Abstract
Suppressor of cytokine signaling-3 (SOCS3) has multiple functions including inhibition of Janus kinase activity, regulation of protein degradation, and suppression of cytokine signaling. SOCS3 modulates macrophage response to cytokines such as IL-6 and leptin that are systemically induced in obesity. Obesity is a suspected risk factor for SOCS3-related pathology such as rheumatoid arthritis and Crohn’s disease as well as zoledronic acid (ZA)-induced osteonecrosis of the jaw (ONJ). Thus, understanding the ability of bisphosphonates to modulate SOCS3 is necessary to qualify their contribution to these disorders. ONJ occurs in up to 10% of patients using intravenous bisphosphonates and has an unknown pathogenesis that may be linked to decreased bone turnover, altered vascularity, bacterial invasion, and compromised wound healing. Given the increased risk of ONJ with obesity and importance of macrophages in wound healing, we hypothesized that amino-bisphosphonates could contribute to the pathogenesis of ONJ by regulating macrophage responses to cytokines such as leptin and IL-6. We report that zoledronic acid is a novel inhibitor of SOCS3 in primary macrophages and human ONJ biopsy specimens. Inhibition of SOCS3 by ZA resulted in significant increases in IL-6 production. SOCS3 transcription is regulated by nuclear accumulation of phosphorylated-Stat3 (P-Stat3). We found that ZA decreased phosphorylation of Stat3 in a mevalonate-pathway dependent manner. However, restoration of P-Stat3 was not sufficient to correct SOCS3 inhibition. We propose that disruption of macrophage SOCS3 expression by amino-bisphosphonates such as ZA may be a novel contributor to inflammatory phenotypes in obesity and the pathogenesis of ONJ.
Keywords: Bisphosphonate, SOCS3, Osteonecrosis, Leptin, Zoledronic Acid, Macrophage
INTRODUCTION
Suppressor of Cytokine Signaling-3 (SOCS3), a member of the SOCS family of proteins, has multiple domain-specific functions that include inhibition of Janus kinase (Jak) activity, competition with signal transducer and activator of transcription (Stat) proteins, regulation of protein degradation, and suppression of cytokine signaling [Piessevaux et al., 2008]. Expression of SOCS3 is induced by extracellular binding proteins such as interleukin 6 (IL-6), IL-10, interferon gamma (IFN-y), bacterial lipopolysaccharide (LPS), and leptin. Complete deficiency of SOCS3 in mice is lethal due to placental defects [Yasukawa et al., 2003], however, conditional deletion has revealed a wide role for SOCS3 in cells and tissues including macrophages [Ohishi et al., 2005], the central nervous system [Mori et al., 2004], T cells [Kinjyo et al., 2006], the pancreas [Mori et al., 2007], and the liver [Ogata et al., 2006]. Alterations in SOCS3 protein levels may play a significant role in the pathogenesis of inflammatory diseases including rheumatoid arthritis [Isomaki et al., 2007], inflammatory bowel disease [Suzuki et al., 2001], and Crohn’s disease [Lovato et al., 2003].
Zoledronic acid (ZA) is a commonly used amino-bisphosphonate medication that is approved in the United States for treatment of Paget’s disease, postmenopausal osteoporosis, multiple myeloma, and bone metastases from solid tumors [Ibrahim et al., 2003]. Additional off-label uses such as treatment of osteoarthritis and rheumatoid arthritis are growing increasingly popular [Jarrett et al., 2006; Zoler, 2010]. ZA is highly potent with a half-life potentially exceeding 10 years and becomes incorporated in the bone after initial administration [Khan et al., 1997]. The compound is then gradually released during bone remodeling. This generates potential for long-term systemic effects and has been linked to complications such as osteonecrosis of the jaw (ONJ). ONJ is diagnosed when a patient with a history of bisphosphonate use and without previous radiation treatment presents with exposed bone in the oral cavity that fails to heal after eight weeks [Novince et al., 2009]. A survey of over 700,000 medical claims revealed that those taking IV bisphosphonates were at a four to six-fold increased risk of requiring jaw surgery due to inflammatory changes [Cartsos et al., 2008] and a survey of cancer patients specifically taking ZA found a 30-fold increase in risk of ONJ [Wessel et al., 2008].
In patients taking ZA, obesity is also a risk factor for development of ONJ [Wessel et al., 2008]. Obesity has been linked to significant increases in systemic markers of inflammation such as c-reactive protein and circulating inflammatory cytokines such as leptin, IL-6, and TNF-α [Considine et al., 1996; Das, 2001; Fontana et al., 2007]. Obesity is also a suspected risk factor for SOCS3-related inflammatory disorders such as rheumatoid arthritis and Crohn’s disease [Mendall et al., 2011; Voigt et al., 1994]. Given the increasing prevalence of amino-bisphosphonate use, understanding the ability of these compounds to modulate inflammation and macrophage cytokine production is necessary to qualify their contribution to these disorders.
The main function of amino-bisphosphonates such as ZA is to reduce osteoclast activity both by induction of osteoclast apoptosis [Sudhoff et al., 2003] and inhibition of osteoclast maturation through blockage of protein prenylation and geranyl-geranylation in myeloid precursor cells [Coxon et al., 2000; Russell et al., 2008]. Although the macrophage and the osteoclast originate from a common precursor, much less is known about the effects of bisphosphonates on macrophage gene expression. Macrophages play an important role in the early phase of wound healing. In addition to the phagocytosis of debris and bacteria, macrophages release cytokines that promote proliferation of fibroblasts and endothelial cells. Some studies have suggested that reduced macrophage infiltration may, in part, be responsible for the increased rate of healing in oral mucosa compared to dermal sites [Szpaderska et al., 2003]. We previously demonstrated that macrophages are present in human ONJ biopsy specimens with a trend toward increased macrophage numbers in patients with IV bisphosphonate-induced ONJ [Scheller et al., 2011]. Thus, understanding the mechanisms by which bisphosphonates regulate macrophage functions, such as cytokine secretion, may provide insight into the pathogenesis of ONJ and lead to discovery of new therapeutic targets. Given the increased risk of ONJ with obesity and the importance of macrophages in wound healing, we hypothesized that amino-bisphosphonates could contribute to the progression of ONJ and other inflammatory disorders by regulating macrophage responses to systemic cytokines such as leptin and IL-6.
