Abstract
Purpose
To examine five- and ten-year survival based on cancer-specific geriatric assessment (C-SGA) in older women with early stage breast cancer.
Methods
We evaluated 660 women ≥65-years old diagnosed with stage I-IIIA primary breast cancer and attending physician permission to contact in four geographic regions in the U.S.A. Data were collected over ten-years of follow-up from consenting women’s medical records, telephone interviews, National Death Index, and Social Security Death Index. C-SGA was described by four domains using six measures: socio-demographic (financial resources); clinical (co morbidity, obesity); function (physical function limitations); and psychosocial (general mental health, social support). Survival from all-cause and breast-cancer-specific mortality and receipt of guideline-recommended therapy was assessed for different groups of subjects with C-SGA domain deficits (cut-off ≥3 deficits).
Results
The proportion of women with ≥3 C-SGA deficits surviving ten-years was consistently statistically significantly lower (all-cause 26% versus 46% and breast-cancer-specific 76% versus 89%, p≤0.04). The proportion significantly decreased as number of C-SGA deficits increased (linear trend p<0.0001). Receipt of guideline-recommended therapy decreased with age but not consistently by number of C-SGA deficits. The all-cause and breast-cancer-specific death rate at five- and ten-years was consistently approximately two times higher in women with ≥3 C-SGA deficits even when fully adjusted for confounding factors (HR5-yrAllCauseFullyAdjusted=1.87[1.36–2.57], HR10-yrAllCauseFullyAdjusted=1.74[1.35–2.15], HR5-yrBreastCancerFullyAdjusted=1.95[1.18–3.20], HR10-yrBreastCancerFullyAdjusted=1.99[1.21–3.28]).
Conclusion
Regardless of age and stage of disease C-SGA predicts five- and ten-year all-cause and breast-cancer-specific survival in older women. Hence, C-SGA may provide an effective strategy to guide treatment decision-making and to identify risk factors for intervention.
Keywords: Assessment, breast cancer, cancer-specific geriatric assessment, decision-making, guideline-recommended therapy, geriatric assessment, older women, survival, survivor
INTRODUCTION
In 2009 there were an estimated 192,370 new cases of invasive breast cancer in the United States (US) with 50% of cases diagnosed in women 61-years or older.(1) Due to the high relative survival rates in US women (89% five-years after diagnosis, 82% after ten-years) the vast majority of older women with breast cancer will become long-term survivors.(1–3) Thus, weighing the impact of treatment in relation to treatment tolerance, morbidity, quality of life, and survival should play a central role in the care of older cancer patients.
However, older cancer patients are an extremely heterogeneous group, making management of their cancer care complex and challenging.(4–6) Applying geriatric principles to oncology care attempts to weigh the impact of known care-influencing factors such as co morbidity (i.e., vulnerability to adverse treatment effects) or social support (i.e., ability to get to treatments), in relation to care, quality of life, and overall survival.(7–11) There is a growing evidence that these factors can be effectively assessed by cancer-specific geriatric assessment (C-SGA) and predict treatment tolerance, morbidity, and mortality in older cancer patients.(12–17) Yet little is known about the accuracy of estimating survival based on C-SGA.
Taking advantage of our longitudinal study of older women with breast cancer we conducted a secondary analysis of survival using C-SGA. The current study extends previous analyses through ten-years of follow-up(12) and focuses on the predictive value of C-SGA in relation to age- and cause-specific survival after a breast cancer diagnosis. Our a priori hypothesis was that C-SGA would better predict all-cause and breast-cancer-specific survival than age, particularly that women with C-SGA ≤2 domain deficits would have better survival.
PATIENTS AND METHODS
Study Population
The longitudinal study design and subject recruitment procedures have been previously reported.(18) Six hundred sixty women ≥65-years old with stage I tumor diameter ≥1cm or stage II-IIIA disease and permission from attending physician to be contacted in four geographic regions (Los Angeles, California; Minnesota; North Carolina; Rhode Island) were identified through regular pathology report review at hospitals or collaborating tumor registries. Women could not have a prior primary breast cancer or simultaneously diagnosed or treated second primary tumor, and must have signed a consent form approved by the institutional review board at each site.
Analytic Variables
Data was collected by medical record review (definitive surgery date, surgery type, tumor characteristics) and baseline telephone interview (socio-demographic, psychosocial, health, breast cancer therapy) at least three-months after surgery.
