Abstract
Translocation of bacteria and other luminal factors from the intestine following surgical injury can be a major driver of critical illness. Bile acids have been shown to play a key role in the loss of intestinal epithelial barrier function during states of host stress. Experiments to study the ability of nonionic block copolymers to abrogate barrier failure in response to bile acid exposure are described. In vitro experiments were performed with the bile salt sodium deoxycholate (SDC) on Caco-2 enterocyte monolayers using transepithelial electrical resistance (TER) to assay barrier function. A bisphenol-A coupled tri-block polyethylene glycol, PEG 15–20, was shown to prevent SDC-induced barrier failure. ELISA, LDH, and caspase-3 based cell death detection assays demonstrated that bile acid-induced apoptosis and necrosis were prevented with PEG 15–20. Immunofluorescence microscopic visualization of the tight junctional protein zonula occludens-1 (ZO-1) demonstrated that PEG 15–20 prevented significant changes in tight junction organization induced by bile acid exposure. Preliminary TER-based studies examining structure-function correlates of polymer protection against bile acid damage were performed with a small library of polyethylene glycol-based copolymers. Polymer properties associated with optimal protection against bile acid-induced barrier disruption were PEG- based compounds with a molecular weight > 10 kDa and amphiphilicity. The data demonstrate that PEG-based copolymer architecture is an important determinant that confers protection against bile acid injury of intestinal epithelia.
Keywords: Enterocyte, sodium deoxycholate, polyethylene glycol block copolymers, PEG 15–20, gut-derived sepsis
INTRODUCTION
The gut has frequently been described as the “motor” of multiple organ failure. Loss of intestinal epithelial barrier function can be both a cause and consequence of critical illness and can result in local and systemic dissemination of luminal toxins, microbes, and yet- to- be identified pro-inflammatory agents. The barrier function of the intestinal epithelium is affected by luminal factors, microbes, and various other factors including nerves, immune cells, and local and circulating cytokines. Many of these factors converge to affect critical elements within the cell cytoskeleton that alter tight junction integrity (1). Loss of tight junctional integrity following severe injury and inflammation has been shown to have potent pro-inflammatory potential and to adversely affect outcome (2).
Virtually all disorders that affect epithelial barrier function appear to be characterized by loss of the overlying protective mucus coat. One hypothesis is that mucus loss exposes the epithelium to cytotoxic bile acids present in the gut lumen leading to loss of barrier function (3, 4). Supporting this theory are studies showing that pancreatic duct ligation, which occludes bile flow, abrogates intestinal epithelial barrier failure following severe catabolic stress such as occurs from injury or hemorrhage (5). Exposure of the underlying epithelium to bile acids can, in addition, induce epithelial apoptosis via the generation of reactive oxygen species (6,7). Bile acids are also known to cause epithelial necrosis. At concentrations above their critical micellar concentration (CMC), bile acids form micelles that emulsify membrane lipids. Below their CMC, bile acids exist as monomers and are capable of destabilizing the cellular membrane by inserting into the outer leaflet (8–10). Additionally, bile acids have been shown to alter various structural elements of the tight junction leading to loss of barrier function (11, 12).
Amphiphilic species normally present in bile play a major protective role against the cytotoxic effects of bile acids on the gut epithelium. Phosphatidylcholine (PC), the predominant phospholipid in bile, has been studied extensively for its ability to prevent bile acid disruption of epithelium (13–16). In addition to providing a cytoprotective effect in bile, a phospholipid layer composed primarily of PC covers the GI surface and provides a barrier between the epithelium and the aqueous luminal contents (17, 18). There is evidence to suggest that this critical barrier is disrupted during states of extreme physiologic stress (19).
Here we study the ability of nonionic block copolymers (NBCs) to provide cytoprotective effects against bile acid injury in the same way that PC provides protection in the normal physiologic state. NBCs are commercially available amphiphilic macromolecules composed of two blocks of hydrophilic polymer, typically polyethylene glycol (PEG), flanking a core hydrophobic block in an ABA configuration. Because the number and identity of the monomer units in each block can be varied, many different copolymers can be synthesized with resulting differences in chemical properties. NBCs have recently been studied for their potential to preserve host-bacteria commensalism in the face of host stress, thus providing an alternative option to antimicrobials for suppressing post-operative infections. One NBC with a bisphenol A central hydrophobic unit, PEG 15–20, has been shown to prevent P. aeruginosa–induced barrier disruption in vitro and to significantly reduce mortality and bacterial virulence in a surgically stressed murine model of gut-derived sepsis (20).
In light of NBCs’ capability to reduce mortality from gut-derived sepsis, research into their behavior in the gut lumen, including putative interactions with bile acids, merits further study. Elucidation of the exact mechanism of NBC protection could enable the optimization of polymer architecture for use as a therapeutic agent to prevent sepsis in post-surgical patients. Therefore the aim of this study was to determine the structural requirements of cytoprotective polymers to protect the intestinal epithelial barrier using bile acids as the barrier disrupting agent.