MATERIALS AND METHODS
Transgenic Mice
All procedures were approved by the University Committee on the Use and Care of Animals. Mice with the coding sequence for exon17 of the long-form leptin receptor (ObRb) flanked by loxP were obtained from Dr. Martin Myers (University of Michigan) with permission of Dr. Streamson Chua (Columbia University) [McMinn et al., 2004]. Mice with germline transmission of the recombined floxed region and inactivation of leptin receptor signaling were used in our study and are referred to as knock-out (KO) mice. ObRb transgenic s/s (point mutation at tyrosine 1138) and l/l (point mutation at tyrosine 985) mice were the generous gift of Dr. Peter Mancuso (University of Michigan) and are also available from Jackson Labs (Stock No. 008518 and 008385). Genotyping of s/s, l/l, and ObRb KO mice was performed from tail biopsy as described previously [Bates et al., 2003; Bjornholm et al., 2007; McMinn et al., 2004]. Recombination and deletion of the loxP flanked allele was determined using a three-primer system designed by McMinn et al [McMinn et al., 2004].
Cell Culture
Human
Primary human bone marrow macrophages (hBMMs) were differentiated from primary marrow of iliac crest specimens (60 year old male) obtained with University of Michigan Institutional Review Board (IRB) approval. Fragments were washed extensively with PBS and marrow suspension processed with Lympholyte® Mammal gradient cell separation media (Cedarlane® Laboratories, Cat:CL5110) to remove dead cells and erythrocytes. Briefly, 4mL cell suspension was layered on 3mL Lympholyte® and centrifuged at 800g for 20 minutes at room temperature. The lymphocyte layer was harvested with a Pasteur pipet and washed three times in media. Cells were plated in macrophage growth medium (α-Modified Eagle’s Medium (α-MEM; Invitrogen/Gibco), 10% FBS (Gibco, Lot no.451459), 100U/ml penicillin, 100mg/ml streptomycin sulfate (Gibco Cat:15140)) at a density of 700,000 cells/cm2 in 100ng/mL recombinant human macrophage colony stimulating factor (M-CSF) (Peprotech, Cat:300–25). Media was replenished after two days. At day four serum free media ± ZA was added overnight before stimulation with 50ng/mL IL-6 (Peprotech, Cat:200–06) for five hours.
Mouse
Bone marrow macrophages (BMMs) were generated by plating the total marrow from two mice (femora, tibiae, and humeri) to 36-wells of two 24-well plates for ELISA or five 60mm dishes for Western blot analysis. Cells were derived in macrophage growth medium plus 70ng/mL M-CSF (Peprotech, Cat:315–02)) for 2–3 days. Primary BMMs were serum starved in the presence of ZA ((Novartis, Zometa®) overnight (16–18 hours) before treatment with 100nM recombinant mouse leptin (R&D Systems, Cat:398-LP-01M), 20% fetal bovine serum, or 25ng/mL IL-6 (PeproTech, Cat:216–16) for 1 hour for qPCR, 3 hours for Western blot analysis, or 24 hours for ELISA. Cells were treated with 5–10 μM trans,trans-Farnesol (FOH) (Sigma:277541) or geranylgeraniol (GGOH) (Sigma:G3278) where indicated.
ELISA
BMMs were plated in 24-well plates, six wells per data point per experiment. All experiments were repeated at least twice. After three days of BMM differentiation, cells were serum starved in the presence of 10−7 to 10−5M ZA overnight. Subsequently, 250uL of α-MEM + 0.5% BSA (Sigma, Cat:A8806) containing fresh ZA and 100nM Leptin as added as indicated. Supernatant was harvested after 24 hours and stored at −80°C until analyzed. Post-supernatant removal, genomic DNA was harvested using the QIAGEN DNeasy kit (Cat:69506) by adding PBS, proteases, and buffer directly to each well followed by incubation of the whole plate at 55°C. Harvested DNA was quantified using a UV spectrophotometer at 260nm. Ratio of 260nm to 280nm absorbance was greater than 1.4 for sample acceptance. Ten to twenty-five microliters of supernatant was analyzed with an IL-6 ELISA kit (R&D Systems, Cat:M6000B). Results from each well were normalized to total genomic DNA.
Western Blot Analysis
Protein was harvested using NP-40 lysis buffer (10% glycerol, 1% NP-40, 50mM Tris pH 7.4, 200mM NaCl, 2mM MgCl2, 1mM PMSF, 1xProtease Inhibitor Cocktail (Sigma Cat:P8340)). Cells were collected and pelleted in 1mL ice cold PBS at 4°C. Cell pellets were resuspended in 25–50 μL of lysis buffer, incubated for 30min on ice, centrifuged at 13,000rpm for 10 minutes, and supernatant harvested. Protein concentration was measured at 595nm with Bio-Rad protein assay dye concentrate (Cat:500–0006). Protein extracts (30–80 μg) were boiled in 2x SDS sample buffer 5min and separated on a 12% Tris-HCl polyacrylamide gel (Bio-Rad). Protein was transferred to PVDF membranes using a wet transfer system (Bio-Rad Cat:170–3930). Membranes were blocked in 5% milk (Stat3, P-Stat3, GAPDH; Bio-Rad, Cat:170–6404) or 5% BSA (SOCS3, Sigma, Cat:A7906) one hour and probed overnight with 1:1000 P-Stat3 (Cell Signaling:9131) or 1:1000 SOCS3 (Cell Signaling:2923) and stripped and re-probed the next day with 1:1000 Stat3 (Cell Signaling:9132) and/or 1:1000 GAPDH (Chemicon® International:MAB374). Signals were amplified with 1:3000 HRP-conjugated secondary antibody (Santa Cruz Biotechnology) and SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific, Pierce Product) and developed using film exposure.
