Abstract
Human aging is characterized by a marked decrease in circulating levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate (DHEAS), hormonal changes associated with cognitive decline. Despite beneficial effects of DHEA supplementation in rodents, studies in elderly humans have generally failed to show cognitive improvement after treatment. In the present study we evaluate the effects of age and estradiol supplementation on expression of genes involved in the de novo synthesis of DHEA and its conversion to estradiol in the rhesus macaque hippocampus. Using RT-PCR we demonstrate the expression of genes associated with this synthesis in several areas of the rhesus brain. Furthermore, real-time PCR reveals an age-related attenuation of hippocampal expression level of the genes CYP17A1, STS, and 3BHSD1/2. Additionally, short-term administration of estradiol is associated with decreased expression of CYP17A1, STS, SULT2B1, and AROMATASE, consistent with a downregulation not only of estrogen synthesis from circulating DHEA, but also of de novo DHEA synthesis within the hippocampus. These findings suggest a decline in neurosteroidogenesis may account for the inefficacy of DHEA supplementation in elderly humans, and that central steroidogenesis may be a function of circulating hormones and menopausal status.
Keywords: Aging, Dehydroepiandrosterone, Hormone replacement, Menopause, Neurosteroidogenesis
1. Introduction
Human aging is associated with several physiological and cognitive changes, but the underlying etiology is poorly understood. Many of these age-associated disorders and pathologies are even more pronounced in females, due to the marked decrease of circulating estradiol (E2) concentrations that occurs around the time of menopause. Consequently, common therapies developed for postmenopausal women involve estrogen replacement. In the brain, ovarian steroids are known to increase synaptic plasticity (Brann et al., 2007) and may improve cognition in postmenopausal women (Fillit et al., 1986; Tang et al., 1996). However, due to potential health risks associated with estrogen-based hormone replacement therapy (HRT) (Manson et al., 2003), there is need for safer alternative therapies to help alleviate postmenopausal disorders, such as cognitive decline.
One potential alternative therapy involves the adrenal steroid dehydroepiandrosterone (DHEA) and its ester, DHEA-sulfate (DHEAS), both of which decline with age in humans (Labrie et al., 1997). Importantly, administration of these steroids (DHEA/S) to old mice has shown promise in restoring cognitive function to a level observed in young animals (Flood and Roberts, 1988; Markowski et al., 2001). However, as mouse and rat adrenal glands do not produce measurable levels of circulating DHEA/S, they may not be ideal models for human aging. In humans, high baseline levels of DHEA/S are associated with increased longevity in men, and in elderly women they have been associated with better cognitive performance (Davis et al., 2008; Sanders et al., 2010). As DHEA/S treatment carries fewer risks than estrogen replacement (Labrie et al., 2003; Panjari et al., 2009), supplementation with this hormone may represent a relatively safe alternative therapy compared to traditional forms of HRT. Despite promising associations with cognition, however, clinical studies in the elderly have failed to detect significant cognitive benefits of DHEA/S supplementation (Grimley-Evans et al., 2006). The reason for this is unclear.
One potential mechanism responsible for the pro-cognitive effects observed in rodents involves local conversion of DHEA/S to E2 (Sorwell and Urbanski, 2010). This phenomenon is a process known as intracrine conversion, or the conversion of a circulating prohormone to an active hormone that acts locally in an auto- or paracrine manner (Labrie, 1991). This steroid synthesis pathway involves the actions of the following enzymes, all of which are expressed in the rodent brain (Mellon and Griffin, 2002): sulfyl transferase (SULT2B1) and steroid sulfatase (STS) convert DHEA to DHEAS and vice versa, while 17β-hydroxysteroid dehydrogenase type 5 (17BHSD5), 3β-hydroxysteroid dehydrogenase types 1 and 2 (3BHSD1/2), and aromatase, are the primary enzymes involved in the central conversion of DHEA to E2. This locally produced E2 can have significant effects on hippocampal spine density and synapse frequency in vivo, suggesting metabolism of DHEA to E2 is a likely mechanism of the observed cognitive effects of DHEA (Hajszan et al., 2004; Hirshman et al., 2004; Rune and Frotscher, 2005). Although well established in rodents, no studies have directly examined this steroidogenic mechanism in humans or nonhuman primates (NHPs). Thus, it is possible that an inability to convert DHEA/S to E2 in the aged human brain underlies the lack of cognitive efficacy observed in clinical studies of DHEA/S supplementation (Sorwell and Urbanski, 2010). Additionally, an age-related decline in the de novo central synthesis of DHEA/S from cholesterol, without the contribution of peripheral hormone precursors, and a resulting loss of central E2 may add to the cognitive effects of the loss of peripheral E2 and serve as a potential target for therapeutic intervention.
The aim of the present study was to shed new light on neurosteroidogenesis in the aging primate brain, and to lay the foundation for potential novel therapies for age-associated cognitive decline. To overcome limitations associated with the rodent model of human aging, we utilized macaque monkeys. Specifically, our goal was to address the following questions: (a) Does de novo DHEA production in the brain, like that in the adrenal cortex, change with age; (b) could the lack of cognitive improvement in human DHEA replacement studies stem in part from reduced conversion of DHEA to E2 in the hippocampus; and (c) does traditional HRT in adult females negatively impact neurosteroidogenesis in the hippocampus.
2. Materials and Methods
2.1. Experimental animals
This study was performed using plasma and tissues samples obtained from Japanese macaques (Macaca fuscata) and rhesus macaques (M. mulatta), maintained at the Oregon National Primate Research Center (ONPRC). The animals were fed a specially formulated monkey chow (Agway, Ithaca, NY) twice daily, supplemented with fresh fruits and vegetables. Animal care was provided by the ONPRC Division of Animal Resources (DAR) in accordance with the NRC Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the Oregon Health and Science University (OHSU) Institutional Animal Care and Use Committee.
Throughout the study, the age groups were defined as young adult (5–7 years), middle-aged (8–17 years), old (18–24 years) and oldest old (25 years and above).