METHODS
Intestinal epithelial cells
Experiments were performed with highly differentiated human colonic carcinoma Caco-2/C2bbe, or C2, cells at passage 51–60 (21). C2 cells produce minimal mucus (22) and therefore mimic conditions of the intestinal epithelium exposed to catabolic stress (23, 24). Cells were grown at 37° C in 5% CO2 to 95% air atmospheric conditions. Cells were subcultured on a weekly basis by trypsinization, and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA), supplemented with 10% fetal bovine serum, 2 mM L-Glutamate, 50 µg/mL Penicillin/Streptomycin, and 1.3 µg/mL transferrin.
C2 cells were seeded on collagen-coated Transwell porous polycarbonate membranes (6.5 mm diameter, 0.4 µm pore size, 24 well format) obtained from Corning Incorporated (Corning, NY, USA), and Falcon PET (poly[ethylene terephthalate]) track-etched porous membranes (6.5 mm diameter, 0.4 µm pore size, 24 well format) obtained from Becton Dickinson Labware (Franklin Lake, NJ, USA). Seeding density was 6 – 9 × 105 cells/cm2. Inserts were incubated for 5 – 10 days and the transepithelial electrical resistance (TER) was periodically checked with a Millicel-ERS (Electrical Resistance System, Millipore, Bedford, MA, USA). Experiments were performed once the C2 cells achieved confluence (TER ≥ 300 Ohms*cm2). C2 cells were serum starved for 24 hours prior to experimentation in DMEM with 2 mM L-Glutamate and 0.1% fetal bovine serum (referred to as SS media hereafter).
Transepithelial electrical resistance (TER)
Resistance was measured with a Millicel-ERS using cadmium “chopstick” electrodes with one electrode placed in the apical medium and one electrode in the basal medium. Resistance measurements were also taken with blank (enterocyte-free) membranes exposed to each experimental condition and these values were subtracted from the resistance of the cell-laden membranes yielding the resistance of only the enterocyte monolayer.
Baseline monolayer resistance was measured and calculated immediately prior to exposure to experimental conditions to establish “baseline resistance.” Subsequent TERs were measured at the indicated times and cell monolayer resistance calculated and reported. To allow for comparison of polymer protection, TERs are presented as a percentage of the individual well baseline resistance where appropriate.
Preparation of bile salt and polymer solutions
Immediately prior to the experiment, the bile salt sodium deoxycholate (3a, 12a-Dihydroxy-5b-cholan-24-oic acid, Sigma Chemical Co., St. Louis, MO, USA) was prepared in a solution of SS media. All bile acid solutions were vortexed for 15 seconds immediately prior to drawing any volume for dilution or application to cell monolayers to maximize homogeneity in suspension concentration.
Polymers were purchased from Sigma Chemical Co. and used as received: PEG 15–20 (catalog no. P2263), PEG-35 (catalog no. 81310), and from Spectrum Chemical Mfg. Co. (Gardena, CA, USA): Poloxamer 181 (catalog no. P1162), Poloxamer 188 (catalog no. P1169), Poloxamer 338 (catalog no. P1172), and Poloxamer 407 (catalog no. P1166) (Pluronics L61, F68, F108, and F127, respectively). PEG 15–20 is composed of a center hydrophobic block of bisphenol A flanked by hydrophilic arms made of polyethylene glycol (PEG). The bisphenol a moiety in PEG 15–20 was studied since we discovered that its aromatic rings anchor and insert into cholesterol- rich domains in cell membranes facilitating its usefulness as a mucoadhesive polymer in the gut (25). For comparison, additional polymers were selected using PEG as the flanking hydrophilic arms, but instead of bisphenol-A they are constructed with polypropylene oxide (PPO) as the center core. Copolymers of the PEG-PPO-PEG composition are also known as poloxamers, or by the trade name Pluronics. These copolymers find a wide range of uses in industrial applications and thus many different combinations of PEG arm sizes and PPO center sizes are commercially available and convenient for use in experimentation. A sampling of these poloxamers was selected to span a range of sizes of the hydrophilic PEG blocks, the size of the hydrophobic PPO core block, and the amphiphilicity (Table 1). All of these properties can affect not only how the polymer interacts with the epithelium and changes membrane function but also can affect conformational changes in the polymer as a result of its interaction with epithelial cells thereby altering its repellant and protective effect against xenobiotic agents and bile salts within the intestinal lumen.
Table 1.
Representative schematics of polymers tested for protective ability against bile acid-induced degradation of epithelial barrier function.
| Structure | Schematic | Polymers |
|---|---|---|
| PEGn |  |
PEG-35 |
| PEGn-BPA-PEGn |  |
PEG 15–20 |
| PEGn-PPO-PEGn |  |
P181, P188, P338, P407 |
PEG=polyethylene glycol; BPA=bisphenol A; PPO=polypropylene oxide. In the schematics, red=PEG; yellow=BPA; green=PPO.
Less than a week prior to experiment, polymers were prepared in a solution of SS media and stored at 4° C. Appropriate amounts of polymer and SDC solutions were combined and vortexed for 15 seconds immediately prior to application to ensure proper mixing.