Human Tissue Collection
Biopsies were accessioned with University of Michigan IRB approval. Biopsies were identified as described previously [Scheller et al., 2011] and assigned to one of two groups, no BP history (control) or ONJ. H&E stained slides of all cases were examined to ensure that each specimen contained epithelium and connective tissue. Histological identification of non-vital bone with associated bacterial colonies, consistent with osteonecrosis, was also required.
Immunofluorescence
Cells were fixed for 5 minutes in methanol at −20°C before PBS wash and permeabilization with 1% Triton-X-100 in PBS. Cells were blocked in PBS+2.5% goat serum+2.5% donkey serum+0.1% Triton-X-100 for 30 minutes. CD68 primary antibody (1:500, AbCam:ab955) was added in 1:10 diluted block for one hour and after washing signal was amplified with secondary antibody (1:500 Donkey anti-mouse Cy™3, Jackson ImmunoResearch) for 45 minutes. Nucleic acids were visualized with DAPI.
Immunohistochemistry
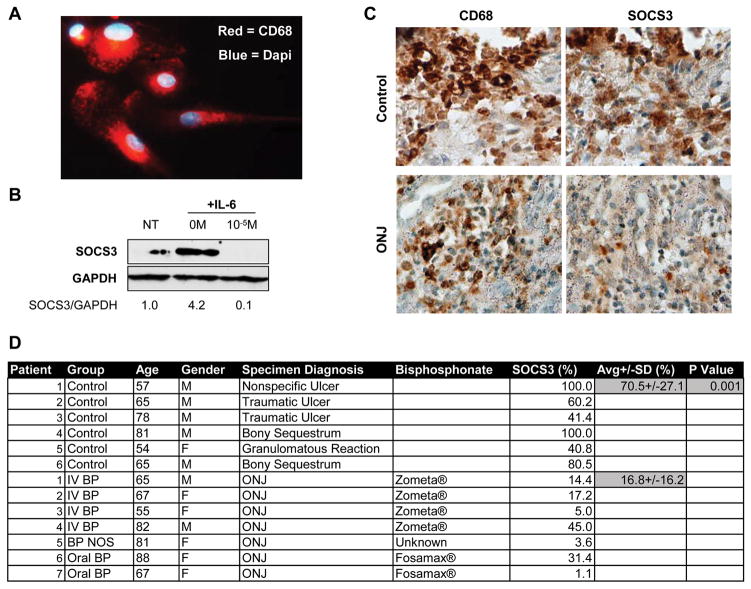
Immunohistochemistry was performed as described previously [Scheller et al., 2011]. Formalin-fixed sections were deparaffinized in xylene and rehydrated. Antigen retrieval was performed in pH 6.0 citrate buffer. Endogenous peroxidase was quenched in 3% hydrogen peroxide for 30 minutes followed by permeabilization in 0.1% Triton-X-100 in PBS. Slides were blocked with 2.5% normal horse serum. Primary antibodies were added overnight at 4°C: CD68 (1:500, Abcam:ab955) and SOCS3 (1:500, Abcam:ab16030). Signal was amplified with ImmPRESS reagent (VectorLabs:MP-7500) and imaged with ImmPACT DAB substrate (VectorLabs:SK-4105). Slides were counterstained with hematoxylin. Serial sections were stained for CD68 and SOCS3. Comparison was performed by generating a representative image composite of all CD68 or SOCS3 stained sections to ensure identical analysis of all images (40x magnification), images were then processed with Adobe Photoshop CS3 by using select→color range→reds to remove background blue hematoxylin staining. The resulting composite was thresholded with ImageJ (Scion Corp., Frederick, MD, USA) and total pixel density of stain per total field size of each specimen recorded. Results are expressed as the ratio of SOCS3 stain density to CD68 stain density in percent.
PCR
Total RNA was extracted from primary BMMs using TRIzol reagent (Invitrogen). Total RNA (0.6 μg) was converted to cDNA using the SuperScript® III First-Strand Synthesis SuperMix Kit (Invitrogen:11752). Resulting cDNA was diluted 1:20 and used for 20 μL reactions in SYBR® Green PCR master mix (Applied Biosystems). PCR and determination of the computed threshold was performed on an iCycler (Bio-Rad). SOCS3 primers based on sequence NM_007707 are as follows: Right_AACTTGCTGTGGGTGACCAT, Left_AAGGCCGGAGATTTCGCT.
Statistical Analysis
A two-tailed, homoscedastic t test was used to calculate statistical differences between control and experimental groups. Values are reported as the mean ± the standard deviation. P<0.050 was considered statistically significant. For western blot, band density was measured with UVP® VisionWorksLS™ image acquisition and analysis software. For Figure 2 and 3, SOCS3 band density was normalized to GAPDH band density. For Figure 3, P-Stat3 band density was normalized to Stat3 band density. The resulting value was then normalized to GAPDH. To evaluate significance, the control ratio of [(P-Stat3/Stat3)/GAPDH] for each time point was set to 1.0. The value from the corresponding ZA treated time point was then assigned a proportional fraction of 1.0. This fraction was then compared between multiple independent blots, three to five blots per time point, and statistics calculated as above.