2.2. Annual DHEAS and cortisol measurements
For several decades the ONPRC has maintained an outdoor colony of Japanese macaques (M. fuscata), and during their annual physical examination plasma samples are collected from each animal (between 09:00 h and 15:00 h) and stored frozen at −80 °C. Using these archived samples, and previously described assay procedures (Downs et al., 2008; Lemos et al., 2009), we examined the longitudinal plasma DHEAS and cortisol changes that occur across the adult lifespan of females from 6 to 29 years. Cortisol was measured using electrochemiluminescence (ECL) with the Elecsys 2010 Platform (Roche Diagnostics, Indianapolis, IN). DHEAS was measured with radioimmunoassay (RIA) using a highly specific antibody against DHEA-17-(O-carboxymethyl)oxime-BSA (Endocrine Sciences, Tarzana, CA) and [3H] DHEAS (SA, 22Ci/mmol). Intra- and inter-assay coeffecients of variation were less than 10% for each assay and the assay detection limits were 3 ng/ml. A total of 172 measurements from 14 animals were performed for each hormone.
2.3. Twenty-four hour plasma DHEAS and cortisol measurements
This experiment involved a total of 16 adult female rhesus macaques (M. mulatta), that were maintained indoors under a controlled lighting regimen comprising 12 hours of light and 12 hours of darkness per day (12L:12D, lights on a 7:00 h). Daily menses records and plasma E2 and P4 measurements were used to characterize the reproductive neuroendocrine status of these animals, as previously described (Downs and Urbanski, 2006). Accordingly, the animals were divided into the following four groups: young adult (n=5), middle-aged (n=4), old premenopausal (n=4), and old perimenopausal (n=3) animals. In the latter group, the animals either showed elongated (>30 days) or highly irregular menstrual cycles, but had not yet attained menopause.
To obtain detailed 24-hour hormone profiles, each animal was fitted with a subclavian vein catheter and connected to a remote blood sampling system, as previously described (Urbanski et al., 1997; Urbanski, 2011). This system enabled hourly blood samples to be remotely collected from non-sedated animals, from 7:00 h to 7:00 h on the following day. Blood samples were collected in EDTA-coated borosilicate glass tubes, centrifuged at 4 °C, and the plasma supernatant stored at −20 °C until assay for cortisol and DHEAS (see above). For each hormone, group mean values were determined from the overall mean of the individual hormone values spanning the entire 24-h sampling period. Group maximum hormone values were determined by first identifying the maximum and adjacent values, spanning five hours, for an individual and taking the mean of those individual maximum values. Group amplitude values were determined by first calculating the minimum, based on five adjacent values, and then calculating half the difference between the maximum and minimum values. Between-group differences in mean, maximum, and amplitude were analyzed (Mann-Whitney U-test) for the cortisol and DHEAS concentrations, and also for the DHEAS:cortisol ratio.
2.4. Archived rhesus macaque tissue
Rhesus macaque tissues used in the following two experiments were obtained through the ONPRC Tissue Distribution Program. Qualitative reverse transcription polymerase chain reaction (RT-PCR) analysis of neurosteroid enzyme gene expression was performed on sub-dissected brain regions from ovariectomized adult rhesus macaques. For quantitative real-time PCR analysis of age-related gene expression changes, hippocampi from 38 male and female rhesus macaques (ages 8 to 32 years) were collected at necropsy between 09:00 h and 15:00 h; they were quickly immersed in liquid N2 and stored frozen at −80 °C for later analysis. These samples were divided into the following three age groups: middle-aged (n=16; 7 females and 9 males), old (n=13; 8 females and 5 males) and oldest old (n=9; 6 females and 3 males).
2.5. Hormone replacement therapy (HRT)
Twelve female rhesus macaques (9.4 ± 0.3 years) were used to assess the effects of HRT on hippocampal steroidogenic gene expression. Each animal underwent bilateral ovariectomy and was used 6.7 ± 0.6 months later. At this time, 8 animals were implanted with subcutaneous Silastic capsules containing E2; 15 days later, 4 of these animals received subcutaneous Silastic implants containing P4. This experimental design has been used previously to mimic circulating hormone levels of the late follicular (in the E2-only group) and mid-luteal (in the E2 plus P4 group) phases (Kohama and Bethea, 1995). To confirm the efficacy of the implants, both sex steroids were assayed using the Elecsys 2010 Platform, as previously described (Downs and Urbanski, 2006). The animals were sacrificed 28 days after receiving the initial subcutaneous implant and hippocampi were frozen in liquid N2 for later analysis.
2.6. Assessment of gene expression
Total RNA was extracted from frozen tissues using RNeasy columns (Qiagen). Final concentrations and purity were determined using an Agilent 2100 Bioanalyzer (Agilent Technology, Palo Alto, CA). RT-PCR was used to confirm the presence of steroidogenic gene expression in various brain regions from ovariectomized female rhesus macaques. RNA samples from rhesus macaque adrenal gland and testis were used as positive controls, while water was used as a negative control. The reaction mixtures contained 22.5 μl of SuperMix, 0.5 μl of 25 μM specific forward and reverse primers, and 1 μl of cDNA, using the Platinum PCR SuperMix kit (Invitrogen). The RT-PCR primer sequences for the various genes and transcript variants are shown in Table 1.
Table 1.
