were resolved by SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. The membrane was incubated in PBS containing 0.05% Tween 20 and 5% (w/v) nonfat dry milk and then exposed to the desired primary antibody (1:1000 dilution) for 1 hr at room temperature. After treatment with appropriate secondary antibody (1:5000 dilution), the immunoreactive bands were visualized using the enhanced chemiluminescence method.
2.8 TUNEL assay
To evaluate apoptosis among irradiated and inhibitor-treated cells, we performed the terminal deoxynucleotide transferase (TdT)-mediated biotin-dUTP nick end labeling (TUNEL) assay using the in situ cell death detection kit according to the manufacturer’s recommendations (Roche Applied Science, Indianapolis, IN). Briefly, 5,000 cells were seeded onto 8-well chamber slides, treated with Chk2 phosphorylation inhibitor, irradiated after 1 hr, and incubated for 36 hrs. The cells were then washed, fixed and permeabilized with freshly prepared 0.1% Triton X-100 containing 0.1% sodium citrate. Later, the cells were incubated with TUNEL reaction mixture for 1 hr at 37°C in a humidified chamber. The slides were washed three times with PBS, and the incorporated biotin-dUTP was detected under a fluorescent microscope. Cell death was quantified as the relative percent of apoptosis as compared to the controls.
2.9 Immunofluorescence
Cells were fixed in 3% (w/v) paraformaldehyde for 10 min, washed twice in PBS, permeabilized in PBS-T (PBS containing 0.5% (v/v) Triton X-100), and blocked in 2% BSA in PBS. The Chk2 antibody was diluted 1:100 in PBS containing 1% BSA. The cells were incubated overnight with the antibody at 4°C, then rinsed three times in PBS-T, and incubated for 1 hr at room temperature with a Fluorophore-conjugated goat anti-rabbit antibody at a dilution of 1:500 in PBS containing 1% BSA. The cells were washed three times in PBS-T and incubated with Slow Fade Antifade Kit with DAPI (Molecular Probes, Eugene, OR).
2.10 In vivo studies
The Institutional Animal Care and Use Committee at the University of Illinois College of Medicine in Peoria approved all experimental procedures involving the use of animals. Intracranial implantation of the luciferase-expressing cells and normal IOMM Lee cells was accomplished as described previously [29;30;30]. Briefly, luciferase-expressing stable IOMM Lee and CH 157 MN cells were subjected to 7 Gy radiation in two sets. Irradiated cells from the first set were trypsinized and infused into the brains of one group of animals on the same day. The second set of cells were allowed to recover for 72 hrs with a regular replenishment of fresh medium every 24 hrs and infused into another group of animals. Nude mice infused with non-irradiated cells served as controls for the respective groups. The animals were observed for changes in morphological characteristics and luminescence was tracked with in vivo imaging system on a daily basis for two weeks. Similarly, IOMM Lee cells which are irradiated or uPAR knocked down were implanted in different groups of nude mice. After 2 weeks, the brains were harvested and either snap frozen or formalin fixed for further analyses.
2.11 Rt-PCR and Immunohistochemistry
Total RNA was extracted from frozen brain tissues and subjected to cDNA synthesis using Transcriptor first strand cDNA synthsis kit (Roche Applied Science). PCR was performed using Cyclin B1 human specific primers and products were analyzed on 1.8% agarose gels. Sections of formalin fixed and paraffin embedded brain tissue (FFPE) were subjected to H&E staining to verify the tumor formation and immunostaining for Cyclin B1 (1:100 dilution) and HRP-conjugated secondary antibody (1:200 dilution) as described elsewhere [31].
2.12 tStatistical analysis
All data are presented as means ± standard errors (SE) of at least three independent experiments (each performed at least in triplicate). One way analysis of variance (ANOVA) combined with the Tukey post-hoc test of means were used for multiple comparisons of cell culture experiments. Statistical differences are presented at probability levels of p<0.05, p<0.01 and p<0.001.
3. Results
3.1 Radiation treatment induces G2/M cell cycle arrest in meningioma cells
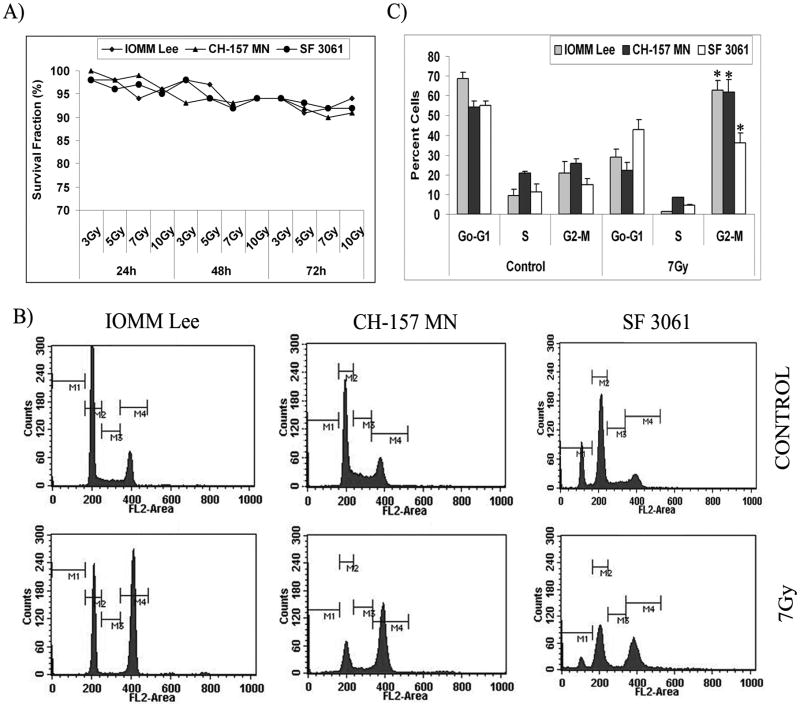
We have previously demonstrated that radiation treatment shows signs of significant initial decrement in the proliferation of meningioma cells [28]. In the present study, we used IOMM Lee, CH 157 MN and SF3061 cells to determine whether the initial inhibition of cell proliferation was caused by cell death or by delay in proliferation. Colony formation assays showed that more than 92% of cells retained colony formation potential with an insignificant portion of cell death due to radiation treatment at all the given doses (3 to 10 Gy) (Fig. 1A). Next, we determined whether the delayed proliferation was a result of perturbations in cell cycle progression. The effect of radiation on cell cycle distribution was determined using flow cytometry for DNA content after staining the cells with propidium iodide. A 24-hr exposure of cells to a 7 Gy dose of radiation resulted in a statistically significant increase in cells in the G2/M phase (Fig. 1B). The increase in percentage of cells in G2/M was accompanied by a resultant decrease in cells in the G0/G1 or S phase in all three cell lines (Fig. 1B). IOMM Lee and CH 157 MN cells showed more than 55% cells in the G2/M phase while SF3061 cells showed more than 35% cells in the G2/M phase after radiation treatment (Fig. 1C). We found that meningioma cells exhibited a high basal line of S phase, and this fraction decreased by more than two-fold in response to radiation (Figs. 1B–C). Furthermore, the sub-G0/G1 population, which is an indicator of cell death, among irradiated cells was insignificant when evaluated against the respective controls, thereby confirming that only cell cycle progression had been affected significantly.