Mortality all-cause and breast-cancer-specific
Decedents were identified by first and last name, middle initial, social security number, date of birth (DOB), sex, race, marital status, and state of residence matched against National Death Index (NDI) and Social Security Death Index (SSDI) records. Survival time was number of days from date of definitive surgery until date of death (DOD). Breast-cancer-specific follow-up time was censored on DOD from another cause or at end of follow-up, whichever came first.
Socio-demographic characteristics
We classified patient age as 65–69, 70–79, ≥80-years; race as white, other; education as <12-years, 12-years, >12-years; marital status as married (yes/no); and having adequate finances to meet needs (yes/no).
Breast cancer characteristics
We categorized stage as I-III using TNM classification.(19) Definitive primary therapy was mastectomy plus maxillary lymph node dissection (ALND) or lumpectomy with radiation therapy plus ALND. Guideline-recommended therapy (yes/no) based on modifications of 1990 and 2000 NIH breast cancer treatment guidelines(20, 21) was defined as receipt of one of the following:(22) (1) definitive primary therapy; (2) tamoxifen therapy in hormone receptor positive breast cancer; (3) chemotherapy in node positive disease; and (4) chemotherapy in breast cancers with tumor size ≥1cm and receptor negative status. Under-treatment was defined as no guideline-recommended therapy with breast-cancer-specific death.
Health-related characteristics
We determined underlying diseases present at diagnosis using the Charlson Co morbidity Index (CCI) scaled from 0–3 with higher scores indicating more co morbidity.(23–25) Self-rated health status before diagnosis was assessed using a single-item measure dichotomized as “excellent/very good/good” versus “fair/poor”. Body mass index (BMI) was derived as 30kg/m2 versus obesity >30kg/m2. We calculated total number of limiting physical functions based on the ten-item Physical Function Index of the Medical Outcomes Study Short Form (MOS-SF-36) and categorized as 0 or ≥1 limitation.(26) General mental health was assessed by the Mental Health Index (MHI5), a five-item measure of mental health from the MOS-SF-36 scored on 0–100 scale. Higher scores indicate better mental health and a score of ≥80 considered good general mental health.(26) Social support was measured using a reduced set of eight-items derived from the 19-item Medical Outcomes Study Social Support Scale (MOS-SSS) scored from 0–100 with higher scores indicating more support and ≥80 considered good social support.(27)
Cancer-specific geriatric assessment
C-SGA was described by four domains using six individual measures: (1) socio-demographic by adequate financial resources; (2) clinical using CCI and BMI; (3) function by number of physical function limitations; and (4) psychosocial as MHI5 and MOS-SSS. Criteria for determining domain deficits were: inadequate finances; CCI≥2 and/or obesity; number of physical limitations ≥1; and MHI5 and/or MOS-SSS<80. Deficits in C-SGA domains were summed (maximum sum of 4) then dichotomized as ≤2 versus ≥3.(12)
Analytic Strategy
We first examined descriptive statistics on all study variables then evaluated bivariate distributions between independent and mortality outcome variables using Spearman correlations, chi-square test, log-rank test, and Cochran-Armitage test-of-trend. Five- and ten-year survival was analyzed using Kaplan-Meier survivor functions. Unadjusted and multivariable adjusted Cox proportional hazards regression models were fitted to predict five- and ten-year all-cause and breast-cancer-specific mortality with baseline C-SGA; reported as hazard ratio (HR) and 95% confidence interval (95%CI). Model selection was based on either a theoretical basis for, or knowledge of a relation between causal variables and effects or statistical testing (i.e., elimination of highly correlated variables, inclusion of a necessary minimum set of statistically meaningful variables, and overall model fit).(28) Participants with missing data for independent or outcome variables were excluded from models (N<19). Multivariable adjusted models were validated using stepwise and backward regression analyses. All analyses were performed using SAS version 9.2.
RESULTS
Characteristics of the Study Population
Socio-demographic, breast cancer, and health-related characteristics of the baseline study population (N=660) are shown in Table 1. The majority of women were ≥70-years, white, and had at least 12-years education. Approximately half had stage I disease and 46% received guideline-recommended therapy. Fifty-eight percent had a CCI of 0, 85% self-reported good health, and 21% were obese. More than half of the women exhibited high levels of general mental health and physical function.
Table 1.