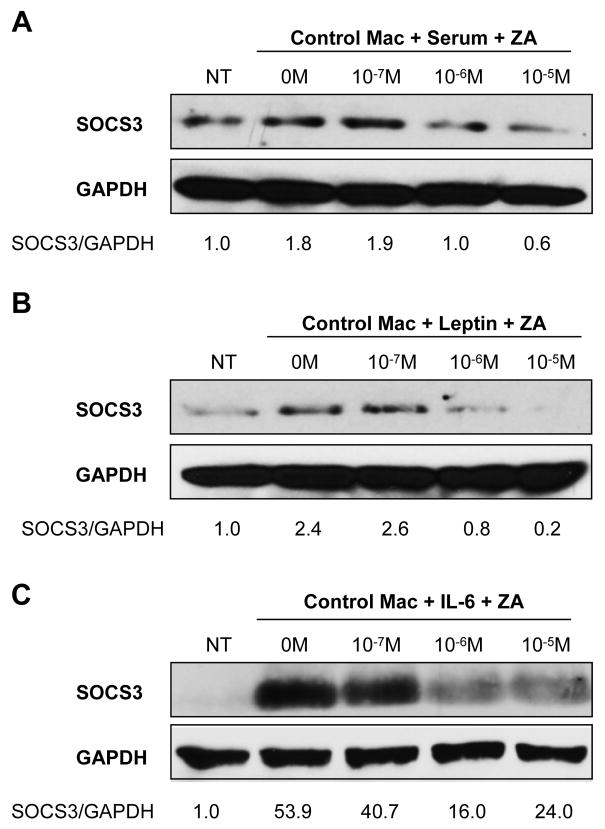
Figure 2. ZA inhibits SOCS3 protein accumulation in primary bone marrow macrophages.
Western blot densitometry data was normalized to GAPDH control. (A,B,C) ZA pretreatment for 16–18 hours from 10−7 to 10−5M dose-dependently inhibited induction of SOCS3 in BMMs by 20% serum, 100nM leptin, or 25ng/mL IL-6 respectively after three hours. Representative blots shown, all experiments repeated at least twice.
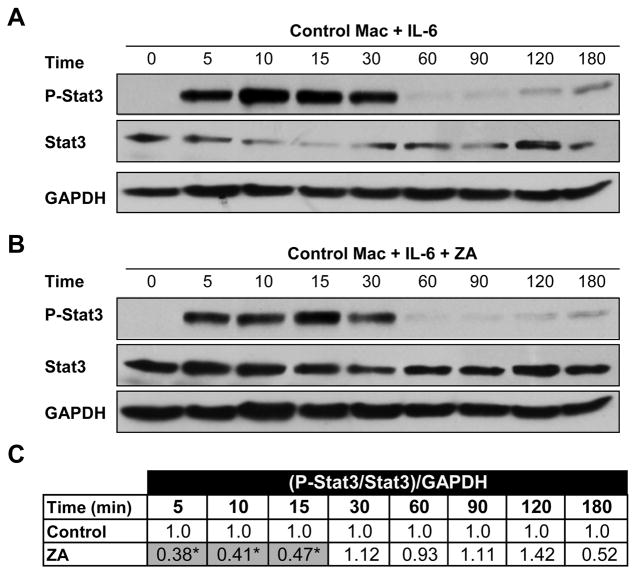
Figure 3. ZA inhibits Stat3 phosphorylation.
(A) Control western blot of P-Stat3, Stat3, and GAPDH after BMM stimulation with 25ng/mL IL-6 for 0 to 180 minutes. (B) Paired western blot after treatment with 10−5M ZA. (C) Statistical analysis of the ratio of phosphorylation of Stat3 after ZA treatment to control at each time point (N=3). Control densitometry ratio [(P-Stat3/Stat3)/GAPDH] was set to 1.0 and corresponding ZA treated ratio given a proportional value before statistical comparison of independent blots.
RESULTS
Zoledronic Acid enhances leptin-induced IL-6 production in an ObRb Tyr985-dependent manner
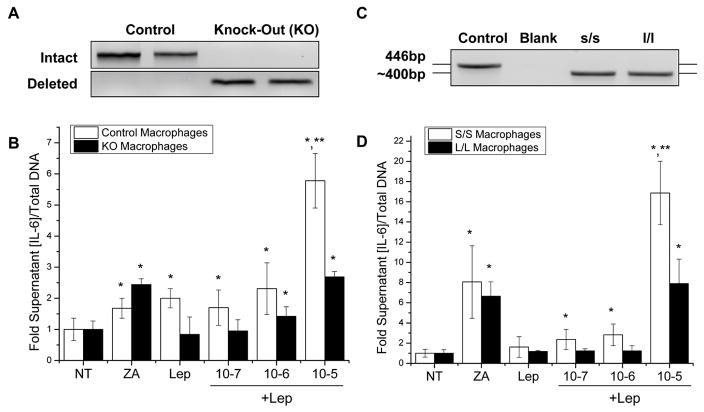
Primary bone marrow macrophages (BMMs) from control and complete long-form leptin receptor (ObRb) knock-out (KO) mice (Fig. 1A) were stimulated with 100nM leptin and 10−7 to 10−5M zoledronic acid (ZA) (Fig. 1B). The conditioned medium was subsequently analyzed for IL-6 and normalized to total genomic DNA per well. ZA alone induced a 1.68 fold (p=0.006) increase of IL-6 in control cells and a 2.44 fold (p<0.001) increase in KO cells (Fig. 1B). Administration of 100nM leptin induced a leptin-specific 2.01 fold (p<0.001) increase of IL-6 in control cells with no change in ObRb KO cells confirming the ability of leptin to induce IL-6 through ObRb (Fig. 1B). Combination treatment with 10−5M ZA and 100nM leptin produced a synergistic 5.78 fold (p<0.001) increase in IL-6 above that induced by ZA or leptin alone (Fig. 1B). This synergy was not observed in the KO cells (Fig. 1B).