Figure 1. Radiation treatment induces G2/M cell cycle arrest in meningioma cells.
(A) IOMM Lee, CH 157 MN and SF3061 cells were irradiated with different doses of radiation (3, 5, 7 and 10 Gy). The irradiated cells were seeded (1×104 cells) in 150-mm culture plates and allowed to form colonies for 24, 48 and 72 hrs. Cells were fixed and stained with Giemsa, followed by colony counting. Survival fraction was calculated and the values are means of three different experiments. (B) Cell cycle analysis of IOMM Lee, CH 157 MN and SF3061 cells treated with 7 Gy, stained with propidium iodide, and measured 24 hrs after treatment. The y axis denotes cell count and the x axis represents DNA content. The percentages of cells out of 10,000 events were calculated without gating. (C) Quantification of the distribution of cells in the G0/G1, G2/M, and S phases of the cell cycle. Data presented are means ± SEM calculated from three independent experiments.
3.2 G2/M phase arrest is mediated by Chk2, Cdc25C, cyclin B1 and Cdc2
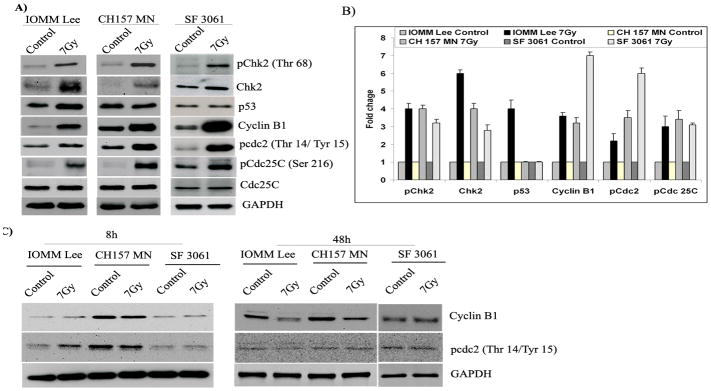
Activation of checkpoints in response to DNA damage typically leads to cell cycle arrest to allow time to repair damage. Checkpoints are initiated to ensure orderly and timely completion of critical events such as DNA replication and chromosome segregation of the cell cycle. To examine whether the marked induction of G2/M arrest in meningioma cells by X-rays correlated with cell cycle regulators, we examined the accumulation of active proteins using western blotting (Fig. 2A). We did observe an accumulation of phosphorylated Chk2 (Thr 68), Cdc25C (Ser 216), and Cdc2 (Thr 14/Tyr15) at 24 hours post-irradiation treatment in all three cell lines (Fig. 2A). We also observed a significant rise in the levels of cyclin B1 at the same time point. Further, the total form of Chk2 increased noticeably whereas the level of total Cdc25C did not change over the same time period (Fig. 2A). We observed an irradiation-induced increase of p53 levels in IOMM Lee cells while p53 levels were not altered in the other two cell lines (Fig. 2A). Quantification of the western blots revealed a greater than two-fold increase in all the phosphorylated molecules and cyclin B as compared to the respective untreated cells, possibly regulating G2/M cell cycle arrest (Fig. 2B). To find the time point specificity for the activation of these molecules, we assessed cyclin B1 and pCdc2 (Thr 14/Tyr 15) levels at 8-hr and 48-hr time points using western blotting. As expected, there was no significant difference among cyclin B1 and pCdc2 levels (Thr 14/Tyr 15) at both time points, which indicates that activation/phosphorylation was maximal at 24 hrs after irradiation and declined thereafter (Fig. 2C). These results also suggest the reversible nature of G2/M arrest induced by radiation treatment in meningioma cells.
Figure 2. Expression of cell cycle-related proteins in irradiated cells.
(A) Western blot analysis of Chk2, phospho-Chk2, p53, Cdc25C, phospho-Cdc25C (Ser216), cyclin B1 and phospho-Cdc2 (Thr 14/Tyr15) in IOMM Lee, CH 157 MN and SF3061 cells at 24 hrs post-irradiation. GAPDH served as a loading control. (B) ImageJ quantification of the molecules in arbitrary units; each value is representative of three independent experiments. (C) Western blot analysis of cyclin B1 and pCdc2 (Thr 14/Tyr 15) at 8 and 48 hrs post-irradiation. GAPDH served as a loading control.
3.3 Chk2 inhibitor induces apoptosis in irradiated cells
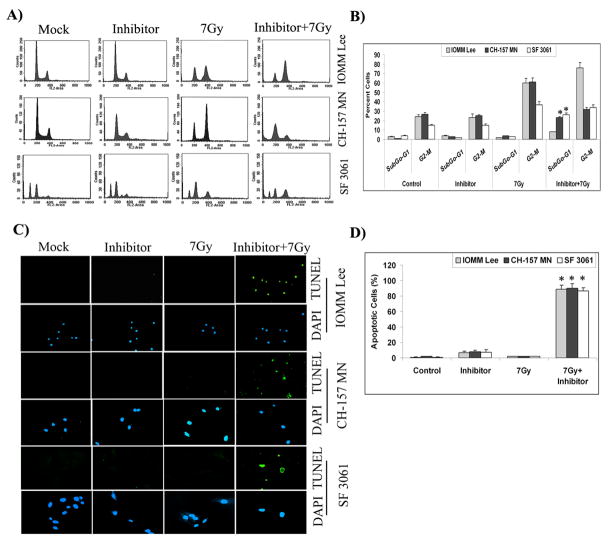
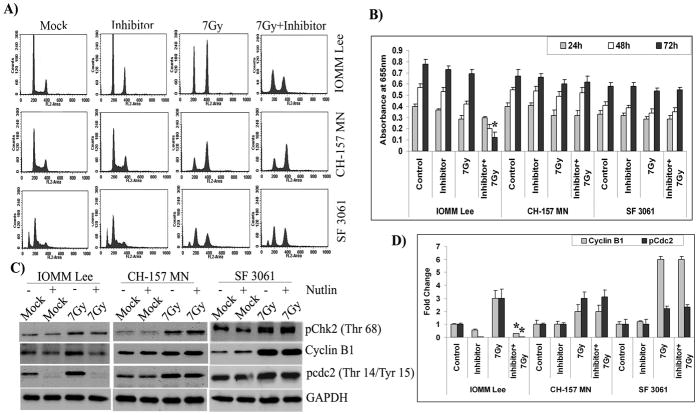
As radiation treatment changed the expression and activity of checkpoint proteins, we chose to evaluate their role in the G2/M cell cycle arrest. To discern the role of Chk2, we used a specific inhibitor of Chk2 kinase activity (NSC 109555 ditosylate) for our studies. Cell cycle analysis of IOMM Lee, CH 157 MN and SF3061 cells exhibited Chk2 inhibitor-associated G2/M arrest occurring at 24 hrs after initial exposure to radiation (not shown). Interestingly, the G2/M arrest was found to be delayed with a large proportion (more than 75%) of IOMM Lee cells remaining in the G2/M phase even at 48 hrs post-irradiation treatment (Figs. 3A–B). In contrast, the inhibitor and radiation-treated CH 157 MN and SF3061 cells showed an observable peak in the sub-G0/G1 population along with a diminishing G2/M peak at the same time point (Fig. 3A). The sub-G0/G1 population was significantly higher in the inhibitor pre-treated, irradiated cells as compared to cells treated with the inhibitor alone (Fig. 3B). However, all of the cell lines showed a lack of proliferation at 48 hrs in comparison with the untreated controls (not shown), indicating that the radiation and inhibitor combination did cause proliferation arrest. Therefore, we next investigated apoptosis in the cells using the TUNEL assay. The results show a large proportion of apoptotic bodies often very small fragments in all three cell lines treated with radiation as well as the inhibitor (Fig. 3C). More than 85% of the cells were found to be TUNEL-positive in the combination treatment. Although there were a few apoptotic cells treated with the inhibitor alone, their proportion was insignificant when compared to the respective control groups (Fig. 3D).