Baseline socio-demographic and health-related characteristics in a population of older women with breast cancer (N=660), 1997–2007
| Characteristic at Baseline | Population at Baseline N (%) |
|---|---|
| Socio-demographic | |
| Enrollment site | |
| LA | 150 (23) |
| RI | 163 (25) |
| MN | 188 (28) |
| NC | 159 (24) |
| Age | |
| 65–69 years | 172 (26) |
| 70–79 years | 372 (56) |
| 80+ years | 116 (18) |
| Race | |
| White | 620 (94) |
| Other | 40 (6.1) |
| Education | |
| Less than 12 years | 115 (17) |
| 12 years | 228 (35) |
| More than 12 years | 316 (48) |
| Married | 304 (46) |
| Adequate finances | 587 (90) |
| Breast Cancer | |
| Stage | |
| I | 336 (51) |
| II | 298 (45) |
| III | 25 (3.8) |
| Guideline-recommended therapy | 305 (46) |
| Health-related | |
| CCI | |
| 0 | 380 (58) |
| 1 | 232 (35) |
| 2 | 48 (7.3) |
| Good self-rated health | 564 (85) |
| Obesity (BMI >30) | 140 (21) |
| Number of physical limitations ≥1 | 247 (37) |
| Good mental health (MHI5 ≥80) | 455 (69) |
| High-level of social support (MOS-SSS ≥80) | 333 (51) |
| Cancer-specific geriatric assessment | |
| Deficits in ≤2 C-SGA domains | 514 (78) |
LA, Los Angeles, California; RI, Rhode Island; MN, Minnesota; NC, North Carolina; CCI, Charlson Co morbidity Index; BMI, Body Mass Index; MHI5, 5-item Mental Health Index; MOS-SSS, Medical Outcomes Study-Social Support Survey; C-SGA, Cancer –Specific Geriatric Assessment.
C-SGA
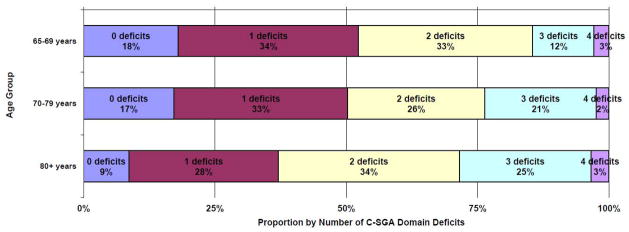
At baseline 15.9% of women had no C-SGA deficit, 32.6% had one, 29.4% two, 19.4% three, and 2.7% four. The proportion of women within C-SGA deficit categories (0–4) by age group is shown in Figure 1. Age and C-SGA deficit count were modestly but statistically significantly correlated (r=0.12, p=0.003) and the proportion of women with ≥3 C-SGA domain deficits increased for each incremental age group (65–69-years 14.5%, 70–79-years 23.7%, 80+-years 28.5%, test-of-trend p=0.01). This relationship was not observed between C-SGA and stage (r=0.03, p=0.59; Stage I 21.7%, Stage II 22.4%, Stage II 24%, test-of-trend p=0.75
Figure 1.
Proportion of women with zero to four cancer-specific geriatric assessment (C-SGA) domain deficits at baseline by age group in a longitudinal study of older women with breast cancer (N=660), 1997-2007
Age and Survival
As expected, the proportion of women surviving five- and ten-years of follow-up decreased with increasing age. The mean age of each group by number of C-SGA deficits (0–4) was similar (age range 72.6–75.6-years, p=0.12), with the highest mean age among those with three C-SGA deficits. There was a decreased probability of survival over follow-up by increasing age group (all-cause/breast-cancer-specific 65–69, 70–79, 80+-years, respectively: five-years: 79%, 76%, 52%/91%, 88%, 77%; ten-years: 55%, 43%, 15%/90%, 87%, 73%. At five-years of follow-up the mean survival time ±standard error in years by age group was: 65–69-years 4.4±0.092, 70–79-years 4.3±0.063, and 80+-years 3.7±0.13, whereas at ten-years it was: 65–69-years 7.5±0.22, 70–79-years 7.3±0.16, and 80+-years 5.3±0.27.