Polymer protection against bile– induced damage to C2 cell monolayer
Prior to each experiment, baseline TER measurements were recorded for all inserts to be used. While establishing the model, we ran multiple dose-response experiments in order to determine the effective damaging dose (EDda) of SDC, defined as the minimum concentration of SDC needed to cause a >50% fall in the TER of C2 monolayers at one hour. A 20 mM solution of SDC was diluted to 0.4, 0.6, 0.8, 1.0, and 1.2 mM for PET inserts and 1.5, 1.75, and 2.0 mM for Transwell inserts. The apical media of the inserts was removed by dipping the insert into a beaker of fresh SS media, and the various bile salt solutions were added to the apical well (200 µL for Transwell inserts, 300 µL for PET inserts; 2 inserts per SDC concentration). SS media served as a negative control. TER measurements were taken every 30 minutes.
Following determination of the EDda, other inserts were used to study the protective effects of the polymers. All inserts used the EDda determined for that insert type in the dose-response experiments. The apical media was removed as described above, and the prepared solutions were added (4 inserts per condition). TER measurements were taken every hour, between which the inserts were incubated.
Polymer protection against bile acid– induced cell death
Initial assays for necrotic and apoptotic cell death were performed using a Roche Diagnostics Cell Death Detection Elisa Plus (catalog no. 11774425001). C2 monolayers were grown on 24 well plates until confluent as visualized by microscopy. Cells were serum starved for 24 hours, after which the medium was replaced by the appropriate experimental condition and the cells allowed to incubate for one hour (4 wells per condition). A portion of apical medium was removed and analyzed for the presence of DNA-histone complexes using the manufacturer’s ELISA assay. The remaining apical medium was pipetted off the cell monolayer, which was then rinsed in sterile PBS three times. The cells were collected and centrifuged at 200g for 10 minutes, and the resulting cell pellet was lysed, centrifuged to isolate the cytoplasmic fraction, and the same ELISA assay used to measure the concentration of DNA-histone complexes. Differences in concentration of DNA-histone complexes between the supernatant (representing necrosis) and the cytoplasmic fraction (representing apoptosis) provided a measure of necrosis vs. apoptosis as compared to the control condition. Confirmatory assays for necrosis (LDH release) and apoptosis (caspase-3) were performed using a Promega CytoTox 96 Non-Radioactive Cytotoxicity Assay (catalog no. G1781) and Calbiochem Caspase-3 Activity Assay (catalog no. QIA70), respectively. For necrosis assays, C2 monolayers were grown on p60 well plates until confluent, serum starved for 24 hours, and then exposed to previously defined experimental conditions (4 wells each). At one hour, medium samples were collected, centrifuged at 4°C for 5 minutes, and incubated with the appropriate kit reagents. The absorbance was then measured with a spectrophotometer at 490 nm and compared to the appropriate controls. For apoptosis assays, cells were grown on p60 well plates until confluent, serum starved for 24 hours, and then exposed to the previously described experimental conditions (4 wells each). At one hour, the cells were centrifuged at 500×g for 5 minutes, and the supernatant discarded. The cell pellet was then resuspended in kit reagents, lysed, and incubated in the appropriate buffer. Absorbance was measured with a spectrophotometer at 390 nm and compared to the appropriate controls.
Immunofluorescence microscopy
Microscopy was performed with fluorescein isothiocyanate (FITC) labeled mouse antibodies to the tight junctional protein zonula occludens-1 (ZO-1) (Invitrogen catalog no. 339111.) We used ZO-1 as a general marker for cytoskeletal integrity according to previously published work (26, 27). C2 monolayers were grown to confluence on P-35 Petri dishes (BD Falcon, catalog no. 351008). Monolayers were incubated in SS media for 24 hours followed by application of the appropriate experimental condition in addition to ZO-1 antibodies. Following incubation for one hour, the tight junctions were visualized using immunofluorescent microscopy.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to compare groups, with a P<0.05 considered to be statistically significant.
RESULTS
Dose- response studies of the effect of bile salt on the TER of C2 monolayers
We investigated the ability of bile salt to degrade the barrier function of a C2 monolayer and the ability of different polymers to prevent barrier disruption. We focused solely on deoxycholic acid in our experiments based on the approach of others and because it comprises approximately 20% of the bile acids (28) and is the main secondary bile acid in humans.
Barrier disruption was assessed by a decrease in the transepithelial electrical resistance (TER) of the monolayer following application of bile salt. As established in previous studies (16), the dose-response pattern for bile salt-induced barrier disruption was studied by incubating monolayers with various concentrations of bile salt and measuring the TER at one hour (Fig. 1).
Fig.1. Dose-response of the TER of C2 monolayers on PET inserts treated with different concentrations of the bile salt Sodium Deoxycholate (SDC) for one hour in serum starved media.

Concentrations ≥ 0.8 mM significantly degraded the TER. *p<0.05 versus 0 mM SDC.
Concentrations of bile salt of 0.8 mM or greater were sufficient to significantly decrease the TER of monolayers grown on PET inserts. Concentrations of 1.75 mM or greater were needed for monolayers grown on collagen-coated Transwell inserts (data not shown). As previously demonstrated, bile acids exist in the human intestine at concentrations up to 14 mM (29), with a more typical range from 1–3 mg/mL (~2–6mM) (30) therefore doses used in the present study fall within the physiologic range.