Figure 3. Chk2 inhibitor induces apoptosis in irradiated cells.
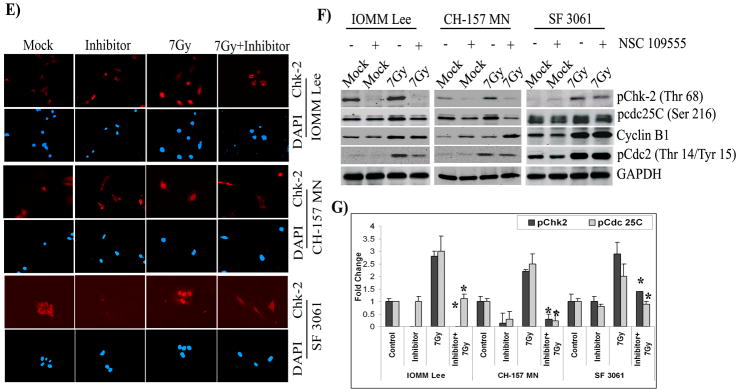
(A) Cell cycle analysis of IOMM Lee, CH 157 MN and SF3061 cells treated with 10 μM inhibitor for 1 hr and/or 7 Gy radiation, stained with propidium iodide, and measured 24 hrs after treatment. The y axis denotes cell count and the x axis represents DNA content. The percentages of cells out of 10,000 events were calculated without gating. (B) Quantification of the cell distribution in the G0/G1, G2/M, and S phases of the cell cycle. Data presented are means ± SEM calculated from three independent experiments. (C) IOMM-Lee, CH 157 MN and SF3061 (1×103) cells were treated with 10 μM inhibitor and 7 Gy radiation, incubated for 48 hrs, and stained for apoptosis using the TUNEL assay. Data shown are from representative fields. (D) Cell death was quantified as percent of apoptotic cells against controls. Values are mean ± SD from three different experiments (p<0.05). (E) Immunofluorescence microscopy for Chk2 staining patterns in the treatment groups using red Fluorophore-tagged secondary antibody. DAPI was used for nuclear staining. Each picture is representative of 15 observed fields (20X). (F) Western blot analysis of pChk2, pCdc25C, cyclin B1, and pCdc2 in the inhibitor-treated cells. GAPDH served as a loading control. Each blot is representative of three independent experiments. (G) Quantification of pChk2 and pCdc25C in the inhibitor-treated cells.
Chk2 is activated at a DNA double-strand break by a mechanism that requires phosphorylation on threonine 68 and localizes diffusely throughout the nucleus of irradiated cells [32]. Consequently, to further establish that Chk2 activation is required for G2/M arrest in meningioma cells, we carried out immunostaining for the localization of Chk2. We observed a diffuse pattern of Chk2 distribution throughout the control cells (Fig. 3E). In contrast, we observed strong cytoplasmic staining for Chk2 with specific exclusion from the nuclear region in the majority of the inhibitor-treated cells with or without radiation (Fig. 3E). However, in comparison to control and inhibitor-treated cells, a large portion of total Chk2 localized to the nuclear region in irradiated cells. These results demonstrate the necessity of Chk2 recruitment into the nucleus under irradiation-induced genotoxic stress. To further validate the influence of Chk2 on downstream effectors, we performed western blotting on inhibitor-treated cells. An apparent decrease in pChk2 (Thr 68) accompanied by reduced levels of pCdc25C was observed in the inhibitor-treated cells (Fig. 3F). This decrease was found to be more than two-fold in all cell lines (Fig. 3G). The expression levels of pCdc2 (Thr 14/Tyr 15) were decreased in IOMM Lee and CH 157 MN cells but remained unaffected in SF3061 cells (Fig. 3F). The expression levels of cyclin B1 varied significantly among the three cell lines and even within in a particular cell line at the 24-hr post-irradiation time point (Fig 3F). Collectively, these data demonstrate the participation of Chk2 phosphorylation and the subsequent recruitment of downstream molecules Cdc25C and Cdc2 in G2/M arrest. Also, the phosphorylation of Chk2 was essential for sustenance of transient arrest in G2/M, which was followed by recovery of irradiated meningioma cells. In contrast, treatment with the inhibitor created an irreversible arrest and drove the cells to apoptotic death.
3.4 p53 acts as downstream effector to Chk2 in IOMM Lee cells
Chk2 functions as a kinase on p53 and performs key roles in connecting p53 to the response to double-strand breaks [33]. Thus, we studied the role of p53 in irradiation-induced G2/M arrest using Nutlin, which is an inhibitor that inhibits p53-mdm2 binding, induces the expression of p53-regulated genes, and exhibits potent anti-proliferative activity in cells with functional p53 but not in p53-mutated or p 53-null cells. We detected a robust increase in sub-G0/G1 phase of IOMM Lee cells treated with the inhibitor and radiation as compared to irradiated controls (Fig. 4A). Although our results demonstrated a decrease of S phase in IOMM Lee cells treated with the inhibitor alone, there was no apparent accompanied increase in cells in the sub-G0/G1 phase (Fig. 4A). In contrast, CH 157 MN and SF3061 cells did not show any response after the combination treatment (Fig. 4A). MTT results demonstrated a rapid decline in proliferation (>80%) of IOMM Lee cells treated with radiation and the inhibitor, thereby indicating that prolonged activation of the p53 pathway leads to apoptosis (Fig. 4B). As anticipated, CH 157 MN and SF3061 cells treated with the combination of radiation and the inhibitor did not show any significant change in cell proliferation when compared to respective controls (Fig. 4B). We next subjected cell lysates to western blotting for analysis of pChk2, cyclin B1 and pCdc2. The activation of Chk2 was not affected in any of the three cell lines (Fig. 4C). However, the expression levels of cyclin B1 and activation of pCdc2 were significantly decreased in IOMM Lee cells (Figs. 4C–D), which indicates the downstream activity of p53 to Chk2. In accord with the results of the MTT assay, cyclin B1 and pCdc2 levels were not affected in CH 157 MN and SF3061 cells treated with the inhibitor and radiation (Fig. 4C), which reveals the non-functional or defective nature of p53 in these cell lines.