C-SGA and Survival
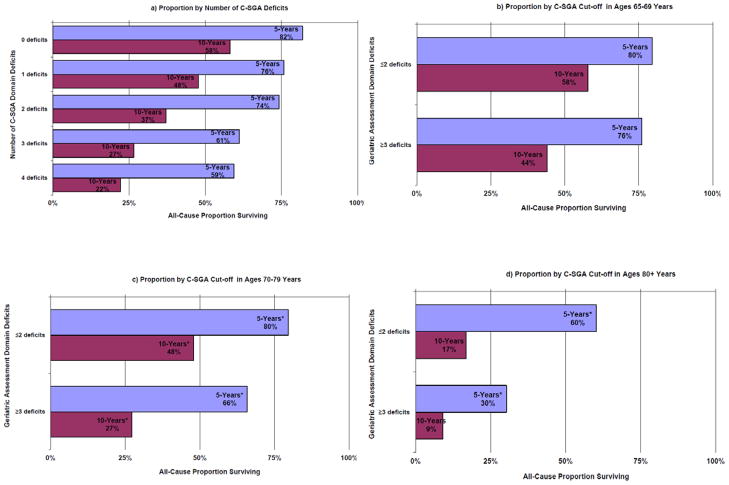
The mean study all-cause and breast-cancer-specific survival time in years for women with ≤2 C-SGA deficits was 4.4±0.053 and 4.8±0.02 at five-years and 7.4±0.13 and 8.7±0.08 at ten-years of follow-up, respectively. Women with ≥3 deficits in C-SGA domains had lower mean all-cause and breast-cancer-specific survival time at both five- and ten-years. As shown in Figure 2, the proportion of survivors significantly decreased as the number of deficits in baseline C-SGA domains increased; while the proportion of women surviving with ≥3 C-SGA domain deficits was consistently lower across age groups and time points.
Figure 2.
Proportion of the total population (N=660) surviving all-cause at five- and ten-years follow-up by number of cancer-specific geriatric assessment (C-SGA) deficits and age (65-69-years, 70-79-years, 80+-years) in a longitudinal study of older women with breast cancer, 1997-2007
a) Proportion by Number of C-SGA Deficits
b) Proportion by C-SGA Cut-off in Ages 65-69 Years
c) Proportion by C-SGA Cut-off in Ages 70-79 Years
d) Proportion by C-SGA Cut-off in Ages 80+ Years
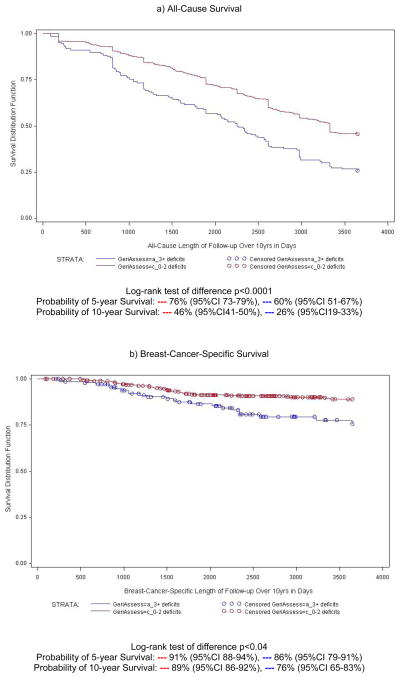
Figure 3 illustrates all-cause and breast-cancer-specific survival curves for C-SGA groups ≤2 and ≥3 C-SGA deficits) over ten-years of follow-up. The probability of ten-year survival was consistently statistically significantly lower in women with ≥3 C-SGA deficits. Across age groups the difference in probability of survival at five- and ten-years favored women with ≤2 C-SGA deficits (range 0.3–2.1-years). As expected age was a stronger predictor of all-cause mortality and stage was a very strong predictor of breast-cancer-specific death (Models 2&3 Table 2). The all-cause and breast-cancer-specific death rates at both five- and ten-years were consistently approximately two-times higher in women with ≥3 C-SGA deficits even when fully adjusted for confounding factors.
Figure 3.
Survival plot of five- and ten-year all-cause and breast-cancer-specific survival based on cancer-specific geriatric assessment (C-SGA) in a longitudinal study of older women with breast cancer (N=660), 1997-2007
Table 2.