PEG 15–20 protection of C2 monolayers against bile salt-induced barrier disruption
C2 monolayers grown on PET inserts were then treated with an effective damaging dose of bile salt together with PEG 15–20 in order to examine the polymer’s protective effects against bile salt-induced barrier disruption. Because PEG 15–20 has been shown to retain its protective effects against radiation-induced damage even after washing of the apical medium (25), we wanted to test the hypothesis that PEG 15–20 anchors into the cell membrane and would protect even after changing of the apical medium. We tested the ability of the polymer to protect against epithelial disruption before, during and after exposure to the bile salt with the following specifications: 1) polymer pre-treatment followed by changing of the apical media at the disrupting dose of bile salt, 2) concurrent application of polymer and bile salt, and 3) post-treatment with polymer following exposure to bile salt (Fig. 2). Results indicate that both pre-treatment and concurrent application of 5% PEG 15–20 protected against SDC-induced barrier disruption. Post-exposure treatment with polymer after 30 minutes of exposure to bile salt provided no protection against TER degradation. A dose-response pattern of protection was observed at one hour past treatment (Fig. 3). Concentrations of PEG 15–20 of 0.9% or greater were needed to preserve barrier function at one hour, although all PEG 15–20 treatments below 1% did not protect well beyond one hour. The protective behavior of PEG 15–20 was found to be identical on collagen-coated Transwell and PET inserts (Supplemental Digital Content Figs. S1 and S2), and henceforth only PET inserts were used for experimentation.
Fig.2. Time course plot of TER protection of C2 monolayers by PEG 15–20.

Application of 0.8mM SDC significantly degraded the TER; pre-treatment for one hour followed by cell washing and application of SDC, or concurrent application of 5% PEG 15–20 with the SDC prevented a drop in TER; post-treatment with PEG 15–20 after 30 minutes of bile acid exposure did not rescue TER; PEG 15–20 alone did not degrade the TER. *p<0.05.
Fig.3. Semi-log plot of dose-response of PEG 15–20 protection against SDC induced barrier disruption at one hour.
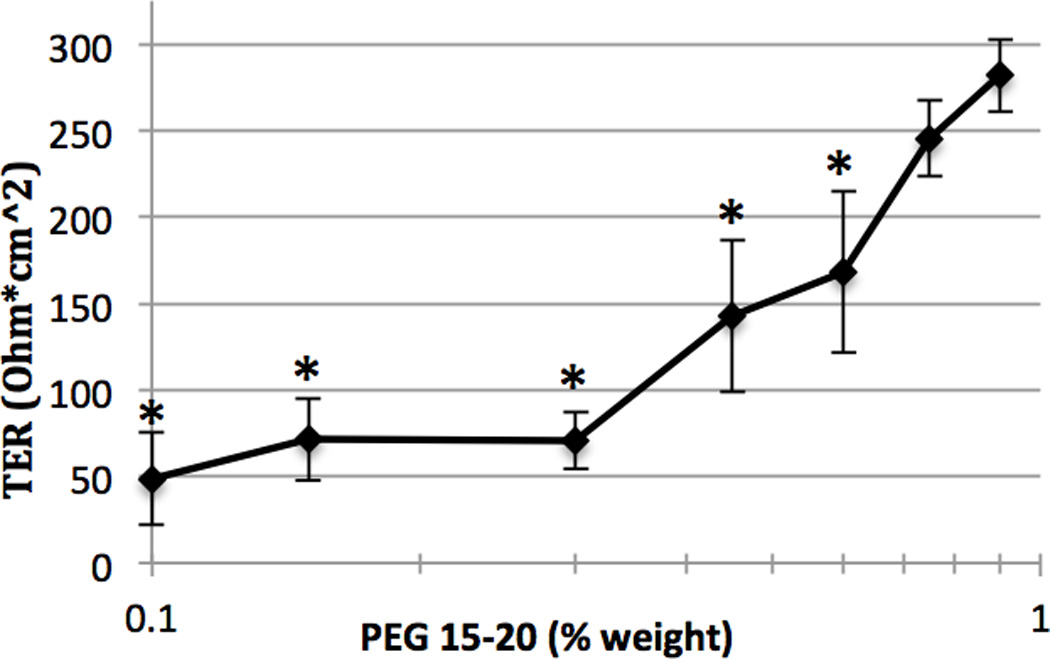
SDC (0.8 mM) was added at each dose response experiment. Significant TER degradation was seen at PEG 15–20 concentrations < 0.9%; there was no significant reduction in TER with PEG 15–20 concentrations ≥ 0.9%. *p<0.05 versus 1% PEG 15–20.
Previous studies have shown basally applied PEG 15–20 to protect C2 monolayers against apically applied reactive oxygen species such as monochloramine (Alverdy, unpublished). For this reason we tested whether basal PEG 15–20 would still protect against bile acid-induced barrier disruption if compartmentally separated from the apical bile salt (Fig. 4). Compartmental separation of the polymer from the bile salt abrogated the protective effects of the polymer.
Fig.4. Time course plot of loss of TER protection with compartmental separation of polymer.

Application of 0.8 mM SDC significantly degraded the TER in both the presence and absence of compartmentally separated 5% PEG 15–20. *p<0.05 versus corresponding control.