Figure 4. p53 acts as downstream effector to Chk2 in IOMM Lee cells.
(A) Cell cycle analysis of IOMM Lee, CH 157 MN and SF3061 cells treated with 10 μM inhibitor for 1 hr and/or 7 Gy radiation, stained with propidium iodide, and measured 24 hrs after treatment. The y axis denotes cell count and the x axis represents DNA content. The percentages of cells out of 10,000 events were calculated without gating. (B) IOMM Lee, CH 157 MN and SF3061 cells (1×105) were seeded in 6-well plates and irradiated. Next, cells were trypsinized and seeded at 1×104 cells per well in 96-well plates. After the indicated hours of incubation, MTT reagent was added, followed by another 4 hrs of incubation and the addition of acid-isopropanol. Absorbance was measured at 550 nm and the values were quantified. (C) Western blot analysis of pChk2, cyclin B1 and pCdc2 in the inhibitor-treated cells. GAPDH served as a loading control. Each blot is a representative of three independent experiments. (D) ImageJ quantification of cyclin B1 and pCdc2 in the inhibitor-treated cells.
3.5 Cyclin B1 and Cdc2 complex maintains G2/M arrest
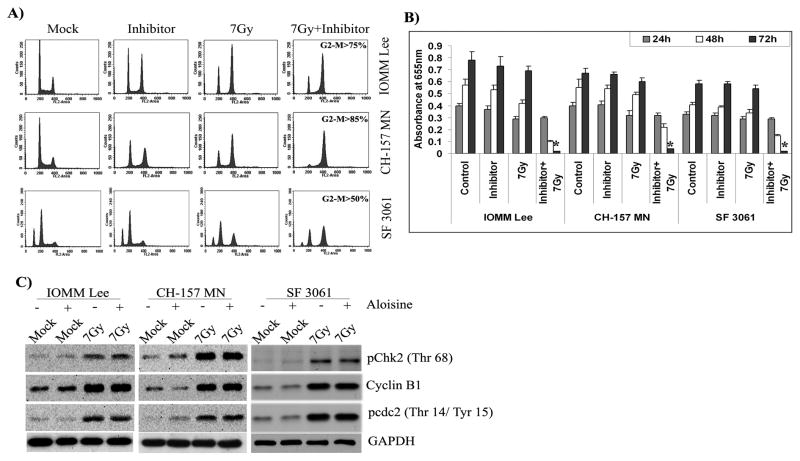
A complex between cyclin-dependent kinase 1 (Cdc2) and cyclin B1 is important for a cell to enter into mitosis in most organisms [34]. To verify the role of the Cdc2-cyclin B1 complex in Chk2-mediated G2/M arrest, we examined whether cyclin B1-associated Cdc2 overrides or causes cell cycle arrest at the G2 checkpoint using the inhibitor Aloisine, which acts as a potent, selective, ATP-competitive inhibitor of Cdk1/cyclin B interaction. Cell cycle analysis of inhibitor-treated cells showed a significant cell cycle arrest in the G2/M phase with or without radiation in IOMM Lee and CH 157 MN cells (Fig. 5A). The inhibitor treatment alone did not induce any significant G2/M arrest in SF3061 cells, but the combination treatment of inhibitor and radiation did result in a marked increase (Fig. 5A). The fraction of cells in G2/M arrest after combination treatment appeared to increase in all three cell lines (Fig. 5A). Further analysis of cell proliferation by MTT assay revealed that nearly all of the cells (>95%) treated with the combination perished by 72 hrs post-irradiation (Fig. 5B). In contrast, cells treated with the inhibitor alone showed signs of recovery in proliferation rates despite their arrest in the G2/M phase (Fig. 5B). Western blot analysis for pChk2, cyclin B1 and pCdc2 showed that the expression or phosphorylation of these proteins was not affected by the inhibitor treatment in any of the three cell lines (Fig. 5C). These data demonstrate that cyclin B1 and Cdc2 interact during irradiation-induced DNA damage and maintain the transitions between mitosis and the G2/M phase for survival in meningioma cells while treatment with the inhibitor potentiated a permanent arrest in the G2/M phase and led to cell death.
Figure 5. Cyclin B1-Cdc2 complex maintains G2/M arrest.
(A) Cell cycle analysis of IOMM Lee, CH 157 MN and SF3061 cells treated with 10 μM inhibitor for 1 hr and/or 7 Gy radiation, stained with propidium iodide, and measured 24 hrs after treatment. The y axis denotes cell count and the x axis represents DNA content. The percentages of cells out of 10,000 events were calculated without gating. (B) IOMM Lee, CH 157 MN and SF3061 cells (1×105) were seeded in 6-well plates and irradiated. Cells were trypsinized and seeded at 1×104 cells per well in 96-well plates. After the indicated hours of incubation, MTT reagent was added, followed by another 4 hrs of incubation and the addition of acid-isopropanol. Absorbance was measured at 550 nm and the values were quantified. (C) Western blot analysis of pChk2, cyclin B1 and pCdc2 in the inhibitor-treated cells. GAPDH served as a loading control. Each blot is representative of three independent experiments.
3.6 Meningioma cells recover from G2/M arrest
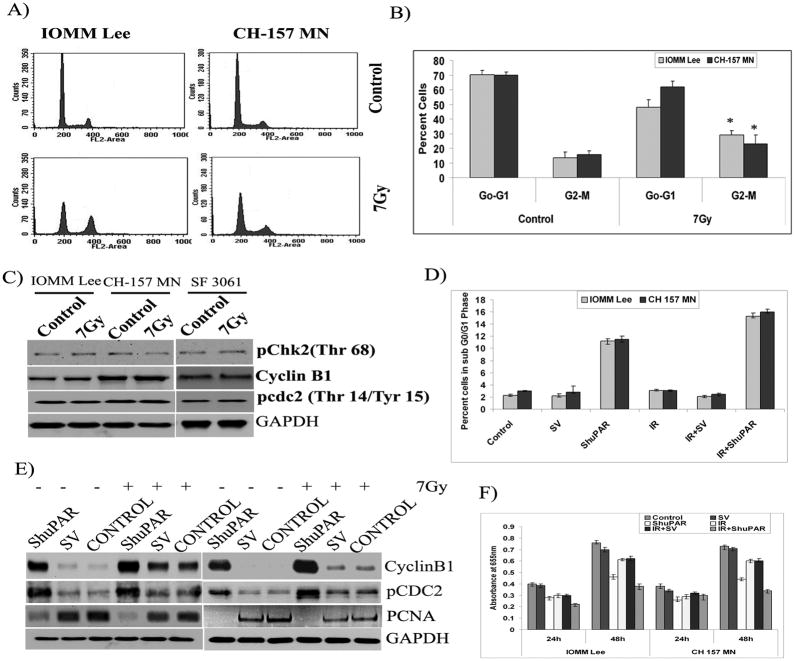
To determine whether the cell cycle arrest induced by radiation is reversible, cells were exposed to radiation, allowed to recover for 72 hrs, and then either processed for analysis of cell cycle distribution or western blotting. Results of DNA cell cycle analysis on IOMM Lee and CH 157 MN cells harvested at the 72-hr time point showed that irradiated cells follow the same pattern of cell distribution in the G0/G1, S and G2/M phases as seen in the respective controls (Fig. 6A). A significant decline (less than 30 percent) in G2/M cells was noted at the 72-hr time point as compared to more than 60 percent of cells in the G2/M phase at 24 hrs post-irradiation, suggesting a withdrawal of G2 delay and subsequent entry into normal cell cycle (Figs. 6A–B). The results of cell cycle analysis were not clear with SF3061 cells as there were multiple additional peaks seen with the irradiated cells (not shown). Nevertheless, the levels of pChk2, cyclin B1 and pCdc2 reached normalcy and were equivalent to the respective controls in all three cell lines (Fig. 6C).