Figure 1. ZA and leptin induce synergistic downstream cytokine production mediated by ObRb tyrosine 985.
(A) Genomic DNA PCR confirming complete cre-lox recombination of the BMMs from the long-form leptin-receptor (ObRb) knock-out (KO) animals and intact ObRb in the controls. (B) IL-6 ELISA of control and KO BMMs after 16–18 hour pretreatment with ZA followed by induction with 100nM leptin for 24 hours. (C) Confirmatory genotyping of s/s (point mutation ObRb Tyr1138) and l/l (point mutation ObRb Tyr985) mice. (D) IL-6 ELISA of s/s and l/l BMMs as above. *: significant over NT control, **: significant over ZA only control. (N=6, all experiments repeated at least twice)
To determine which signaling pathway was responsible for the increased cytokine production with combined leptin and ZA treatment, we utilized two mouse models with gene targeted deletions in the signaling components of the leptin receptor, ObRb. ObRb has two signaling phosphotyrosines, Tyr1138 and Tyr985, on the cytoplasmic domain of the receptor. Point mutation of Tyr1138, which was generated in s/s gene targeted mice, blocks leptin signaling through P-Stat3 and SOCS3. In contrast, mutation of Tyr985 of the leptin receptor in l/l mice blocks 70–100% of downstream Erk signaling [Myers, 2004]. The signaling deficits of s/s and l/l mice have been previously verified in the central nervous system [Bates et al., 2003; Bjornholm et al., 2007] and in peripheral macrophages [Mancuso et al., 2011]. BMMs harvested from s/s and l/l mice were genotyped (Fig. 1C) and treated as described above. Repetition of the ELISA experiment revealed a 16.87 fold (p=0.001) synergistic increase in IL-6 after combined leptin and 10−5M ZA treatment in the s/s BMMs but not in the l/l BMMs (Fig. 1D). This implies that synergistic induction of IL-6 production by Leptin and ZA requires Tyr985-directed Erk signaling.
ZA blocks SOCS3 protein accumulation by BMMs
The s/s transgenic mice that lack the ability to signal through P-Stat3 and SOCS3 demonstrated a robust synergistic increase in macrophage IL-6 output. A similar increase was observed after treatment of control macrophages with leptin and 10−5M ZA. Since SOCS3 is a known inhibitor of leptin signaling, we hypothesized that ZA may be regulating cellular SOCS3 accumulation. SOCS3 is a negative-feedback regulator of signaling molecules such as leptin and IL-6, and induction of SOCS3 expression can decrease macrophage cytokine production [Yoshimura et al., 2007]. We found that stimulation of BMMs with 20% FBS for three hours increased SOCS3 protein 1.8-fold (Fig. 2A). Treatment with 10−6M or 10−5M ZA inhibited this increase in a dose dependent manner (Fig. 2A). Leptin induction of SOCS3 protein levels by 2.4-fold at three hours was also inhibited dose dependently by 10−6 or 10−5M ZA (Fig. 2B). Similarly, SOCS3 inhibition after stimulation with 25ng/mL IL-6 by ZA was noted (Fig. 2C).
ZA reduces phosphorylation of Stat3 in a mevalonate-pathway dependent manner
Since SOCS3 RNA is induced by nuclear accumulation of P-Stat3 and previous reports have suggested that ZA can modulate P-Stat3 levels in clonal macrophages [Reuben et al., 2011], we investigated the ability of ZA to regulate P-Stat3 in primary BMMs. Addition of 25ng/mL IL-6 for 0 to 180 minutes increased phosphorylation of Stat3 from 5–30 minutes and again from 120–180 minutes (Fig. 3A). Pre-treatment with 10−5M ZA for 16–18 hours resulted in both an increase in total Stat3 at all time points and a reduction in Stat3 phosphorylation (Fig. 3B). This approximately 50% reduction in the ratio of P-Stat3 to Stat3, normalized to GAPDH, was statistically significant from 5 to 15 minutes (Fig. 3C). Many of the functions of amino-bisphosphonates are mediated by their ability to inhibit the mevalonate pathway. Therefore, mevalonate pathway intermediates GGOH and FOH were added to determine the extent to which specific intermediates may rescue the bisphosphonate-induced P-Stat3 defect. Addition of 10 μM GGOH did not alter phosphorylation of Stat3. In contrast, the addition of 10 μM FOH fully restored levels of P-Stat3 in the presence of 10−5M ZA (Fig. 4A).
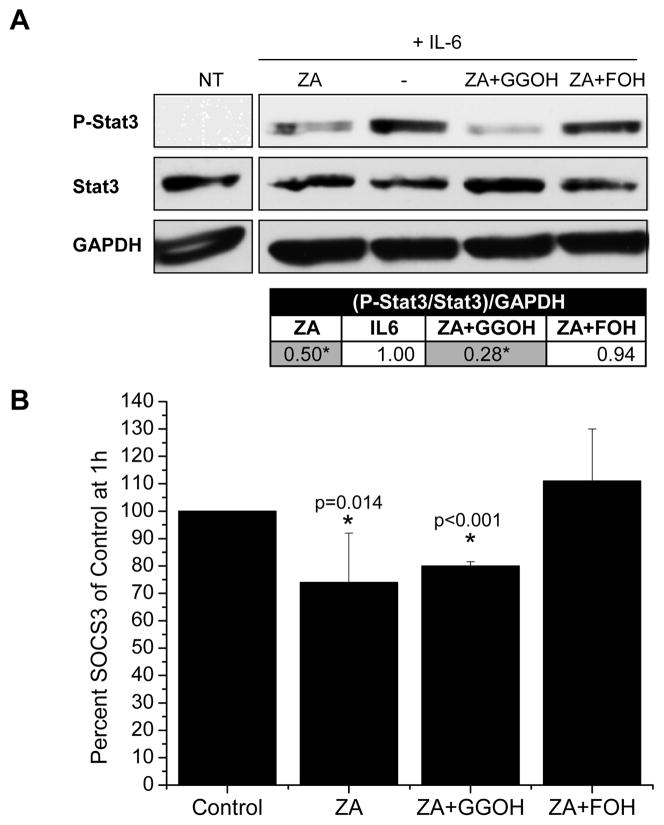
Figure 4. Farnesyl intermediates rescue ZA inhibition of P-Stat3 and SOCS3 mRNA.