The necessity of the polymer to be compartmentally co-located with the bile acid implies that PEG 15–20 may confer protection by directly interacting with the SDC as opposed to affecting cell signaling or function. To address this possibility, we first performed ELISA–based cell death assays to measure bile acid induced apoptosis and necrosis in the presence and absence of 5% PEG 15–20 (Fig. 5A). Bile acids increased apoptosis 2.5 fold and necrosis 9 fold in the absence of polymer whereas PEG 15–20 maintained cell death measurements at their baseline level. Next we performed caspase 3 activity and LDH release assays to measure bile acid-induced apoptosis and necrosis, respectively, in the presence of different polymer treatment regimens (Fig. 5B,C). In these experiments, bile acids increased necrosis ~80 fold and apoptosis ~3 fold with respect to control conditions. The addition of polymer in both pre-treatment and co-treatment protocols significantly decreased necrosis, although levels were significantly increased relative to control conditions. The polymer markedly reduced apoptosis in both treatment regimens.
Fig.5. Results of cell death assays for SDC- induced damage and protection by PEG 15–20.
All experiments performed with 0.8 mM SDC and 5% PEG 15–20. A) ELISA- based cell death assays demonstrate that exposure to SDC increased both necrosis and apoptosis that was inhibited with PEG 15–20 co-treatment. B) LDH assay confirmed SDC– induced necrosis and a significant decrease in necrosis both with PEG 15–20 pre– treatment (i.e polymer exposure followed by cell washing) and with co-treatment. C) Caspase 3 assay demonstrates SDC-induced apoptosis, and significant reduction in Caspase 3 levels with PEG 15–20 pretreatment and co-treatment. *p<0.05.
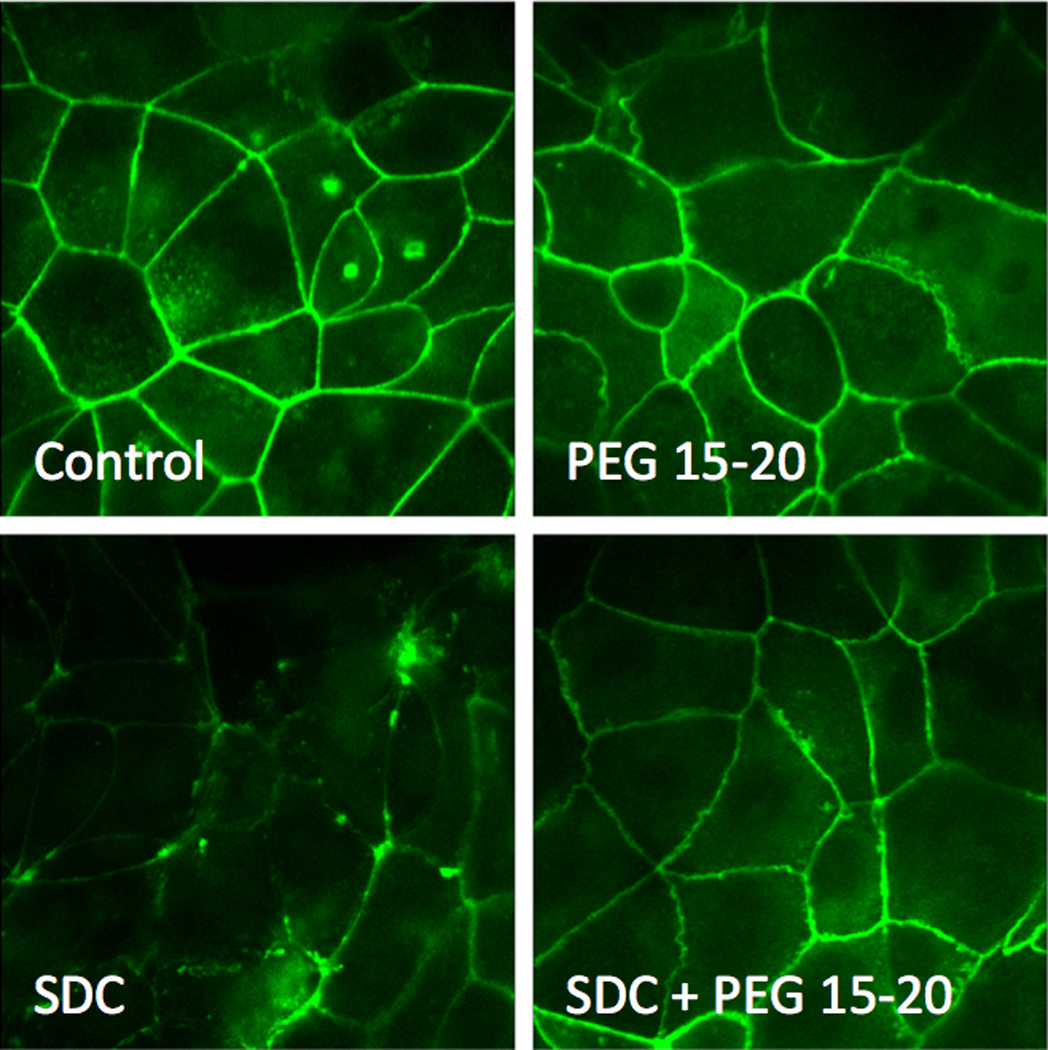
Finally we performed immunofluorescence microscopy to visualize changes in tight junctions exposed to bile acids in the presence and absence of PEG 15–20 (Fig. 6). Results demonstrated that incubation of C2 monolayers in 0.8 mM SDC significantly disrupted cytoskeletal architecture. Co-application of 5% PEG 15–20 maintained normal appearing cytoskeletal architecture following exposure to bile acids.