Figure 6. Meningioma cells recover from radiation induced cell cycle arrest and uPAR knock down induces cell death.
(A) Cell cycle analysis of IOMM Lee and CH 157 MN cells treated with 7 Gy radiation, stained with propidium iodide, and measured 72 hrs after treatment. The y axis denotes cell count and the x axis represents DNA content. The percentages of cells out of 10,000 events were calculated without gating. (B) Quantification of distribution of cells in the G0/G1 and G2/M phases of the cell cycle. Data presented are means ± SEM calculated from three independent experiments. (C) Western blot analysis of pChk2, cyclin B1 and pCdc2 at 72 hrs after irradiation. GAPDH served as a loading control. Each blot is representative of three independent experiments. (D) Cell cycle analysis of IOMM Lee and CH 157 MN cells transfected with Scrambled vector (SV), ShuPAR, irradiated (7Gy) stained with propidium iodide after 24 hrs treatment as indicated. Percentage of cells in SubG0/G1 population in each treatment group is plotted. Data presented are means ± SEM calculated from three independent experiments. E) IOMM Lee and CH 157 MN cells were transfected either with SV or ShuPAR expressing plasmids alone or in combination with radiation. After 6h radiation treatment cells were transferred into 96 well plates and MTT assay was performed at indicated period of times. Data presented are means ± SEM calculated from three independent experiments. F) Western blot analysis in transfected cells for cyclin B1 and pCdc2 at 24 hrs after irradiation. GAPDH served as a loading control. Each blot is representative of three independent experiments.
3.7 Knockdown of uPAR induces sustained G2/M cell cycle arrest followed by cell death
uPAR is an important signaling orchestrator and known to have an important role in cancer cell survival and invasiveness while its knockdown was shown to be lethal for different cancer cells. It is because of that we knocked down the uPAR from IOMM Lee and CH157 MN cells and analyzed for cell cycle progression. Silencing of uPAR expression has shown the accumulation of cells in G2/M phase in presence or absence of radiation treatment with significant portion (>12%) of cells falling into Sub G0/G1 population compared to their normal and irradiated controls (Fig 6D). Cyclin B1 and pCdc2 levels were found to be significantly high in both the cell lines transfected with uPAR siRNA expressing plasmids (Fig. 6E). Further, proliferating cell nuclear antigen (PCNA) levels were very low in uPAR knock down cells indicating the onset of cell death (Fig. 6E). MTT assays for 72h on uPAR knockdown cells showed a marked decrease in the cell proliferation (Fig. 6F) in both the cell lines indicating that uPAR knock down induces permanent arrest of cells in G2/M phase either alone or in combination with radiation treatment, as a result of which cell proliferation is ceased.
3.8 Recovered cells form aggressive tumors in vivo while uPAR knockdown induced Sustained expression of Cyclin B1
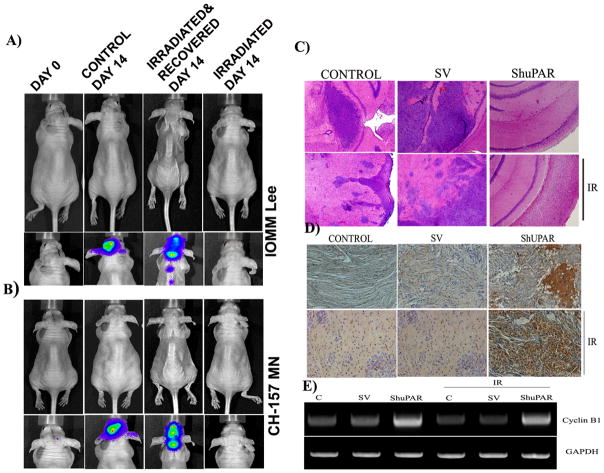
Furthermore, the in vitro signs of recovery and proliferation prompted us to confirm the tumor-forming capability of the irradiated cells in vivo. Our studies with irradiated cells were conducted with two different lines. The irradiated cells, which were allowed to recover for 72 hrs and then implanted intracranially, formed highly aggressive tumors. These tumors were found to be metastatic with several secondary centers along the spinal cord, while the tumors formed by untreated IOMM Lee and CH 157 MN cells were localized to the brain and did not show any morphological features in the animals at 14 days post-implantation (Figs. 7A–B). The aggressive tumor growth in animals implanted with irradiated and recovered IOMM Lee and CH 157 MN cells was accompanied by gross morphological changes in animal size and behavioral response with greater than 40 percent loss in body weight and difficulty in feed uptake (upper panel of Figs. 7A–B). Interestingly, animals infused with irradiated cells that were not allowed to recover before implantation did not show any signs of tumor formation even after 14 days. Also, there were no changes in the morphological characteristics of these animals as compared to the controls (upper panel of Figs. 7A–B). These in vivo results and our previous studies (Gogineni et al., 2009) on pre-established intracranial tumors suggest that radiation resistance is an inherent characteristic of meningioma cells.
Figure 7. Irradiated meningioma form aggressive tumors while uPAR knock down induces sustained G2/M arrest in vivo.
(A-B) IOMM Lee and CH 157 MN luciferase expressing stable cells (1×105) were implanted into nude mice (4 to 6 weeks old). The first group was treated with irradiated (7 Gy) cells. The second group was infused with irradiated (7 Gy) cells that were allowed to recover. The third group was infused with non-irradiated cells. Tumor progression and morphological and behavioral patterns were followed daily for two weeks with an in vivo imaging system. (C) Representative photmicrograph (40X)of Immunohistochemistry for Cyclin B1 in the brain sections of animals implanted with transfected IOMM Lee cells in combination with radiation treatment (IR; 7Gy) as indicated (n=5). (D) H&E staining of brain sections for the visualization of tumor formation. (E) Representative image of RT-PCR analysis for Cyclin B1 in the brain tissues of different treatment groups. GAPDH served as loading control (n=3).