RT primer design for steroidogenic enzymes.
| Gene name | GenBank accession ID | Nucleotide sequences (5′ – 3′) |
|---|---|---|
| STS | XM_001088752 | Forward AGGACAGGATCATTGATGGACG |
| Reverse TGGCAAAGCATCCATTGGA | ||
| SULT2B1 | XM_001111839 | Forward CGCCCAGCTAATTTGTGTCCT |
| Reverse TCAGAGCCTTGGTCCCTCTTCT | ||
| 3BHSD1/2 (v.1–3) | XM_001113873 | Forward CCACACGGTGACATTGTCAAAT |
| Reverse CCCACATGCACATCTCTGTCAT | ||
| 17BHSD1 | NM_001047132 | Forward GACCCATCCCAGAGCTTCAAA |
| Reverse TGCGTTACACACCAGCACGT | ||
| 17BHSD2 | XM_001111794 | Forward TCACATAACTCAGGCTGCCTCC |
| Reverse CCATCCAGTTCCACAGCTGC | ||
| 17BHSD3 (v.2) | XM_001105829 | Forward AGGCCCTGCAAGAGGAATATAGAG |
| Reverse CCTGACCTTGGTGTTGAGCTTC | ||
| 17BHSD4 (v.1,3) | XM_001087837 | Forward GTGGATCTTGCACCAACATCTGG |
| Reverse CTCTGGCCTTCAGCCTGCCAC | ||
| 17BHSD5 (v.1,2) | XM_001104543 | Forward GAAGTAAAGCTTTGGAGGTCT |
| Reverse CCATCGTTTGTCTCGCTGAGA | ||
| AROMATASE | XM_001082665 | Forward CCAGCAGACCCAGGACTCTAAA |
| Reverse CCAGGACCTGGTATTGAGGATG |
Reference sequences for the targeted Macaca mulatta genes can be accessed via GenBank accession ID
Taqman real-time PCR was used to further confirm steroidogenic gene expression, and to quantify hippocampal gene expression during aging and after manipulation of the sex-steroid environment. Random-primed reverse transcription was performed, and complementary DNA (cDNA) was diluted 1:3 and analyzed in triplicate. The PCR reaction mixtures contained 5 μl of Taqman Universal PCR Master Mix, 0.3 μl of each specific forward and reverse primers (300 nM final concentration), 0.25 μl of specific probe (250 nM final concentration), and 2 μl of cDNA. The reaction sequence included 2 min at 50 °C, 10 min at 95 °C and 50 cycles of 15 s at 95 °C, followed by 1 min at 60 °C. Automatic baseline and threshold levels were determined by ABI sequence detection system software (version 2.2.1). Standard curve analysis was used to convert critical threshold values into relative RNA concentrations and final expression values were expressed as a ratio relative to the arithmetic mean of the relative RNA concentrations of three reference genes, ALG9, GAPDH, and RPL13A, of each respective sample. This combination of housekeeping genes was used to provide a more stable control than a single reference gene, as slight changes in housekeeping genes may be seen with certain conditions or treatments (Noriega et al., 2010). Real-time primer and probe sequences for the various genes and transcript variants are depicted in Table 2. Of the isozymes of 17BHSD, type 5 was chosen because of its confirmed expression in the brain and its primary direction of action, namely the conversion of androstenedione to testosterone (Labrie et al., 2000; Luu-The and Labrie, 2010; Moeller and Adamski, 2009).
Table 2.
Real-time RT primer and probe design for steroidogenic enzymes.
| Gene name | GenBank accession ID | Nucleotide sequences (5′ – 3′) |
|---|---|---|
| CYP11A1 | XM_001096506 | 6FAM– TGCAGTGGCACTTGTA–MGB-NFQ |
| Forward GAGGACATCAAGGCCAACGT | ||
| Reverse TTCAGGTTGCGTGCCATCT | ||
| CYP17A1 | NM_001040232 | 6FAM– TGAAGAAGAAGCTCTACGAGGA–MGB-NFQ |
| Forward CCTTCCTGCTGCACAATCCT | ||
| Reverse ACGGTTACGGTCACTGATGGTT | ||
| STS | XM_001088752 | 6FAM– CCAGTGCGACAGAGAAAAACAGGATAAGAGA–MGB-NFQ |
| Forward CCTTCCTCCGGCCTGTCT | ||
| Reverse AGCTTTGCCACATGCATCTG | ||
| SULT2B1 | XM_001111839 | 6FAM– TTCTTCAGCTCCAAGGCCAAGGTGATC–MGB-NFQ |
| Forward CAGTACAGCCCTCGCCTCAT | ||
| Reverse GGGTTGCGGCCCATGT | ||
| 3BHSD1/2 (v.1–3) | XM_001113873 | 6FAM– CATTGATGTCTTTGGTGTCACTCA–MGB-NFQ |
| Forward AGGACGTCTCGGTCGTCATC | ||
| Reverse GAGCTGGGTACCTTTCACATTGA | ||
| 17BHSD5 (v.1,2) | XM_001104543 | 6FAM– CAGAAGCCGTGCGTGTGGATGG–MGB-NFQ |
| Forward TGGAGGGCTTTGCTGAAGTCT | ||
| Reverse GGTCCAGTCACCAGCATACAGA | ||
| AROMATASE | XM_001082665 | 6FAM– AATGCATGGACTTTGCCACTGAGTTGATTTT–MGB-NFQ |
| Forward TAGCAGAAAAAAGACGCAGGATT | ||
| Reverse CGTCAGGTCACCTCGTTTCTC | ||
| GAPDH | XM_001105471 | 6FAM– TGAGCACCAGTGGTCTCCTCCGACT–MGB-NFQ |
| Forward AAGGGCATCCTGGGCTACA | ||
| Reverse GAAGAGTGGGTGTCGCTGTTG | ||
| ALG9 | XM_001106180 | 6FAM– ACTGTCTTCCTGTTCGGG–MGB-NFQ |
| Forward AACAGTGCCACAGAGCGAGAA | ||
| Reverse CGATACCGCCTGGAGCACTA | ||
| RPL13A | XM_001115079 | 6FAM– CCAGGCAGTGACAGCCACCTTGG–MGB-NFQ |
| Forward TCACGAGGTTGGCTGGAAGT | ||
| Reverse GATCTTGGCTTTCTCCTTCCTCTT |
Reference sequences for the targeted Macaca mulatta genes can be accessed via GenBank accession ID
3. Results
3.1. Age-related changes in plasma DHEAS and cortisol levels
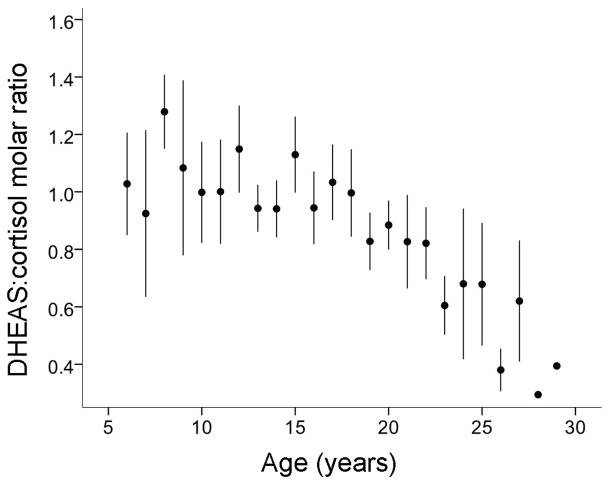
To validate the NHP as an appropriate model of the aging human adrenal gland, we examined changes in adrenal plasma hormone levels of female Japanese macaques (M. fuscata) during aging. Longitudinal examination of plasma DHEAS and cortisol levels in these animals revealed a marked age-related decline in the molar ratio of DHEAS:cortisol (One-way ANOVA effect of age, P<0.05; Fig. 1). This ratio was calculated by multiplying the measured ratio of DHEAS:cortisol by the ratio of their molar masses (368.48 g/mol and 362.46 g/mol, respectively). The decline in this ratio stemmed from a specific decrease in circulating DHEAS levels, which is consistent with observations made in humans (Chehab et al., 2007; Guazzo et al., 1996).