Fig.6. Tight junctions visualized with labeled ZO-1 antibodies.



Application of 0.8 mM SDC disrupts tight junctions; application of SDC and 5% PEG 15–20 shows no alteration in tight junctions. Application of 5% PEG 15–20 alone does not alter tight junctions.
Do structurally similar polymers protect against bile salt-induced epithelial barrier disruption?
In order to determine the structure- function relationship of PEG 15–20 to its cytoprotective effects, selected additional polymers were tested for their ability to prevent bile acid–induced loss of barrier function.
Based on preliminary experiments, we lowered the concentration of the polymers to 2% (w/w) to allow for characteristic differences in their protective abilities to be observed in TER assays (Table 2). P407 protected significantly better than PEG 15–20 and P338, which both protected significantly better than P188 and PEG-35 (p<0.05 for each polymer to polymer comparison). P181 was by itself harmful to the barrier function of the C2 monolayers, and conferred no protection against bile acid injury.
Table 2.
Characteristics and protective abilities of a small library of polymers
| Polymer | Core | Core Size (Da) |
Hydrophilic Arms Size (Da) |
Total Mol. Wt (Da) |
HLB | Schematic | TER (% of baseline) |
Standard Deviation of TER % |
|---|---|---|---|---|---|---|---|---|
| PEG 15–20 | BPA | 225 | 16000 | 16225 | 42.2 | 74* | 8 | |
| P181 | PPO | 1800 | 100 | 2000 | 3 | 5 | 0.3 | |
| P188 | PPO | 1800 | 7200 | 9000 | 29 | 33* | 6 | |
| P338 | PPO | 3300 | 13200 | 16500 | 27 | 57* | 8 | |
| P407 | PPO | 4000 | 9330 | 13330 | 22 | 98* | 15 | |
| PEG-35 | N/A | 0 | N/A | 35000 | N/A | 33* | 7 | |
| SDC alone | N/A | N/A | N/A | N/A | N/A | N/A | 7 | 0.7 |
Polymers were all tested at 2% w/w, and were applied concurrently with 0.8 mM SDC. All polymers with the exception of P181 provided significant protection against TER degradation (*p<0.05 vs SDC alone).
HLB= hydrophilic-lipophilic balance; a higher number indicates greater polymer hydrophilicity. While PEG 15–20 has a larger HLB, its hydrophobic core is, by mass, several orders of magnitude more hydrophobic than the PPO core of the other polymers. BPA = bisphenol A; PPO = polypropylene oxide; PEG = polyethylene glycol;  = PEG;
= PEG;  = BPA;
= BPA;  =PPO.
=PPO.
DISCUSSION
Nonionic block copolymers (NBCs) have potential as a means of maintaining GI barrier function during extreme physiologic stress and following injury. Structure–function relationships between polymer architecture and cytoprotection to the intestinal epithelium remain undetermined. PEG 15–20 is an especially promising NBC because it possesses a short, hydrophobic core block, allowing it to anchor into the epithelium in areas where mucus is depleted- a common finding following injury and inflammation (31–33). Most recently we determined that PEG 15–20 anchors to lipid raft domains on epithelial cells and may confer protection via lipid raft induced cell signaling (25). In the current study we initially observed mixed protective results with different cell passage numbers when the polymer was washed off the cells prior to bile acid exposure. The transformed cell line used in experimentation may be prone to down-regulation of cell surface components (i.e. lipid rafts) which we have previously demonstrated to be the binding sites for PEG 15–20. In support of this, we observed a marked reduction in bile acid-induced apoptosis, as compared to a less robust reduction in necrosis, which may point towards a lipid raft mediated protective mechanism in the case of PEG 15–20. We note a greater reduction in apoptosis with exposure to both polymer and SDC as compared with polymer alone. We speculate that this effect may stem from SDC-induced conformational changes in the polymer-lipid raft complexes that further down-regulate apoptosis.
However, precisely how PEG and related compounds protect the intestinal epithelial barrier remains to be determined. While PEG 15–20 specifically has been shown to protect the gut barrier against bacterial invasion, intestinal ischemia reperfusion injury, lethal radiation enteritis, and lethal sepsis more detailed structure function analyses are needed (20, 25, 34, 35). The present study, although incomplete, suggests that screening libraries of related compounds in clinically relevant in vitro models may be a first step approach in this process.