The results of in vitro uPAR knock down also led us to study its effect in vivo. The animals implanted with IOMM Lee cells have shown tumor formation. However, tumors in animals treated with irradiated cells were dispersed with several foci in the brain sections (Fig 7C). Nevertheless, uPAR knock down cells show significantly small or no tumor formation either in radiation combination or alone (Fig 7C). Immunostaining for Cyclin B1 revealed strong reactivity in brain sections of the animals implanted with uPAR knocked down cells (Fig 7D). On the other hand the reactivity was no different between control and irradiated groups indicating low levels of Cyclin B1 (Fig 7D). Further, RT-PCR analyses with human specific Cyclin B1 primers indicate a marked increase in the mRNA levels in animals implanted with uPAR knocked down IOMM Lee cells (Fig 7E). As anticipated the cyclin B1 levels in irradiated and recovered cells were found to be equal to that of the respective control. These results demonstrate the prolonged arrest of ShuPAR treated cells in G2/M phase and culminating in cell death.
4. Discussion
Radiation treatment has been unsuccessful when radiation was not properly fractionated, when less than the total dosage was given, or when radiation was used preoperatively [35]. In addition, effects on bystander cells, radioresistance and radiation-induced development of neoplasia contribute significantly to treatment failure [36;37]. To improve radiotherapy for malignant tumors, it is important to understand the biological reaction of the cells to radiation. Cells exposed to radiation showed a temporary, reversible cell cycle arrest and then continued to proliferate at a slower rate. The initial lag in cell proliferation after radiation treatment has been reported to be the result of the activation of the repair pathways [38]. However, cells that were unable to complete the division successfully appeared to be eliminated through various mechanisms including apoptosis. Checkpoints in the cell cycle regulate the progression or arrest of the cell cycle in response to DNA damage and allow time for DNA repair. These occur either in late G1, which prevents entry to the S phase, or in late G2, which prevents entry to mitosis [39]. Our cell cycle analyses showed a clear arrest of cells in the G2/M phase, which indicates entry into mitosis had been delayed. Thus, given that the G2/M checkpoint serves as a mandatory requirement for survival of meningioma cells, this delay in cell division, in combination with more efficient DNA damage repair, is necessary for maintenance of genome integrity in these cells. Both the extent and the length of this G2/M delay were reported be highly variable based on the cell line, radiation dose and the dose rate [40].
Existing studies suggest a strong correlation between the nature of G2 delay and early cell proliferation. Activated cell membrane receptors phosphorylate numerous substrates at the sites of DNA double-strand breaks and subsequently activate downstream signal transducers [41]. Numerous lines of validation establish a strong link between ionizing radiation exposure, Chk2 phosphorylation (Thr 68), and subsequent G2/M cell cycle arrest [42]. The mammalian checkpoint effectors that are established as substrates of Chk2 in vivo include members of the Cdc25 family, p53 and BRCA1 [43]. Chk2 phosphorylation both inhibits phosphatase activity of Cdc25C and contributes to its cytoplasmic sequestration by an interaction with 14-3-3 proteins [43;44]. Activated Chk2 modulates the activity of Cdc25C via phosphorylation on an inhibitory site (Ser 216), which either enables DNA repair or directs the cell to the apoptotic pathway depending on the extent of perceived genotoxic insult. In the present study, the protein kinase Chk2 was phosphorylated transiently in response to ionizing radiation treatment in meningioma cells, which was also followed by phosphorylation of Cdc25C on Ser 216. Chk2 inhibitor treatment yielded interesting results, and as anticipated, treatment with the inhibitor induced apoptosis in irradiated cells. However, the fraction of cells that remained in the G2/M phase was greater in the inhibitor-treated cells. These findings support the hypothesis that Chk2 is required for maintenance, but not initiation, of G2 arrest induced by DNA damage. Chk2 was shown to synergize with other genes or factors that perpetuate DNA damage repair during the G2/M phase rather than inducing G2/M arrest [33]. A common theme in checkpoint signaling has been that ATM, which is preferentially activated in response to ionizing radiation, often phosphorylates several proteins in the same complex. This trend of cooperative phosphorylation apparently recruits several effectors in achieving G2/M arrest while Chk2 play a key role in maintaining the G2/M phase. The inconsistent pattern of cyclin B1 expression in inhibitor-treated cells might well be a result of the loss of control over the cell cycle. Our immunocytochemistry studies on inhibitor-treated cells demonstrated a clear nuclear exclusion of Chk2, which points to the necessity of Chk2 in double-strand break regions. Checkpoint proteins, including Chk2, often localize to distinct subnuclear bodies or foci, and in fact, ionizing radiation has been shown to trigger accumulation of Chk2 at the sites of DNA strand breaks [43;45].
Phosphorylation of p53 on Ser20 by Chk2 stabilizes the p53 protein [46], which improves its potential to positively regulate the expression of factors involved in DNA repair, cell death and cell cycle control [47]. The importance of Chk2 and p53 in maintaining genome integrity is highlighted by the fact that mutations in Chk2 and p53 are associated with human disease [47]. In the present study, radiation-induced expression of p53 was prominent in IOMM Lee cells, and the inhibitor treatment stimulated apoptosis in irradiated cells. SF3061 is a known p53-mutated cell line, and since CH 157 MN cells also did not respond to the combination treatment, we presume that the mutated status of p53 in this cell line could possibly explain the difference in the response of these cells to radiation treatment. While it is widely reported that p53 is not necessary for G2/M phase arrest, substantial data do indicate p53 itself can modulate G2/M phase delay in a Chk2-dependent manner [33]. Apart from the involvement of Chk2/p53 in maintaining the G2 blockade seems, at least partly, attributable to other redundant mechanisms because either cells, which have mutated p53 or which are p53 null, still undergo a G2/M phase block after irradiation [48].
The decision of cells to either remain in the G2/M phase or go through G2 into mitosis requires the activation of Cdc2 [49] whose activity is regulated by synthesis of cyclin B1 and subsequent complex formation. This complex formation is triggered by a 10-fold increase in cyclin B1 levels in the G2 phase. Cyclin B1 levels fluctuate through the cell cycle and cells with a prolonged G2 phase have reduced cyclin B1 levels when compared to the normal levels [50]. While the presence of cyclin B1 is required for entry into mitosis, its destruction is required for exit from mitosis. Although the rise and fall of cyclin levels are the primary determinant of Cdk activity during the cell cycle, several additional mechanisms are important. Many studies have suggested that inhibition of Cdc2 activity is due to increased inhibitory phosphorylation of Cdc2 [51]. In the present study, radiation treatment seemed to induce cyclin B1 expression along with a simultaneous increase in the inhibitory phosphorylation of Cdc2 on Thr 14/Tyr 15. Activation of cyclin B1 and Cdc phosphorylation are opposites in function. However, the increase in cyclin B1 expression, Cdc2 inhibitory phosphorylation and Aloisine treatment induced irreversible G2/M arrest, plausibly characterizing a dynamic equilibrium that is required between cyclin B1 and Cdc2 to maintain the balance between G2/M phase and mitosis while leaving enough time for the restoration of genome integrity.