Fig. 1.
Plasma DHEAS:cortisol molar ratio decreases with age in adult female Japanese macaques. Circulating DHEAS and free cortisol levels were determined longitudinally from young adulthood to old age (6–29 years, 172 measurements from 14 animals), and the values were converted into the molar ratio of DHEAS:cortisol. The data are presented as means ± SEM, and show a significant effect of age (P<0.05). DHEAS, dehydroepiandrosterone sulfate.
3.2. Twenty-four hour plasma DHEAS and cortisol profiles
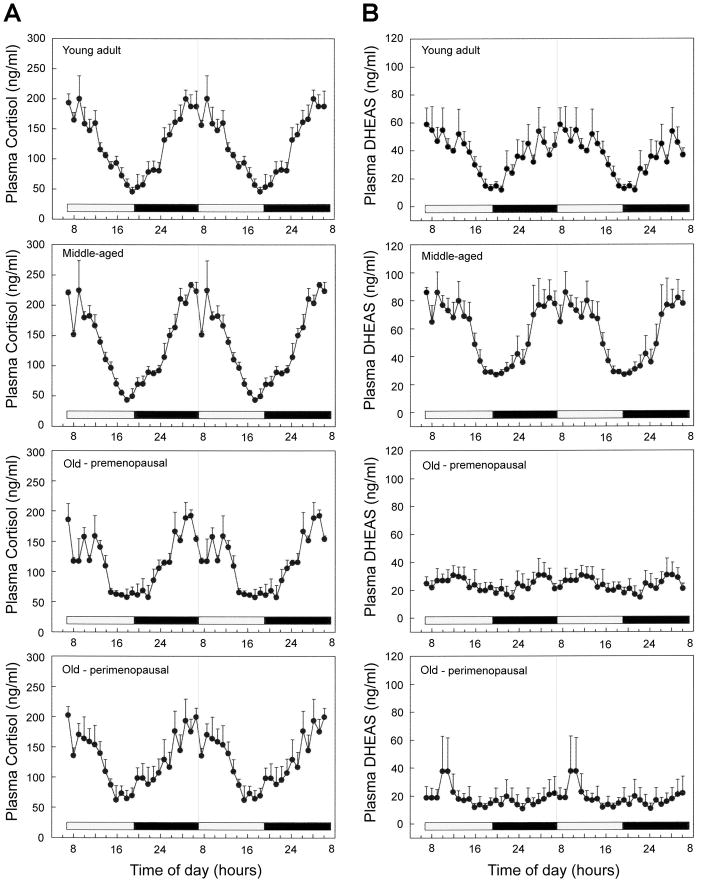
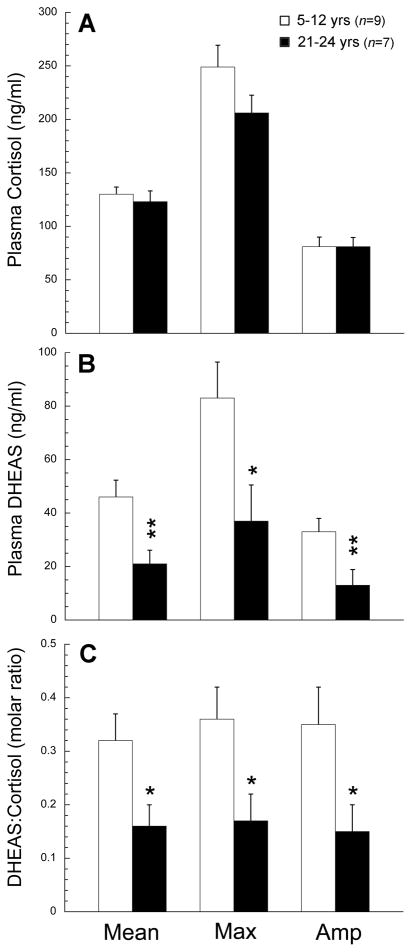
Due to possible interactions between the adrenal and gonadal steroid systems during aging (Crawford et al., 2009; Lasley et al., 2002; Pluchino et al., 2005), we examined adrenal hormone rhythms in relation to menopause in female rhesus macaques (M. mulatta). Twenty-four-hour plasma profiles for cortisol and DHEAS are depicted in Fig. 2 (Panels A and B, respectively). In young adult and middle-aged female rhesus macaques, both hormones showed clear 24-hour rhythms with peak levels occurring in the morning around the time of lights on and a nadir just before the time of lights off. Both old animal groups continued to show a robust cortisol rhythm, whereas the DHEAS rhythm was highly attenuated; mean DHEAS levels were not significantly different between the premenopausal and perimenopausal macaques (P>0.05). Statistical analysis (Fig. 3) of hormone levels between the combined young group (age range: 5–12 years) and combined old group (age range: 21–24 years) showed no age-related change in the plasma cortisol rhythm based on overall mean, maximum, or amplitude level, while plasma DHEAS rhythm showed a significant (P<0.05) age-related decrease in each of these parameters. This was also reflected as a significant (P<0.05) age-related decrease in the molar ratio of DHEAS:cortisol (P<0.05). Importantly, these data demonstrate that the onset of adrenopause in rhesus macaques precedes the onset of menopause.