Five- and ten-year hazard ratios for all-cause and breast-cancer-specific mortality based on cancer-specific geriatric assessment (C-SGA) in a longitudinal study of older women with breast cancer (N=660), 1997–2007
| All-Cause Mortality | Breast-Cancer-Specific Mortality | ||||
|---|---|---|---|---|---|
| 5-years HR (95% CI) |
10-years HR (95% CI) |
5-years HR (95% CI) |
10-years HR (95% CI) |
||
| Model 1: | Deficits in ≥3 C-SGA domains | 1.94 (1.42 –2.65) | 1.81 (1.45 –2.26) | 2.12 (1.30–3.45) | 2.19 (1.34–3.57) |
| Model 2: | Age | ||||
| 65–69 years | 1.0 | 1.0 | 1.0 | 1.0 | |
| 70–79 years | 1.13 (0.77–1.67) | 1.39 (1.07–1.81) | 1.37 (0.74–2.53) | 1.44 (0.78–2.66) | |
| 80+ years | 2.62 (1.71–3.99) | 2.91(2.15 –3.93) | 2.15 (1.07–4.34) | 2.41 (1.19–4.88) | |
| Stage | |||||
| I | 1.0 | 1.0 | 1.0 | 1.0 | |
| II | 1.24 (0.92–1.67) | 1.07 (0.87–1.32) | 3.84 (2.15–6.86) | 3.8 (2.13–6.79) | |
| III | 1.26 (0.61–2.61) | 1.73 (1.11 –2.73) | 5.40 (2.09–13.99) | 5.87 (2.27–15.22) | |
| Deficits in ≥3 C-SGA domains | 1.95 (1.42–2.67) | 1.76 (1.40–2.20) | 2.08 (1.27–3.39) | 2.13 (1.30–3.49) | |
| Model 3: | Age | ||||
| 65–69 years | 1.0 | 1.0 | 1.0 | 1.0 | |
| 70–79 years | 1.11 (0.75–1.64) | 1.37 (1.05–1.78) | 1.32 (0.71–2.44) | 1.38 90.75-2.55) | |
| 80+ years | 2.37 (1.53–3.69) | 2.60 (1.90–3.55) | 1.74 (0.84–3.60) | 1.94 (0.94–4.01) | |
| Stage | |||||
| I | 1.0 | 1.0 | 1.0 | 1.0 | |
| II | 1.21 (0.89–1.64) | 1.05 (0.85–1.29) | 3.76 (2.10–6.74) | 3.71 (2.07–6.65) | |
| III | 1.26 (0.12–2.60) | 1.74 (1.10–2.74) | 5.51 (2.12–14.29) | 6.01 (2.31–15.58) | |
| Education | |||||
| Less than 12 years | 1.0 | 1.0 | 1.0 | 1.0 | |
| 12 years | 0.81 (0.54–1.22) | 0.78 (0.59–1.04) | 0.75 (0.39–1.46) | 0.72 (0.37–1.40) | |
| More than 12 years | 0.85 (0.58–1.24) | 0.79 (0.61–1.03) | 1.01 (0.55–1.84) | 0.96 (0.53–1.75) | |
| Married | 0.82 (0.59–1.03) | 0.81 (0.65–1.00) | 0.57 (0.33–0.97) | 0.56 (0.33–0.95) | |
| Deficits in ≥3 C-SGA domains | 1.87 (1.36–2.57) | 1.74 (1.35–2.12) | 1.95 (1.18–3.20) | 1.99 (1.21–3.28) | |
HR, Hazard Ratio; CI, Confidence Interval; C-SGA, Cancer-Specific Geriatric Assessment
Model 1 includes only C-SGA measure, Model 2 (age and stage adjusted) includes age, stage and C-SGA measure as individual variables, and Model 3 (fully adjusted) includes age, stage, education, martial status, and C-SGA measure as individual variables
Guideline-Recommended Therapy
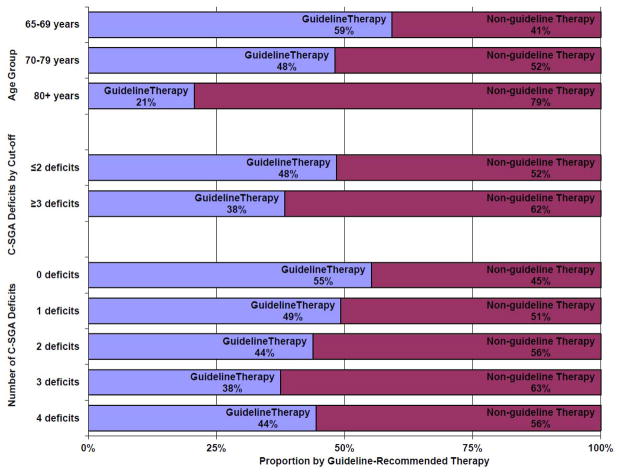
Receipt of guideline-recommended therapy decreased with age but not consistently with number of C-SGA deficits (Figure 4). If treatment had been assigned by low C-SGA (≤2 deficits), over 65% of under-treated women who died of breast cancer (N=48) would have received guideline-recommended therapy. On the other hand, if treatment had also been assigned by a high C-SGA (≥3 deficits), 42% of guideline-treated women who died of causes other than breast cancer within three-years might have received less intensive treatment.