Perhaps the principal biological task of the DNA damage checkpoint is to allow sufficient time for the damage to be repaired so that checkpoint-arrested cells can ultimately resume cell cycle progression and continue their physiological program. The initial arrest of meningioma cells in the G2/M phase was coupled to the activation of various mediators at 24 hours post-irradiation. Recruitment of the effectors at particular time points also appeared to be very important as the activation or expression of these molecules was not observed at earlier (8 hrs) or later (72 hrs) time points. The reversal of cells to normal cell cycle phases 72 hours after radiation treatment suggests that the G2/M phase in these cells is a temporary transition, which allowed for sufficient recovery and brought the effectors to basal levels upon checkpoint termination. These results suggest that G2/M arrest is an important determinant in the cytostatic action of ionizing radiation and that repair of DNA double-strand breaks is sufficient to ensure cell survival as reported in Cervical carcinoma [16] and Glioma cells [15]. Apart from checkpoint activation, radiation stimulates signaling cascades through other receptors that support enhanced repair and cell proliferation.
uPAR is primarily a receptor for urokinase plasminogen activator. However, its participation in multiple ligand interactions, role as ligand, as chemotactic factor and as prognostic marker in variety of disease conditions including cancer makes it as an attractive therapeutic target [52]. Earlier studies on uPAR knock down by our group delineated its potential as a therapeutic target in brain cancers [27;53]. It is with this back ground we analyzed for the cycle progression in uPAR transfected meningioma cells in combination with radiation. Either radiation treatment or uPAR silencing result in cellular G2/M arrest associated with inhibition of Cdc2 activity. Nevertheless, Inhibitory phosphorylation and Cyclin B1 accumulation was transient in radiation treatment yet these are sustained events in uPAR knockdown leading to meningioma cell death. Additionally, our in vitro experiments show that the combination treatment has an advantage over either treatment alone. With these results, our study points to the importance of combination treatment strategies for cancer treatment in order to attain better therapeutic outcome even in the radiation resistant cells. The in vivo behavior of the cells was consistent with our earlier studies in which we reported the aggressive characteristics of growth accompanied by expression of invasive molecules [28;54]. The non-recovered irradiated cells could not form tumors while the recovered cells formed aggressive tumors with metastatic capability, which once again imply the transient nature of cell cycle arrest. Furthermore, brain sections of animals implanted with uPAR knockdown cells showed high degree of cyclin B1 expression with relatively small or no tumor formation suggesting the permanent arrest in G2/M phase and subsequent cell death. On the other hand it is evident that cyclin B1 levels in tumors formed by irradiated and recovered cells are low suggesting the transient nature of the arrest while the invasive tumors were seen in brain sections.
In conclusion, our results indicate that the radioresistance of meningioma cells is closely correlated with the induction of G2/M arrest, which may be regulated by Chk2, cyclin B1 and Cdc2. uPAR knock down sensitizes or complement the radiation treatment so that effective therapy of the tumors can be achieved. The clinical effectiveness of therapies for patients with malignant meningioma could be enhanced by the development of agents that target the G2/M arrest as characterized by the present study.
Acknowledgments
Funding
This research was funded by National Institutes of Neurological Disorders and Stroke, NS061835 to Jasti S. Rao.
We thank Shellee Abraham for manuscript preparation, and Diana Meister and Sushma Jasti for manuscript review.
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Alexiou GA, Gogou P, Markoula S, Kyritsis AP. Management of meningiomas. Clin Neurol Neurosurg. 2010;112:177–182. doi: 10.1016/j.clineuro.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Norden AD, Drappatz J, Wen PY. Advances in meningioma therapy. Curr Neurol Neurosci Rep. 2009;9:231–240. doi: 10.1007/s11910-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 3.Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, Flickinger JC. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53–58. doi: 10.1227/01.NEU.0000311061.72626.0D. [DOI] [PubMed] [Google Scholar]
- 4.Malik I, Rowe JG, Walton L, Radatz MW, Kemeny AA. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13–20. doi: 10.1080/02688690500080885. [DOI] [PubMed] [Google Scholar]
- 5.Milker-Zabel S, Zabel-du BA, Huber P, Schlegel W, Debus J. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68:858–863. doi: 10.1016/j.ijrobp.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 6.Colvett KT, Hsu DW, Su M, Lingood RM, Pardo FS. High PCNA index in meningiomas resistant to radiation therapy. Int J Radiat Oncol Biol Phys. 1997;38:463–468. doi: 10.1016/s0360-3016(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 7.Askoxylakis V, Zabel-du BA, Schlegel W, Debus J, Huber P, Milker-Zabel S. Patterns of failure after stereotactic radiotherapy of intracranial meningioma. J Neurooncol. 2010;98:367–372. doi: 10.1007/s11060-009-0084-1. [DOI] [PubMed] [Google Scholar]
- 8.Marucci G, Betts CM, Frank G, Foschini MP. Oncocytic meningioma: report of a case with progression after radiosurgery. Int J Surg Pathol. 2007;15:77–81. doi: 10.1177/1066896906295824. [DOI] [PubMed] [Google Scholar]
- 9.Preston DL, Ron E, Yonehara S, Kobuke T, Fujii H, Kishikawa M, Tokunaga M, Tokuoka S, Mabuchi K. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 2002;94:1555–1563. doi: 10.1093/jnci/94.20.1555. [DOI] [PubMed] [Google Scholar]
- 10.Shintani T, Hayakawa N, Kamada N. High incidence of meningioma in survivors of Hiroshima. Lancet. 1997;349:1369. doi: 10.1016/S0140-6736(05)63205-9. [DOI] [PubMed] [Google Scholar]
- 11.Osipov V, Ho KC, Krouwer HG, Meyer G, Shidham VB. Post-radiation dedifferentiation of meningioma into osteosarcoma. BMC Cancer. 2002;2:34. doi: 10.1186/1471-2407-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svajdler M, Bohus P, Rychly B, Sulla I, Moram M. Post-radiation dedifferentiation of meningioma into chondroblastic osteosarcoma. Cesk Patol. 2009;45:20–23. [PubMed] [Google Scholar]
- 13.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 14.Cann KL, Hicks GG. Regulation of the cellular DNA double-strand break response. Biochem Cell Biol. 2007;85:663–674. doi: 10.1139/O07-135. [DOI] [PubMed] [Google Scholar]
- 15.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 16.Tamamoto T, Ohnishi K, Takahashi A, Wang X, Yosimura H, Ohishi H, Uchida H, Ohnishi T. Correlation between gamma-ray-induced G2 arrest and radioresistance in two human cancer cells. Int J Radiat Oncol Biol Phys. 1999;44:905–909. doi: 10.1016/s0360-3016(99)00072-3. [DOI] [PubMed] [Google Scholar]