Fig. 2.
Mean 24-hour plasma profiles of cortisol and DHEAS show age-related changes in female rhesus macaques. The data are presented as means ± SEM, and to aid in the visualization of the night and day variations in hormone concentrations the values have been double plotted; the horizontal white and black bars on the abscissa correspond to the 12L:12D day-night lighting regimen. The panels show cortisol (A) and DHEAS (B) profiles from young adult (n=5), middle-aged (n=4), old premenopausal (n=4), and old perimenopausal (n=3) animals. Statistical analysis of the data is depicted in Fig. 3.
Fig. 3.
Analysis of age-related changes in 24-hour plasma cortisol and DHEAS profiles in female rhesus macaques. The mean, maximum, and amplitude of the hormonal rhythms were calculated for each animal, and comparisons made between the two age groups. No age-related changes in cortisol concentrations were detected (A), but the overall mean concentration, maximum concentration and amplitude of the DHEAS rhythm (B) and the DHEA:cortisol ratio (C) were all significantly attenuated in the old animals. Values are expressed as mean ± SEM. *P <0.05, **P <0.01.
3.3. Steroidogenic gene expression in the rhesus macaque brain
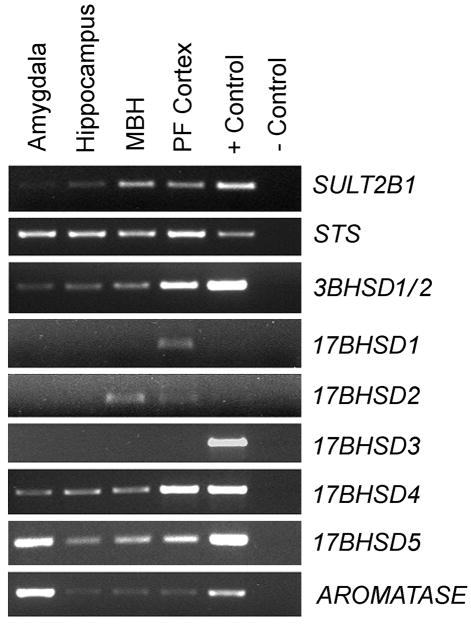
To establish the possibility of a steroidogenic mechanism in the NHP brain, we sought to identify the expression of steroidogenic gene transcripts in four key brain areas. RT-PCR was performed on the amygdala, hippocampus, medial basal hypothalamus, and prefrontal cortex of ovariectomized female rhesus macaques using monkey-specific primers (Table 1). Genes encoding each of the enzymes involved in the conversion of DHEA and DHEAS to E2 were found to be clearly expressed, and the qualitative intensity of expression showed regional specificity (Fig. 4). For example, 3BHSD1/2 was most highly expressed in the prefrontal cortex, whereas 17BHSD5 and AROMATASE were most highly expressed in the amygdala. The data emphasize the potential of the primate brain to synthesize sex-steroids using DHEA and DHEAS as precursors.
Fig. 4.
RT-PCR results showing expression of key genes involved in the intracrine conversion of DHEA/S to 17β-estradiol, within the rhesus macaque amygdala, hippocampus, arcuate nucleus of the medial basal hypothalamus (MBH), and prefrontal (PF) cortex; mRNA from the adrenal gland was used as the positive control for all reactions except 17BHSD3, which used mRNA from the testis. Brain areas shown are from a representative ovariectomized female rhesus macaque. The bands in the gels corresponded to the predicted size of 200–500 nucleotides. The data suggest that DHEA and DHEAS are major precursors in primate neurosteroidogenesis.
3.4. Effect of age on hippocampal expression of steroidogenic genes
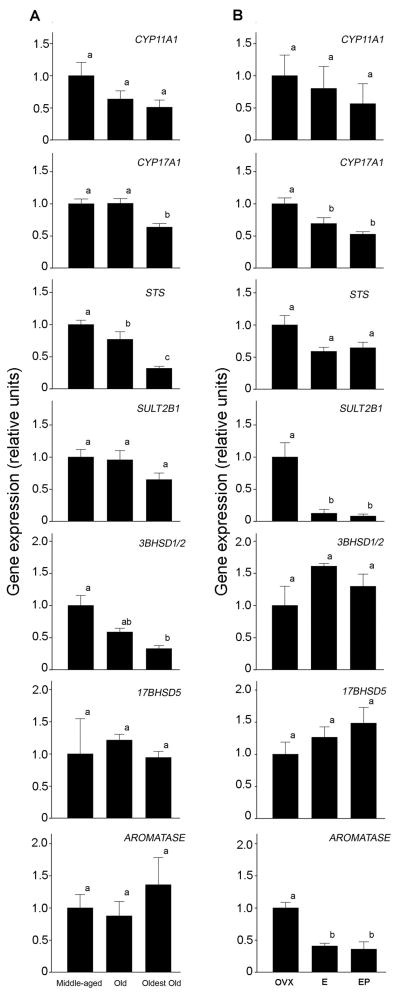
Because adrenal and gonadal steroidogenesis decreases with age, we hypothesized that central steroidogenesis would similarly be attenuated in aged animals. To examine this, as well as to corroborate results of the RT-PCR study, real-time PCR was performed on hippocampal samples from male and female rhesus macaques aged 8 to 32 years, to quantify potential age-related changes in the expression of genes associated with sex-steroid synthesis. No statistically significant differences were detected in gene expression between the males and females (Mann-Whitney U-test). Consequently, gene expression results from both sexes were combined to increase statistical power. The data were assigned to one of three groups, based on the animal’s age (middle-aged, n=16; old, n=13; oldest old, n=9), and group means were compared using ANOVA and the Tukey’s HSD test (Fig. 5A). Fig. S1 shows the expression levels from individual males and females at various ages, as well as the results of correlation analyses. An age-related decrease in hippocampal STS expression was significant in the old animals and continued to drop further in the oldest old. For CYP11A1, CYP17, and 3BHSD1/2, however, a significant age-related decrease only became evident in the oldest old group.