(A) Western blot of BMMs pre-treated with 10−5M ZA and 10 μM GGOH or FOH overnight, ‘−’ indicates lack of ZA treatment. Pre-treated cells were stimulated with 25ng/mL IL-6 for 10–15 minutes. Tabulated results report the ratio of phosphorylation of Stat3 after ZA treatment to control at each time point as in figure 3 (N=3). (B) Quantitative PCR analysis of SOCS3 mRNA after one hour, treatments as in (B). Results are reported as percent of control SOCS3 mRNA (N=3–5). *: significantly less than control.
Correction of P-Stat3 signaling is not sufficient to rescue SOCS3 protein inhibition
P-Stat3 nuclear accumulation induces SOCS3 mRNA transcription [Banks et al., 2000]. Therefore, we sought to determine if cytokine treatment had a similar effect in our system. Addition of 25ng/mL IL-6 significantly induced SOCS3 mRNA accumulation after one hour (Data not Shown). This increase was significantly blunted by treatment with 10−5M ZA and rescued by concomitant treatment with 10 μM FOH, but not 10 μM GGOH (Fig. 4B). Despite the complete rescue of P-Stat3 protein and SOCS3 mRNA levels (Fig. 4A,B), FOH at 5 μM or 10 μM was not sufficient to rescue SOCS3 protein production (Fig. 5).
Figure 5. Rescue of P-Stat3 and SOCS3 mRNA with FOH does not correct SOCS3 protein inhibition.
Representative western blot of SOCS3 after ZA/GGOH/FOH overnight pre-treatment where indicated and three hour 25ng/mL IL-6 stimulation of BMMs. GGOH and FOH concentrations are reported as μM and combined with 10−5M ZA. Experiment was repeated three times with similar results.
Macrophage-associated SOCS3 levels are decreased in human specimens
To determine if alterations in SOCS3 were present in human cells and tissue, we analyzed both primary human macrophages and ONJ biopsy specimens. Human bone marrow macrophages (hBMMs) were harvested from iliac crest bone marrow of a 60 year old male donor. Harvested cells were strongly positive for CD68, a macrophage marker, when analyzed with immunofluorescence (Fig. 6A). SOCS3 protein accumulation induced after five hours of 50ng/mL IL-6 treatment was inhibited by pre-incubation of the cells with 10−5M ZA (Fig. 6B) similar to studies with mouse BMMs (Fig. 2A–C). Immunohistochemistry for SOCS3 and CD68 was performed on serial sections of biopsies derived from control human oral ulcerations or ulcerations associated with ONJ. Comparison of SOCS3 staining density in the areas of macrophage infiltration revealed a significant decrease in SOCS3 in the ONJ specimens (16.8±16.2%, p=001) when compared to controls (70.5±27.1%) (Fig. 6C,D).
Figure 6. Bisphosphonates decrease SOCS3 in human CD68+ macrophages and human ONJ biopsies.
(A) Cellular immunofluorescence of primary human macrophages. Red = CD68, Blue = Dapi. 40x magnification. (B) Representative western blot of primary macrophage SOCS3 after treatment with 50ng/mL IL-6 alone or IL-6 + 10−5M ZA. Experiment was performed in duplicate with similar results. (C) Representative serially stained sections of control ulcerated tissue and an ONJ lesion. 40x magnification. (D) Patient information, condition, treatment, and SOCS3 percent staining density.
DISCUSSION
SOCS3 is an essential regulator of diverse cellular functions including cytokine production and has been implicated in the pathogenesis of inflammatory pathology such as Crohn’s disease and rheumatoid arthritis. We found that the amino-bisphosphonate zoledronic acid has the potential to enhance macrophage cytokine secretion by inhibiting SOCS3 protein accumulation. Due to the extended half-life of bisphosphonates and their ability to incorporate into the bone matrix [Kozloff et al., 2010], it is possible that long-term systemic persistence of these drugs may contribute to inflammatory pathologic processes through the inhibition of SOCS3.
Indeed, ONJ, an increasingly recognized complication of amino-bisphosphonate treatment, may be linked to altered macrophage SOCS3 activity, as preliminary evidence suggests that SOCS3 protein levels are decreased in ONJ biopsy specimens when compared to controls. However, it is important to recognize a limitation of this study. Except for specimen number five that contained a granulomatous reaction (Fig. 6D), both the controls and the ONJ biopsy specimens represented clinical ulcerations of similar appearance. In all specimens one would expect to see an inflammatory reaction including induction of SOCS3. However, the duration of the ulcers is unknown and their status as acute vs chronic inflammatory lesions may also influence SOCS3 protein levels. Despite this level of information, the approximately four-fold decrease in SOCS3 protein levels in the ONJ specimens suggests that further investigation in this area may provide additional insights on the mechanisms responsible for ONJ.