- 17.Aguirre-Ghiso JA. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21:2513–2524. doi: 10.1038/sj.onc.1205342. [DOI] [PubMed] [Google Scholar]
- 18.Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell. 2003;4:67–79. doi: 10.1016/s1535-6108(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Aguirre-Ghiso JA, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 20.Kjoller L, Hall A. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J Cell Biol. 2001;%19;152:1145–1157. doi: 10.1083/jcb.152.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koshelnick Y, Ehart M, Hufnagl P, Heinrich PC, Binder BR. Urokinase receptor is associated with the components of the JAK1/STAT1 signaling pathway and leads to activation of this pathway upon receptor clustering in the human kidney epithelial tumor cell line TCL-598. J Biol Chem. 1997;272:28563–28567. doi: 10.1074/jbc.272.45.28563. [DOI] [PubMed] [Google Scholar]
- 22.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178:425–436. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivas L, Chiaraviglio E. The effects of reversible lidocaine-induced lesion of the tissue surrounding the anterior ventral wall of the third ventricle on drinking in rats. Behav Neural Biol. 1992;57:124–130. doi: 10.1016/0163-1047(92)90617-d. [DOI] [PubMed] [Google Scholar]
- 24.Lee WH. Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery. 1990;27:389–395. [PubMed] [Google Scholar]
- 25.Baia GS, Slocum AL, Hyer JD, Misra A, Sehati N, VandenBerg SR, Feuerstein BG, Deen DF, McDermott MW, Lal A. A genetic strategy to overcome the senescence of primary meningioma cell cultures. J Neurooncol. 2006;78:113–121. doi: 10.1007/s11060-005-9076-y. [DOI] [PubMed] [Google Scholar]
- 26.Tsai JC, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: induced secretion by EGF, PDGF-BB, and bFGF. J Neurosurg. 1995;82:864–873. doi: 10.3171/jns.1995.82.5.0864. [DOI] [PubMed] [Google Scholar]
- 27.Gondi CS, Lakka SS, Dinh DH, Olivero WC, Gujrati M, Rao JS. RNAi-mediated inhibition of cathepsin B and uPAR leads to decreased cell invasion, angiogenesis and tumor growth in gliomas. Oncogene. 2004;23:8486–8496. doi: 10.1038/sj.onc.1207879. [DOI] [PubMed] [Google Scholar]
- 28.Kargiotis O, Chetty C, Gogineni V, Gondi CS, Pulukuri SM, Kyritsis AP, Gujrati M, Klopfenstein JD, Dinh DH, Rao JS. uPA/uPAR downregulation inhibits radiation-induced migration, invasion and angiogenesis in IOMM-Lee meningioma cells and decreases tumor growth in vivo. Int J Oncol. 2008;33:937–947. [PMC free article] [PubMed] [Google Scholar]
- 29.McCutcheon IE, Friend KE, Gerdes TM, Zhang BM, Wildrick DM, Fuller GN. Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg. 2000;92:306–314. doi: 10.3171/jns.2000.92.2.0306. [DOI] [PubMed] [Google Scholar]
- 30.Ragel BT, Elam IL, Gillespie DL, Flynn JR, Kelly DA, Mabey D, Feng H, Couldwell WT, Jensen RL. A novel model of intracranial meningioma in mice using luciferase-expressing meningioma cells. Laboratory investigation. J Neurosurg. 2008;108:304–310. doi: 10.3171/JNS/2008/108/2/0304. [DOI] [PubMed] [Google Scholar]
- 31.Gogineni VR, Nalla AK, Gupta R, Gorantla B, Gujrati M, Dinh DH, Rao JS. Radiation-inducible silencing of uPA and uPAR in vitro and in vivo in meningioma. Int J Oncol. 2010;36:809–816. doi: 10.3892/ijo_00000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Momcilovic O, Choi S, Varum S, Bakkenist C, Schatten G, Navara C. Ionizing radiation induces ataxia telangiectasia mutated-dependent checkpoint signaling and G(2) but not G(1) cell cycle arrest in pluripotent human embryonic stem cells. Stem Cells. 2009;27:1822–1835. doi: 10.1002/stem.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 34.Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–274. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kupersmith MJ, Warren FA, Newall J, Ransohoff J. Irradiation of meningiomas of the intracranial anterior visual pathway. Ann Neurol. 1987;21:131–137. doi: 10.1002/ana.410210205. [DOI] [PubMed] [Google Scholar]
- 36.Knizetova P, Darling JL, Bartek J. Vascular endothelial growth factor in astroglioma stem cell biology and response to therapy. J Cell Mol Med. 2008;12:111–125. doi: 10.1111/j.1582-4934.2007.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umansky F, Shoshan Y, Rosenthal G, Fraifeld S, Spektor S. Radiation-induced meningioma. Neurosurg Focus. 2008;24:E7. doi: 10.3171/FOC/2008/24/5/E7. [DOI] [PubMed] [Google Scholar]
- 38.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 39.Pietenpol JA, Stewart ZA. Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology. 2002;181–182:475–81. 475–481. doi: 10.1016/s0300-483x(02)00460-2. [DOI] [PubMed] [Google Scholar]
- 40.DiPaola RS. To arrest or not to G(2)-M Cell-cycle arrest: commentary re: A. K. Tyagi et al., Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G(2)-M arrest, and apoptosis. Clin. cancer res., 8: 3512–3519, 2002. Clin Cancer Res. 2002;8:3311–3314. [PubMed] [Google Scholar]
- 41.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melchionna R, Chen XB, Blasina A, McGowan CH. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat Cell Biol. 2000;2:762–765. doi: 10.1038/35036406. [DOI] [PubMed] [Google Scholar]
- 43.Bartek J, Falck J, Lukas J. CHK2 kinase--a busy messenger. Nat Rev Mol Cell Biol. 2001;2:877–886. doi: 10.1038/35103059. [DOI] [PubMed] [Google Scholar]
- 44.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 45.Rhind N, Russell P. Roles of the mitotic inhibitors Wee1 and Mik1 in the G(2) DNA damage and replication checkpoints. Mol Cell Biol. 2001;21:1499–1508. doi: 10.1128/MCB.21.5.1499-1508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 47.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 48.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 49.Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–543. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Timofeev O, Cizmecioglu O, Settele F, Kempf T, Hoffmann I. Cdc25 phosphatases are required for timely assembly of CDK1-cyclin B at the G2/M transition. J Biol Chem. 2010;285:16978–16990. doi: 10.1074/jbc.M109.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ragno P. The urokinase receptor: a ligand or a receptor? Story of a sociable molecule. Cell Mol Life Sci. 2006;63:1028–1037. doi: 10.1007/s00018-005-5428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chetty C, Lakka SS, Bhoopathi P, Gondi CS, Veeravalli KK, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS. Urokinase Plasminogen Activator Receptor and/or Matrix Metalloproteinase-9 Inhibition Induces Apoptosis Signaling through Lipid Rafts in Glioblastoma Xenograft Cells. Mol Cancer Ther. 2010;9:2605–2617. doi: 10.1158/1535-7163.MCT-10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gogineni VR, Kargiotis O, Klopfenstein JD, Gujrati M, Dinh DH, Rao JS. RNAi-mediated downregulation of radiation-induced MMP-9 leads to apoptosis via activation of ERK and Akt in IOMM-Lee cells. Int J Oncol. 2009;34:209–218. [PMC free article] [PubMed] [Google Scholar]