Many of the tri-block copolymer agents exist as polydisperse mixtures composed of polymers with a range of component block sizes clustered around the specified dimensions. Due to the nature of the synthetic process, these polymers are extremely difficult to obtain in the pure, monodisperse state. Structure-function studies to determine the precise biophysical mechanism of action of these various agents have been methodologically difficult. We studied various related polymers in our system to attempt to generate structure-functional correlates that might provide insight into how polymers interact with epithelial membranes. We have previously hypothesized that PEG 15–20 behaves as a synthetic mucin sterically preventing bacterial surface structures and, in the case of the present study, bile acids from reaching the epithelial cell membrane. Yet in the present study, it is possible that polymers also form a complex with the bile acids in solution and reduce their emulsifying action, thereby preventing epithelial barrier disruption. If polymers form a synthetic mucus layer, then the hydrophilic arms that compose over 98% of PEG 15–20 should make up the bulk of the mucous layer. In this case we would expect that PEG-35, a 35,000 kD PEG polymer that lacks a hydrophobic core, would behave in the same manner and be protective. PEG-35, however, was markedly less protective than other amphiphilic polymers. We considered the possibility that polymer amphiphilicity was needed only to attract the polymer to the epithelium and form the synthetic mucous layer, but if that were the case then all amphiphilic polymers should have been equally protective. P188 and P181, however, did not protect well. It is likely that P181, due to its hydrophobicity, degraded the TER by inserting into and destabilizing the cell membrane.
These data, together with the fact that the large amphiphilic polymers P338 and P407 also protected well, indicate that criteria for protection include a molecular weight greater than ~10 kD and amphiphilicity. The requirement of amphiphilicity points towards the formation of polymer-bile acid complexes as the mechanism of protection. The size requirement, and the difference in protection between the very similar P338 and P407, indicate that the formation of the complexes is sensitive to the polymer architecture. Although the structures of P338 and P407 are very similar, even seemingly subtle differences in architecture can greatly influence polymer behavior in the aggregate. The structural details of the block copolymer –bile salt complexes are currently under investigation and will be reported in a future publication.
Future experiments will need to be completed to investigate the protective effects of NBCs against bile acids on other cell lines in order to determine if and how polymer protection depends on cellular membrane composition.
ACKNOWLEGEMENTS
The authors would like to thank Kenneth Drabik, MS for graciously providing cell culture expertise. This research was supported by the University of Chicago Pritzker School of Medicine’s Summer Research Program via NIDDK grant DK062719-22 and NIH grant 5R01GM062344–11 (JCA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DIGITAL CONTENT
1. Figure S1: Dose-response of the TER at one hour of C2 monolayers on collagen-coated inserts treated with SDC.
2. Figure S2: Time course plot of TER protection of C2 monolayers by PEG 15–20 on collagen-coated inserts.
REFERENCES
- 1.Clark JA, Coopersmith CM. Intestinal crosstalk: A new paradigm for understanding the gut as the "motor" of critical illness. Shock. 2007;28(4):384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mekalanos JJ. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174(1):1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dial EJ, Romero JJ, Villa X, Mercer DW, Lichtenberger LM. Lipopolysaccharide-induced gastrointestinal injury in rats: Role of surface hydrophobicity and bile salts. Shock. 2002;17(1):77–80. doi: 10.1097/00024382-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Pfeiffer CJ, Qiu B, Lam SK. Reduction of colonic mucus by repeated short-term stress enhances experimental colitis in rats. J Physiol Paris. 2001;95(1–6):81–87. doi: 10.1016/s0928-4257(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 5.Caputo FJ, Rupani B, Watkins AC, Barlos D, Vega D, Senthil M, et al. Pancreatic duct ligation abrogates the trauma hemorrhage-induced gut barrier failure and the subsequent production of biologically active intestinal lymph. Shock. 2007;28(4):441–446. doi: 10.1097/shk.0b013e31804858f2. [DOI] [PubMed] [Google Scholar]
- 6.Araki Y, Katoh T, Ogawa A, Bamba S, Andoh A, Koyama S, et al. Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the caco-2 cell line. Free Radic Biol Med. 2005;39(6):769–780. doi: 10.1016/j.freeradbiomed.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Jean-Louis S, Akare S, Ali MA, Mash EA, Jr, Meuillet E, Martinez JD. Deoxycholic acid induces intracellular signaling through membrane perturbations. J Biol Chem. 2006;281(21):14948–14960. doi: 10.1074/jbc.M506710200. [DOI] [PubMed] [Google Scholar]
- 8.Duane WC, Wiegand DM. Mechanism by which bile salt disrupts the gastric mucosal barrier in the dog. J Clin Invest. 1980;66(5):1044–1049. doi: 10.1172/JCI109932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuman DM, Bajaj RS, Lin Q. Adsorption of mixtures of bile salt taurine conjugates to lecithin-cholesterol membranes: Implications for bile salt toxicity and cytoprotection. J Lipid Res. 1996;37(3):562–573. [PubMed] [Google Scholar]