Figure 4.
Proportion of total population (N=660) with and without guideline-recommended therapy by age group, C-SGA cut-off, and number of C-SGA deficits in a longitudinal study of older women with breast cancer, 1997-2007
DISCUSSION
In this study of older women with breast cancer, C-SGA was a predictor of both five- and ten-year all-cause and breast-cancer-specific survival. As hypothesized, women at or above the ≥3 C-SGA domain deficit cut-off had markedly worse survival than women below. The difference in survival based on C-SGA did not appear to be due to age or stage. The mean age by C-SGA deficit category did not notably differ. Moreover neither the proportion of C-SGA deficit categories between age groups nor the HR stratified by or adjusted for age and stage varied appreciably. The results also suggest that treatment decision-making is heavily weighted by age and decision-making informed by C-SGA might reduce under- or over-treatment with guideline-recommended therapy in older women with breast cancer patients.
These findings extend and complement our previous work as well as other C-SGA studies affirming the potential of C-SGA use in older cancer patients.(12, 14–16, 29) Importantly, C-SGA not only predicted cancer-related outcomes, but it identified opportunities for intervention that could improve treatment tolerance, morbidity, and survival (both all-cause and breast-cancer-specific) as newly suggested in this study. Even though advancing age also predicted five- and ten-year survival (all-cause more strongly than breast-cancer-specific), it is an un-modifiable risk factor and poor predictor of treatment outcomes.(6) When implemented, C-SGA can provide a unique patient-specific proactive component to the care of older cancer patients.(30, 31) It overcomes the problem that age or simple performance measures insufficiently capture the complexity of older cancer patients’ issues.(10) It provides a more comprehensive measure of risk and can influence the prognostic ability of the physician.(32, 33) As this and other research shows, C-SGA can aid in optimizing treatment because it encompasses multiple domains which help to identify and manage issues which can interfere with cancer treatment and impact survival.
Age is a known risk factor for breast cancer under-treatment.(34, 35) Bickell et al showed that breast cancer under-treatment is multi-factorial with one third attributable solely to physicians’ perceptions that treatment was not indicated.(36, 37) In this study, under-treatment was related to age and breast-cancer-specific death; possibly indicating that physicians were relying too heavily on the wrong information (e.g. age) for cancer treatment decision-making and that C-SGA may represent an opportunity to reduce breast-cancer-specific deaths. Correspondingly, there was suggestion of potential over-treatment that may have been avoided with C-SGA based decision-making (decrease burden of therapy without benefit). This underscores the accelerated need for use of evidence-based decision-making tools like C-SGA and highlights the potential serious consequences of under-treatment on the survivorship experience of older women with breast cancer.
The C-SGA predictive value was similar for all-cause and breast-cancer-specific-mortality which may be related to good overall health, the high proportion of women not receiving guideline-recommended therapy, the pronounced relation between GA domains and all-cause mortality, or even un-measurable residual confounding. It also suggests the domains and corresponding GA measures used herein might combine into a useful easy to implement (i.e. low physician/patient burden) GA instrument in more general settings as well.(4, 38) At present there is no consensus on which domains and/or tools should be standardized for GA use in general practice or geriatric-oncology. We do not propose those used in this study are the optimal solution.