The pathogenesis of ONJ is currently unknown but may be linked to decreased bone turnover, altered vascularity, bacterial invasion, and compromised wound healing [Novince et al., 2009]. Osteoimmunology is an emerging field of research that views regulation of bone metabolism within the context of immune function [Lorenzo et al., 2008]. While the macrophage has long been appreciated as a key component of the immune system, an essential role for macrophages in bone homeostasis was only recently confirmed when induced depletion of macrophages in a mouse model ablated mature osteoblast bone-forming surface [Chang et al., 2008]. In addition to regulation of osteogenesis, macrophage-released cytokines are regulators of fibroblast and endothelial cell proliferation and studies have speculated that reduced macrophage infiltration may, in part, be responsible for increased rates of healing of oral mucosa compared to keratinized dermal sites [Szpaderska et al., 2003]. Thus, dysregulation of critical macrophage functions such as cytokine production may contribute to altered bone remodeling and decreased healing potential observed at sites of ONJ.
We found that zoledronic acid at concentrations of 10−6 to 10−5M is a novel inhibitor of serum and cytokine-induced SOCS3 in macrophages in vitro. Inhibition of SOCS3 was also observed in human ONJ biopsy specimens in vivo. SOCS3 is a potent regulator of macrophage responses to systemic cytokines such as IL-6 and leptin that are upregulated in states of obesity. Analysis of alendronate release kinetics in rat bone have demonstrated local bisphosphonate concentrations of 10−3 to 10−4M at sites of active resorption after 0.4mg/kg treatment [Sato et al., 1991]. In addition, secreted concentrations of 10−5M ZA are readily achieved with ZA-loaded bone cement that has been proposed for local treatment of giant cell tumor and multiple myeloma [Zwolak et al., 2010]. This study reveals that the future use of such devices must consider complications due to inhibition of SOCS3 and enhancement of a destructive inflammatory response. Though our study has focused on SOCS3 inhibition in the context of ONJ, this may also play important roles in other inflammatory conditions such as rheumatoid arthritis [Isomaki et al., 2007], inflammatory bowel disease [Suzuki et al., 2001], and Crohn’s disease [Lovato et al., 2003].
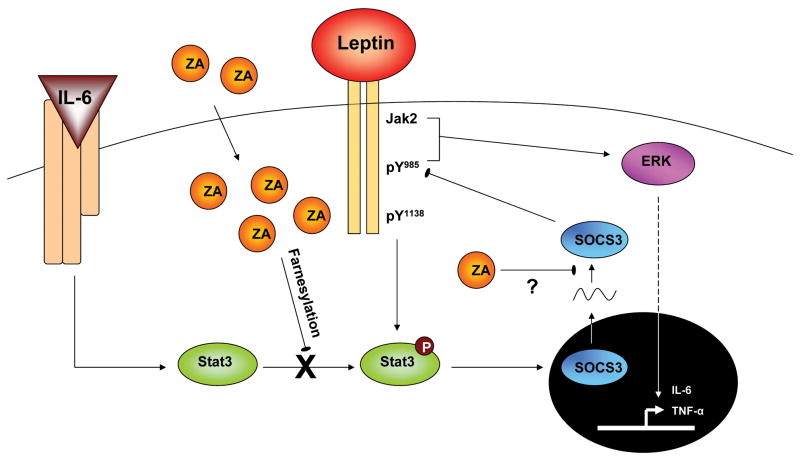
In summary, we conclude that ZA is a novel inhibitor of SOCS3 protein accumulation in macrophages, essentially ‘inhibiting the inhibitor’ of signaling by molecules such as leptin and IL-6 (Fig. 7). Functionally, administration of ZA with leptin promoted a synergistic induction of downstream IL-6 production by primary BMMs that was not present in BMMs lacking the long-form leptin receptor (ObRb). This effect was dependent on ObRb tyrosine 985, likely mediated by the downstream Erk pathway (Fig. 7). Though ZA was found to decrease both phosphorylation of Stat3 and initial induction of SOCS3 mRNA in a mevalonate-pathway dependent manner, rescue of these defects with FOH was not sufficient to restore SOCS3 protein levels (Fig. 4,5). Thus, the specific mechanism of SOCS3 inhibition is unclear at this point but may involve a post-transcriptional process. Though this study focused on SOCS3 induction by obesity-related cytokines leptin and IL-6, our results suggest wider implications for regulation of additional SOCS3 dependent signaling molecules such as IL-10, LPS and IFN-γ. In conclusion, we propose that inhibition of SOCS3 and dysregulation of macrophage cytokine output may contribute to obesity-associated inflammatory disorders and potentially the pathogenesis of ZA-induced ONJ.
Figure 7. Theoretical model of ZA regulation of SOCS3.
ZA taken up by the cell blocks phosphorylation of Stat3 in a farnesylation-dependent manner. ZA, via a secondary mechanism that may involve inhibition of protein translation, blocks protein accumulation of SOCS3. Functionally, this results in increased cytokine output of leptin-induced IL-6 in an ObRb Tyr985, Erk signaling dependent manner.
Acknowledgments
Supported by R01 DE13835 (PHK/KDH), F30 DE019577 (ELS) and the Baylor Oral Health Foundation (JSR). Special thanks to Paul Edwards and Sean Edwards for their help with the human biopsies and primary marrow samples respectively.