Fig. 5.
Effect of age (A) and HRT (B) on hippocampal steroidogenic gene expression in rhesus macaques, as assessed by real-time PCR. (A) Males and females were divided into three groups: middle-aged (n=16), old (n=13), oldest old (n=9). The mRNA levels were first normalized to an index of housekeeping genes consisting of ALG9, GAPDH, and RPL13A, and then expressed relative to the middle-aged group. (B) Females were divided into three groups: ovariectomized controls (OVX, n=4), ovariectomy + E2 treated animals (E, n=4), and ovariectomy + E2 and P4 treated animals (EP, n=4). The mRNA levels were normalized as above, and then expressed relative to the OVX group. Bars represent mean ± SEM values, and those labeled with different letters (a,b) are significantly different (P<0.05).
3.5. Hippocampal expression of steroidogenic genes after short-term HRT
In humans, circulating DHEAS levels increase transiently during menopause (Crawford et al., 2009; Lasley et al., 2002) and decrease in response to HRT (Pluchino et al., 2005). To examine the possibility of a similar central mechanism of hormonal steroidogenesis regulation in the brain, we quantified steroidogenic gene transcripts following short-term (one month) HRT. Subcutaneous E2 implants in the E rhesus macaque group yielded an average plasma concentration of 118 ± 6.7 pg/ml E2 over the 28 days of treatment. Implants in the EP group yielded E2 levels at an average of 130 ± 9.5 pg/ml over 28 days and P4 levels of 3.8 ± 0.9 ng/ml over the last 14 days of treatment. E2 and P4 levels in the OVX animals were undetectable (i.e., <5 pg/ml, and <0.03 ng/ml, respectively).
To investigate changes in gene expression that occur in response to HRT, real-time PCR was conducted on whole hippocampal RNA extracts obtained from female rhesus macaques after ovariectomy (OVX), ovariectomy + E2 treatment (E), and ovariectomy + E2 + P4 treatment (EP). The data are normalized to an index of housekeeping genes consisting of ALG9, GAPDH, and RPL13A, and are expressed relative to the mean of the OVX group (Fig. 5B). No significant between-treatment differences or trends toward significance were seen in CYP11A1, 3BHSD1/2, or 17BHSD5. ANOVA revealed a significant inhibitory effect of E and EP hormone treatment on expression of CYP17A1, SULT2B1, and AROMATASE. Contrast analysis revealed individual group differences between OVX and both hormone treatment groups; specifically, there was a significant decrease in expression of CYP17A1, STS, and AROMATASE following either hormone treatment. Individual group comparisons revealed no significant difference in gene expression between E and EP groups, but a significant decrease with E2 treatment in expression of SULT2B1 and AROMATASE and a significant decrease in CYP17A1, SULT2B1, and AROMATASE with E2 and P4 treatment.
4. Discussion
In the present study we found that macaque monkeys, like humans, show a marked age-related decrease in DHEAS whereas circulating cortisol levels remain elevated. Therefore, the low DHEAS:cortisol ratio that has been linked to human aging (Chehab et al., 2007; Phillips et al., 2010) also occurs in NHPs, making them suitable animal models in which to study the effects of adrenal aging. The finding from Japanese macaques is particularly significant because it represents the first demonstration of a progressive age-related decrease in DHEAS levels in a NHP model, using an extensive longitudinal experimental design. Because cortisol does not decline during human or NHP aging (Lupien et al., 1996), the decline in DHEA/S is primarily due to specific changes in the zona reticularis rather than general changes in the adrenal cortex. Similarly, the 24-hour hormonal data from female rhesus macaques are significant as they confirm the existence of a robust daily plasma DHEAS rhythm (Downs et al., 2008; Lemos et al., 2006; Lemos et al., 2009). More importantly, they establish that the age-related decline of DHEAS in females clearly precedes the onset of menopause.
The attenuation of circulating DHEAS levels during aging has the potential to influence physiological functions through at least three mechanisms. First, the decrease in circulating DHEAS levels results in a lower DHEAS:cortisol ratio, which is likely to potentiate the negative effects of cortisol in the central nervous system (Ferrari and Magri, 2008). Second, the decrease in DHEAS levels may represent a potential loss of humoral circadian cues and thereby contribute to disruption of sleep-wake cycles (George et al. 2006; Pawlikoski et al., 2002) and resulting impairment of cognitive function. We have recently found that increased perturbation of 24-hour activity-rest cycles in old female rhesus macaques is negatively correlated with performance in a cognitive spatial maze task (Haley et al., 2009), and so it is plausible that the marked attenuation of the circadian plasma DHEAS rhythm plays a causal role. Note, in humans the rhythmic release of DHEAS is less pronounced than in rhesus macaques, possibly due to more rapid clearance; however, the human 24-hour DHEA rhythm in a similar pattern to what is seen in the present study is well documented (Ceresini et al., 2000; Liu et al., 1990; Rosenfeld et al., 1975). Although a link between DHEA/S and sleep/wake cycles has been suggested, the causality of this is complicated by concurrent changes in cortisol; thus, more studies are needed to investigate this relationship. Third, while DHEA/S itself has many neurobiological effects that may underlie cognition (for review, see Webb et al., 2006 and Wolf and Kirschbaum, 1999), DHEA and DHEAS are key substrates in the synthesis of E2, which exerts a major influence on neurons (Brann et al., 2007). The data from our RT-PCR study demonstrate that the genes associated with the enzymatic conversion of DHEA/S to E2 are all expressed within brain, and notably in the hippocampus. Consequently, it is plausible that the adrenal gland contributes to the maintenance of cognitive function via local intracrine conversion of DHEA/S to E2. Moreover, an age-related decline in the availability of these sex-steroid precursors is likely to potentiate the negative impact of the menopausal loss of E2 production by the ovaries.