Furthermore, the feasibility of implementing C-SGA in everyday clinical practice, unaddressed by this research, is of practical concern. Administering C-SGA as part of routine clinical practice to all older cancer patients can be a time and resource intensive procedure. It may not be reimbursed by health insurance, oncologists and/or their staff are not often trained in GA, and there may be limited collaboration with and/or availability of geriatric specialists over the course of cancer care. These obstacles likely contribute to the slow translation of C-SGA evidence into every day practice. Nevertheless C-SGA may be a useful guide to estimate survival as recommended for medical decision-making. Ongoing C-SGA research is addressing these important questions producing a sufficient knowledge base for more rapid translation of C-SGA evidence. A compilation of such evidence is vital to the treatment and survivorship experience of the growing numbers of older cancer patients.(39)
Several limitations and strengths of this study should be considered. The measures in this study were not administered as part of C-SGA for patient care purposes and were collected by telephone interview. As such the findings cannot be directly compared with other C-SGA studies using different data collection strategies. Although there was no reporting of deficits identified by C-SGA back to physicians, so their assessment could not have directly influenced patient care or outcomes. Since only early stage disease was included it is possible that women may have been prejudged as fit for surgical treatment. Additionally, tumor biology information was unavailable. These findings may not be generalizable because of study eligibility criteria and since the population was a largely white, well-educated, and healthy group of older women. Lastly, given small numbers we had limited power to statistically meaningfully evaluate results stratified by age and C-SGA domain deficit counts. The strengths of this research include comprehensive standardized C-SGA information, unusually long follow-up of older women with breast cancer, evaluation of all-cause and breast-cancer-specific deaths, and use of NDI and SSDI for mortality outcomes maximizing accuracy and minimizing loss to follow-up.
In conclusion, this study provides some of the first longitudinal evidence that C-SGA can predict five- and ten-year all-cause and breast-cancer-specific survival in older women with breast cancer. Hence, C-SGA may provide an effective strategy to guide treatment decision-making and to identifying important risk factors for intervention. Further studies of C-SGA, survival, and decision-making in various populations of older adults with cancer are warranted.
Acknowledgments
This work was supported by grants CA106979, CA/AG 70818, and CA84506 from the National Cancer Institute. This manuscript contains original material that was in part presented previously as a poster at the 10th Annual International Society of Geriatric Oncology (SIOG) Meeting in Berlin, Germany.
ROLE OF THE FUNDING SOURCE: The sponsors had no role in the design, methods, subject recruitment, data collection, analysis, or paper preparation.
Footnotes
CONFLICT OF INTEREST STATEMENT: None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breast Cancer Facts & Figures. Atlanta, GA: 2009–2010. [Google Scholar]
- 2.Lash TL, Silliman RA. Re: prevalence of cancer. J Natl Cancer Inst. 1998;90(5):399–400. doi: 10.1093/jnci/90.5.399. [DOI] [PubMed] [Google Scholar]
- 3.Reis L, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 4.Hurria A, Lichtman SM, Gardes J, Li D, Limaye S, Patil S, et al. Identifying vulnerable older adults with cancer: integrating geriatric assessment into oncology practice. J Am Geriatr Soc. 2007;55(10):1604–8. doi: 10.1111/j.1532-5415.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 5.Balducci L, Beghe C. Cancer and age in the USA. Crit Rev Oncol Hematol. 2001;37(2):137–45. doi: 10.1016/s1040-8428(00)00109-8. [DOI] [PubMed] [Google Scholar]
- 6.Klepin H, Mohile S, Hurria A. Geriatric assessment in older patients with breast cancer. J Natl Compr Canc Netw. 2009;7(2):226–36. doi: 10.6004/jnccn.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huss A, Stuck AE, Rubenstein LZ, Egger M, Clough-Gorr KM. Multidimensional preventive home visit programs for community-dwelling older adults: a systematic review and meta-analysis of randomized controlled trials. J Gerontol A Biol Sci Med Sci. 2008;63(3):298–307. doi: 10.1093/gerona/63.3.298. [DOI] [PubMed] [Google Scholar]
- 8.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342(8878):1032–6. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- 9.Wieland D, Hirth V. Comprehensive geriatric assessment. Cancer Control. 2003;10(6):454–62. doi: 10.1177/107327480301000603. [DOI] [PubMed] [Google Scholar]