Grant Support:
Contract grant sponsor: NIDCR; Contract grant number: R01 DE13835 (PHK/KDH)
Contract grant sponsor: NIDCR; Contract grant number: F30 DE019577 (ELS)
Contract grant sponsor: Baylor Oral Health Foundation (JSR)
References
- Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Bjornholm M, Munzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjorbaek C, Myers MG., Jr Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–60. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartsos VM, Zhu S, Zavras AI. Bisphosphonate use and the risk of adverse jaw outcomes: a medical claims study of 714,217 people. J Am Dent Assoc. 2008;139:23–30. doi: 10.14219/jada.archive.2008.0016. [DOI] [PubMed] [Google Scholar]
- Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–44. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467–76. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–66. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, Leighton J, Booth B, Gobburu JV, Rahman A, Hsieh Y, Wood R, Vause D, Pazdur R. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9:2394–9. [PubMed] [Google Scholar]
- Isomaki P, Alanara T, Isohanni P, Lagerstedt A, Korpela M, Moilanen T, Visakorpi T, Silvennoinen O. The expression of SOCS is altered in rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1538–46. doi: 10.1093/rheumatology/kem198. [DOI] [PubMed] [Google Scholar]
- Jarrett SJ, Conaghan PG, Sloan VS, Papanastasiou P, Ortmann CE, O’Connor PJ, Grainger AJ, Emery P. Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum. 2006;54:1410–4. doi: 10.1002/art.21824. [DOI] [PubMed] [Google Scholar]
- Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV, Beneton MN, Gertz BJ, Sciberras DG, Holland SD, Orgee J, Coombes GM, Rogers SR, Porras AG. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res. 1997;12:1700–7. doi: 10.1359/jbmr.1997.12.10.1700. [DOI] [PubMed] [Google Scholar]
- Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T, Yoshimura A. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med. 2006;203:1021–31. doi: 10.1084/jem.20052333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff KM, Volakis LI, Marini JC, Caird MS. Near-infrared fluorescent probe traces bisphosphonate delivery and retention in vivo. J Bone Miner Res. 2010;25:1748–58. doi: 10.1002/jbmr.66. [DOI] [PubMed] [Google Scholar]
- Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29:403–40. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, Eriksen KW, Woetmann A, Odum N. Constitutive STAT3 activation in intestinal T cells from patients with Crohn’s disease. J Biol Chem. 2003;278:16777–81. doi: 10.1074/jbc.M207999200. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Peters-Golden M, Goel D, Goldberg J, Brock TG, Greenwald-Yarnell M, Myers MG., Jr Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J Immunol. 2011;186:1081–90. doi: 10.4049/jimmunol.1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, Chua SC., Jr An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome. 2004;15:677–85. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- Mendall MA, Gunasekera AV, John BJ, Kumar D. Is obesity a risk factor for Crohn’s disease? Dig Dis Sci. 2011;56:837–44. doi: 10.1007/s10620-010-1541-6. [DOI] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–43. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- Mori H, Shichita T, Yu Q, Yoshida R, Hashimoto M, Okamoto F, Torisu T, Nakaya M, Kobayashi T, Takaesu G, Yoshimura A. Suppression of SOCS3 expression in the pancreatic beta-cell leads to resistance to type 1 diabetes. Biochem Biophys Res Commun. 2007;359:952–8. doi: 10.1016/j.bbrc.2007.05.198. [DOI] [PubMed] [Google Scholar]
- Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- Novince CM, Ward BB, McCauley LK. Osteonecrosis of the jaw: an update and review of recommendations. Cells Tissues Organs. 2009;189:275–83. doi: 10.1159/000152915. [DOI] [PubMed] [Google Scholar]
- Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene. 2006;25:2520–30. doi: 10.1038/sj.onc.1209281. [DOI] [PubMed] [Google Scholar]
- Ohishi M, Matsumura Y, Aki D, Mashima R, Taniguchi K, Kobayashi T, Kukita T, Iwamoto Y, Yoshimura A. Suppressors of cytokine signaling-1 and -3 regulate osteoclastogenesis in the presence of inflammatory cytokines. J Immunol. 2005;174:3024–31. doi: 10.4049/jimmunol.174.5.3024. [DOI] [PubMed] [Google Scholar]
- Piessevaux J, Lavens D, Peelman F, Tavernier J. The many faces of the SOCS box. Cytokine Growth Factor Rev. 2008;19:371–81. doi: 10.1016/j.cytogfr.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Reuben JS, Dinh L, Lee J, Stateson J, Kamara H, Xiang L, Opperman LA. Bisophosphonates inhibit phosphorylation of signal transducer and activator of transcription 3 and expression of suppressor of cytokine signaling 3: implications for their effects on innate immune function and osteoclastogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:196–204. doi: 10.1016/j.tripleo.2010.09.068. [DOI] [PubMed] [Google Scholar]
- Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–59. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller EL, Baldwin CM, Kuo S, D’Silva NJ, Feinberg SE, Krebsbach PH, Edwards PC. Bisphosphonates Inhibit Expression of p63 by Oral Keratinocytes. J Dent Res. 2011 doi: 10.1177/0022034511407918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhoff H, Jung JY, Ebmeyer J, Faddis BT, Hildmann H, Chole RA. Zoledronic acid inhibits osteoclastogenesis in vitro and in a mouse model of inflammatory osteolysis. Ann Otol Rhinol Laryngol. 2003;112:780–6. doi: 10.1177/000348940311200907. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–81. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J Dent Res. 2003;82:621–6. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5:525–32. [PubMed] [Google Scholar]
- Wessel JH, Dodson TB, Zavras AI. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg. 2008;66:625–31. doi: 10.1016/j.joms.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–6. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zoler ML. Zoledronic acid relieves knee OA pain and shrinks bone marrow lesions: “Elsevier Global Medical News”. International Medical News Group; 2010. [Google Scholar]
- Zwolak P, Manivel JC, Jasinski P, Kirstein MN, Dudek AZ, Fisher J, Cheng EY. Cytotoxic effect of zoledronic acid-loaded bone cement on giant cell tumor, multiple myeloma, and renal cell carcinoma cell lines. J Bone Joint Surg Am. 2010;92:162–8. doi: 10.2106/JBJS.H.01679. [DOI] [PubMed] [Google Scholar]