Early rodent studies reported promising effects of DHEA/S supplementation on cognition (Flood and Roberts, 1988; Roberts et al., 1987), particularly in aged rodents (Farr et al., 2004; Flood et al., 1988; Markowski et al., 2001). Additionally, observations in elderly humans identified a positive correlation between endogenous DHEA/S and certain aspects of cognitive ability (Davis et al., 2008; Sanders et al., 2010), particularly when analyzed in relation to cortisol (Kalmijn et al., 1998). While a similar association study in rhesus macaques failed to find a correlation between cognition and DHEA (Herndon et al., 1999), this study did not take into consideration circulating cortisol levels or the underlying 24-hour modulation of DHEA/S, which may have obscured an association with cognition. Despite promising correlations, most studies of DHEA/S supplementation in elderly humans have failed to replicate the effects seen in rodents, and robust improvements in cognition have yet to be reported (Grimley-Evans et al., 2006).
What then may account for this discrepancy between the rodent and human literature? One possibility may stem from differences between rodent and primate adrenal endocrine systems. For example, most rodents have nearly undetectable circulating levels of DHEA/S (Baulieu, 1998), making any exogenous dose of DHEA/S highly supraphysiological. In contrast, as demonstrated in this and previous studies, adult macaque monkeys do have high circulating DHEA/S levels and also show a marked age-related decline, similar to humans (Downs et al., 2008; Lupien et al., 1996). Because of these species differences, studies of DHEA treatment in rodents might not be translatable to humans and NHPs.
Effect of age on steroidogenic gene expression
Although the ovaries are the predominant source of circulating E2 in women, it has been estimated that up to 75% of active E2 is derived locally through intracrine metabolism of DHEA/S (Labrie, 1991); therefore, plasma E2 levels alone may be poor indicators of central E2. For example, one study of HRT in young ovariectomized rhesus macaques found cognitive benefits for old, but not young, monkeys (Hao et al., 2007), possibly because young animals were able to synthesize sufficient E2 locally de novo or from adrenal DHEA/S precursors. Similarly, in postmenopausal women circulating DHEA/S may be positively correlated with cognition (Davis et al., 2008; Sanders et al., 2010), perhaps due to a greater ability to produce local E2 as compared to women with low circulating DHEA/S. In rodents, locally produced E2 is neuroprotective (Juhász-Vedre et al. 2006) and can have effects on neuronal function (Kretz et al., 2004; Prange- Kiel et al., 2003; Rune and Frotscher, 2005) and plasticity (Prange-Kiel et al., 2009), even beyond that of exogenous E2. An inability to perform this intracrine conversion, especially in old age, may explain the cognitive inefficacy of DHEA supplementation in humans (Sorwell and Urbanski, 2010). The current study supports this hypothesis. Not only are the key steroidogenic enzyme-encoding genes clearly expressed in the primate brain, but also the expression levels of CYP17A1, STS, and 3BHSD1/2 show an age-related decline. Such changes are expected to result in decreased central synthesis of E2, as CYP17A1 is necessary for DHEA synthesis, 3BHSD1/2 is necessary for conversion of DHEA to testosterone, and STS is involved in the conversion of DHEAS to DHEA. Also, while expression of SULT2B1, 17BHSD5, and AROMATASE was similar across the three age groups, it should be emphasized that all of our postmortem hippocampal tissues were collected during the daytime between 09:00 h and 15:00 h; many genes have a 24-hour pattern of expression with a peak occurring during the night (Lemos et al., 2006; Urbanski et al., 2009), and so we cannot exclude the possibility that some genes may show age-related changes that are evident only during the night. Together, the data highlight the potential involvement of DHEA/S in maintaining elevated E2 concentrations within the brain, and show how enzymatic changes could contribute to the etiology of age-associated pathologies.
This study also examined the de novo steroidogenic potential of the macaque hippocampus by quantifying expression of genes involved in the conversion of cholesterol to DHEA throughout aging. Although neurosteroidogenesis has been well established in rodents (Zwain and Yen, 1999), the underlying mechanism may be different in primates because of significant input of DHEA/S of adrenal origin. Some evidence suggests, however, that the NHP brain is in fact capable of de novo neurosteroid synthesis (Robel et al., 1987; Schumacher et al., 2003). Our observation that the brain, and specifically the hippocampus, expresses all of the enzyme-encoding genes necessary for sex-steroid biosynthesis enzymes suggests that primates are able to centrally synthesize DHEA/S de novo. Further, expression of CYP17A1, the enzyme responsible for conversion of pregnenolone to DHEA, was significantly lower in the oldest group of animals studied, suggesting the ability of the hippocampus to synthesize steroids declines with age. Interestingly, a decline in activity of this same enzyme in the adrenal gland is thought to be the underlying cause of age-related declines in circulating DHEA/S (Liu et al., 1990); therefore, the current results may reflect parallel age-related changes in steroid synthesis in the brain and periphery. Based on the expression of key enzyme-encoding genes, these results suggest that de novo synthesis of DHEA/S, as well as conversion of adrenal DHEA/S to E2, is feasible within the primate hippocampus, and that changes in the expression of these genes may contribute to age-associated cognitive decline.
We suggest that multiple neuroendocrine events can contribute to aspects of cognitive decline that are mediated by the loss of steroids during aging, including: (a) loss of gonadal E2 at the time of menopause leads to a direct loss of central E2 availability; (b) the decline in circulating levels of E2 precursors, DHEA and DHEAS, results in reduced intracrine E2 synthesis in the brain; (c) decreased brain expression of steroidogenic enzymes results in less potential for conversion of the diminished levels of DHEA/S to E2; (d) a combined loss of peripheral and local de novo DHEA/S production results in loss of the protective effects of the hormone itself, without conversion to E2 (Flood et al., 1999; Mao and Barger, 1998; Rhodes et al., 1997). Results from this study support the hypothesis that local steroid synthesis within the brain declines with age, through both a decline in circulating DHEA/S and a decline in steroidogenic enzyme expression. Future studies are needed to definitively show that central steroidogenesis is a significant contributor to the brain’s hormonal milieu and further strengthen this hypothesis.