- 10.O'Connor CJ, Wallace RG, Iwamoto K, Taguchi T, Sunamoto J. Bile salt damage of egg phosphatidylcholine liposomes. Biochim Biophys Acta. 1985;817(1):95–102. doi: 10.1016/0005-2736(85)90072-0. [DOI] [PubMed] [Google Scholar]
- 11.Hughes R, Kurth MJ, McGilligan V, McGlynn H, Rowland I. Effect of colonic bacterial metabolites on caco-2 cell paracellular permeability in vitro. Nutr Cancer. 2008;60(2):259–266. doi: 10.1080/01635580701649644. [DOI] [PubMed] [Google Scholar]
- 12.Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, et al. Bile acids modulate tight junction structure and barrier function of caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol. 2008;294(4):G906–G913. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 13.Balint JA, Kyriakides EC, Spitzker HL, Morrison ES. Lecithin fatty acid composition in bile and plasma of man, dogs, rats, and oxen. J Lipid Res. 1965;6:96–99. [PubMed] [Google Scholar]
- 14.Coleman R, Iqbal S, Godfrey PP, Billington D. Membranes and bile formation. composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin GP, Marriott C. Membrane damage by bile salts: The protective function of phospholipids. J Pharm Pharmacol. 1981;33(12):754–759. doi: 10.1111/j.2042-7158.1981.tb13926.x. [DOI] [PubMed] [Google Scholar]
- 16.Dial EJ, Rooijakkers SH, Darling RL, Romero JJ, Lichtenberger LM. Role of phosphatidylcholine saturation in preventing bile salt toxicity to gastrointestinal epithelia and membranes. J Gastroenterol Hepatol. 2008;23(3):430–436. doi: 10.1111/j.1440-1746.2007.05153.x. [DOI] [PubMed] [Google Scholar]
- 17.Hills BA, Butler BD, Lichtenberger LM. Gastric mucosal barrier: Hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983;244(5):G561–G568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- 18.Kao YC, Lichtenberger LM. Localization of phospholipid-rich zones in rat gastric mucosa: Possible origin of a protective hydrophobic luminal lining. J Histochem Cytochem. 1987;35(11):1285–1298. doi: 10.1177/35.11.2443559. [DOI] [PubMed] [Google Scholar]
- 19.Zayat M, Lichtenberger LM, Dial EJ. Pathophysiology of LPS-induced gastrointestinal injury in the rat: Role of secretory phospholipase A2. Shock. 2008;30(2):206–211. doi: 10.1097/shk.0b013e318160f47f. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Zaborina O, Zaborin A, Chang EB, Musch M, Holbrook C, et al. High-molecular-weight polyethylene glycol prevents lethal sepsis due to intestinal pseudomonas aeruginosa. Gastroenterology. 2004;126(2):488–498. doi: 10.1053/j.gastro.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, caco-2. J Cell Sci. 1992;102(Pt 3):581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- 22.Niv Y, Byrd JC, Ho SB, Dahiya R, Kim YS. Mucin synthesis and secretion in relation to spontaneous differentiation of colon cancer cells in vitro. Int J Cancer. 1992;50(1):147–152. doi: 10.1002/ijc.2910500129. [DOI] [PubMed] [Google Scholar]
- 23.Dial EJ, Romero JJ, Villa X, Mercer DW, Lichtenberger LM. Lipopolysaccharide-induced gastrointestinal injury in rats: role of surface hydrophobicity and bile salts. Shock. 2002;17(1):77–80. doi: 10.1097/00024382-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer CJ, Qiu B, Lam SK. Reduction of colonic mucus by repeated short-term stress enhances experimental colitis in rats. J Physiol Paris. 2001;95(1–6):81–87. doi: 10.1016/s0928-4257(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 25.Valuckaite V, Zaborina O, Long J, Hauer-Jensen M, Wang J, Holbrook C, et al. Oral PEG 15–20 protects the intestine against radiation: Role of lipid rafts. Am J Physiol Gastrointest Liver Physiol. 2009;297(6):G1041–G1052. doi: 10.1152/ajpgi.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103(3):755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126(4):741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Rossi SS, Converse JL, Hofmann AF. High pressure liquid chromatographic analysis of conjugated bile acids in human bile: Simultaneous resolution of sulfated and unsulfated lithocholyl amidates and the common conjugated bile acids. J Lipid Res. 1987;28(5):589–595. [PubMed] [Google Scholar]
- 29.Coleman R, Iqbal S, Godfrey PP, Billington D. Membranes and bile formation. composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgstrom B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957;36(10):1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B, Firestone MA. Electron density mapping of triblock copolymers associated with model biomembranes: Insights into conformational states and effect on bilayer structure. Biomacromolecules. 2008;9(6):1541–1550. doi: 10.1021/bm701348r. [DOI] [PubMed] [Google Scholar]
- 32.Gigout A, Buschmann MD, Jolicoeur M. The fate of pluronic F-68 in chondrocytes and CHO cells. Biotechnol Bioeng. 2008;100(5):975–987. doi: 10.1002/bit.21840. [DOI] [PubMed] [Google Scholar]
- 33.Melik-Nubarov NS, Pomaz OO, Dorodnych TY, Badun GA, Ksenofontov AL, Schemchukova OB, et al. Interaction of tumor and normal blood cells with ethylene oxide and propylene oxide block copolymers. FEBS Lett. 1999;446(1):194–198. doi: 10.1016/s0014-5793(99)00208-2. [DOI] [PubMed] [Google Scholar]
- 34.Henry-Stanley MJ, Wells CL. Polyethylene glycol influences microbial interactions with intestinal epithelium. Shock. 2009;31(4):390–396. doi: 10.1097/SHK.0b013e31818348a5. [DOI] [PubMed] [Google Scholar]
- 35.Sheth S, Lu Q, Qin X, Xu D, Reino D, Mason L, et al. Intraluminal administration of high molecular weight polyethylene glycol attenuates trauma-hemorrhagic shock-induced gut injury. Shock. 2010;33(7):44. [Google Scholar]