- 10.Stauder R, Moser K, Holzner B, Sperner-Unterweger B, Kemmler G. Six independent domains are defined by geriatric assessment in elderly cancer patients. Crit Rev Oncol Hematol. 74(2):97–105. doi: 10.1016/j.critrevonc.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol. 2000;35(3):147–54. doi: 10.1016/s1040-8428(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 12.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 28(3):380–6. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–31. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 14.Brunello A, Sandri R, Extermann M. Multidimensional geriatric evaluation for older cancer patients as a clinical and research tool. Cancer Treat Rev. 2009;35(6):487–92. doi: 10.1016/j.ctrv.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Balducci L, Colloca G, Cesari M, Gambassi G. Assessment and treatment of elderly patients with cancer. Surg Oncol. 2009 doi: 10.1016/j.suronc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Chen CC, Kenefick AL, Tang ST, McCorkle R. Utilization of comprehensive geriatric assessment in cancer patients. Crit Rev Oncol Hematol. 2004;49(1):53–67. doi: 10.1016/s1040-8428(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 17.Winkelmann N, Petersen I, Kiehntopf M, Fricke HJ, Hochhaus A, Wedding U. Results of comprehensive geriatric assessment effect survival in patients with malignant lymphoma. J Cancer Res Clin Oncol. doi: 10.1007/s00432-010-0933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silliman RA, Guadagnoli E, Rakowski W, Landrum MB, Lash TL, Wolf R, et al. Adjuvant tamoxifen prescription in women 65 years and older with primary breast cancer. J Clin Oncol. 2002;20(11):2680–8. doi: 10.1200/JCO.2002.08.137. [DOI] [PubMed] [Google Scholar]
- 19.Fleming IDC, Henson JS, Hutter DE, Kennedy RVP, Murphy BJ, GP . AJCC Cancer Staging Manual. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 1997. [Google Scholar]
- 20.Clinical Practice Guidelines in Breast Cancer. National Comprehensive Cancer Network; 2006. [Google Scholar]
- 21.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21(17):3357–65. doi: 10.1200/JCO.2003.04.576. [DOI] [PubMed] [Google Scholar]
- 22.Owusu C, Lash TL, Silliman RA. Effect of undertreatment on the disparity in age-related breast cancer-specific survival among older women. Breast Cancer Res Treat. 2007;102(2):227–36. doi: 10.1007/s10549-006-9321-x. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic co morbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG., Jr Assessing illness severity: does clinical judgment work? J Chronic Dis. 1986;39(6):439–52. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 25.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can co morbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 27.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 28.Greenland S. Chapter 21: Introduction to regression modeling. In: Rothman KGS, editor. Modern Epidemiology. 2. Philadelphia, PA: Lippincott-Raven; 1998. pp. 401–434. [Google Scholar]
- 29.Pallis AG, Fortpied C, Wedding U, Van Nes MC, Penninckx B, Ring A, et al. EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer. 46(9):1502–13. doi: 10.1016/j.ejca.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25(14):1936–44. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 31.Pal SK, Hurria A. Impact of Age, Sex, and Co morbidity on Cancer Therapy and Disease Progression. J Clin Oncol. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 32.Wedding U, Kodding D, Pientka L, Steinmetz HT, Schmitz S. Physicians’ judgement and comprehensive geriatric assessment (CGA) select different patients as fit for chemotherapy. Crit Rev Oncol Hematol. 2007;64(1):1–9. doi: 10.1016/j.critrevonc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Tucci A, Ferrari S, Bottelli C, Borlenghi E, Drera M, Rossi G. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer. 2009;115(19):4547–53. doi: 10.1002/cncr.24490. [DOI] [PubMed] [Google Scholar]
- 34.Hurria A, Leung D, Trainor K, Borgen P, Norton L, Hudis C. Factors influencing treatment patterns of breast cancer patients age 75 and older. Crit Rev Oncol Hematol. 2003;46(2):121–6. doi: 10.1016/s1040-8428(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 35.Mandelblatt JS, Hadley J, Kerner JF, Schulman KA, Gold K, Dunmore-Griffith J, et al. Patterns of breast carcinoma treatment in older women: patient preference and clinical and physical influences. Cancer. 2000;89(3):561–73. [PubMed] [Google Scholar]
- 36.Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25(18):2516–21. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 37.Bickell NA, McEvoy MD. Physicians’ reasons for failing to deliver effective breast cancer care: a framework for underuse. Med Care. 2003;41(3):442–6. doi: 10.1097/01.MLR.0000052978.49993.27. [DOI] [PubMed] [Google Scholar]
- 38.Overcash JA, Beckstead J, Moody L, Extermann M, Cobb S. The abbreviated comprehensive geriatric assessment (aCGA) for use in the older cancer patient as a prescreen: scoring and interpretation. Crit Rev Oncol Hematol. 2006;59(3):205–10. doi: 10.1016/j.critrevonc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Clough-Gorr KM, Silliman RA. Translation Requires Evidence: Does Cancer-Specific CGA Lead to Better Care and Outcomes? Oncology (Williston Park) 2008;22(8):925–928. [PMC free article] [PubMed] [Google Scholar]