Effect of acute HRT on steroidogenic gene expression
In addition to an examination of the effect of age, we used an ovariectomy + HRT model to examine the effects of surgical menopause on steroidogenic gene expression in the hippocampus. Interestingly, short-term (30 days) treatment with E2, or E2 combined with P4, resulted in a decline in expression of the steroidogenic enzymes CYP17A1, STS, SULT2B1, and AROMATASE. The lack of difference between E2 and E2 + P4 treatment suggests that progesterone has no additive effect on E2’s regulation of steroidogenic enzyme expression, however, effects of P4 alone on steroidogenic gene expression are unknown. This result suggests that end-product inhibition occurs with treatment, and/or there is a compensatory increase in neurosteroid production following ovariectomy. This compensatory mechanism could explain the lack of a negative effect of ovariectomy on performance of estrogen-sensitive cognitive tasks in young monkeys, as they still produce sufficient DHEA/S for intracrine neurosteroidogenesis (Hao et al., 2007). Similar end-product inhibition is seen in peripheral tissues, with E2 acting via estrogen receptor alpha (ERα) in ovarian follicles to downregulate transcription of CYP17A1 and ultimately to reduce local steroidogenesis (Taniguchi et al., 2007). Interestingly, while the present study demonstrates a down-regulation of aromatase following E2 treatment, other tissues cells exhibit a positive feedback mechanism between E2 and aromatase. For example, activation of ERα increases aromatase gene expression through the I.1 promoter in placenta (Kumar et al., 2009) and the I.f promoter in mouse hypothalamic cells (Yilmaz et al., 2009). This discrepancy may be explained by the tissue-specificity of these promoter regions, or by variations in regulation with E2 dosing and timing, as hypothalamic cells exhibit decreased aromatase expression 12 hours after E2, but increased aromatase after 24 hours.
In light of this evidence of end-product inhibition, the loss in circulating DHEA and E2 in the oldest animals would be expected to result in increased expression of steroidogenic genes. However, the current data suggest an inability to increase synthesis with this loss of negative feedback. There is evidence of age-related promoter damage that leads to down-regulation of certain genes (Lu et al., 2004), which may explain the lack of steroidogenic regulation in the oldest animals. Additionally, as local E2 synthesis has been shown to be regulated by GnRH input to the hippocampus (Prange-Kiel et al., 2008; Prange-Kiel et al., 2009; Rosati et al., 2011), a dysregulation of GnRH signaling, or reduced GnRH responsiveness with age or ovariectomy, may further modulate steroidogenic enzyme expression. The present observation that exogenous E2 down-regulates neurosteroidogenic enzyme expression is consistent with a previous study demonstrating E2 treatment of ovariectomized rhesus macaques results in a decrease in GnRH pulse amplitude (Mizuno and Terasawa, 2005); thus, peripheral or exogenous E2 may downregulate local hippocampal steroidogenesis via GnRH suppression.
Furthermore, recent human studies have reported a transient increase in adrenal DHEA/S secretion between the early and late stages of perimenopause (Crawford et al., 2009; Lasley et al., 2002), followed by a return to a pattern of decline postmenopausally. Due to the relatively short post-ovariectomy endpoint of the current study, these results may reflect a compensatory mechanism common to the adrenal gland and central nervous system during perimenopause in which the adrenal gland produces more DHEA and the hippocampus produces more E2 to compensate for a loss of ovarian E2. Additionally, treatment of healthy postmenopausal women with estrogen significantly reduced circulating DHEAS after 6 and 12 months of treatment (Pluchino et al., 2005), suggesting that the phenomenon observed in the current study may not reflect an increase in steroidogenesis with ovariectomy or menopause, but instead a decrease in steroidogenesis with E2 treatment.
Conclusions
Our hormonal data are consistent with previous findings and further demonstrate the value of the macaque model of aging. More importantly, they provide insights into potential mechanisms by which neurosteroids contribute to cognitive function during menopause, thereby laying a foundation for the development of alternative HRT. Specifically, we suggest three possible sources of E2 in the primate brain: (1) ovarian or circulating origin, (2) local conversion of adrenal DHEA to E2, and (3) local de novo synthesis from cholesterol. Data from the HRT portion of our study suggest an ability of the hippocampus to compensate for loss of ovarian E2 following ovariectomy, and human studies suggest additional compensation from the adrenal glands (Crawford et al., 2009; Lasley et al., 2002; Pluchino et al., 2005). However, aging is associated with a decline in ovarian E2 as well as adrenal DHEA, leaving the only source of central E2 to be de novo synthesis. Our data suggests that this source of steroids may also be impaired, as aged animals expressed significantly lower levels of steroidogenic enzymes in the hippocampus. Thus, a progressive loss of E2 contribution from these three sources may lead to a total loss of central E2, possibly potentiating cognitive decline. Additionally, an age-related decline in expression of the enzymes necessary to convert DHEA to E2 provides a possible explanation for the cognitive inefficacy of DHEA supplementation in the elderly.
Supplementary Material
Hippocampal expression of key steroidogenic genes correlates with age in adult rhesus macaques. Relative mRNA levels were quantified using real-time PCR from males and females and due to small group sizes the sexes were collectively analyzed, using Pearson correlation. Significant age-related decreases were detected in the expression of CYP17A1, STS, and 3BHSD2 (R = −0.454, P = 0.009; R = −0.0583, P < 0.001; and R = −0.514, P = 0.001, respectively). No significant sex differences were observed.
Acknowledgments
We wish to thank Vasilios Garyfallou for help with blood sample processing and with the design of gene primers and probes. This work was supported by NIH grants AG023477, AG029612, AG036670, HD018185, HD029186, and RR000163.
Footnotes
Author contributions: K.G.S., S.G.K. and H.F.U. designed and performed the research; K.G.S. and H.F.U. analyzed the data and wrote the paper.
Conflicts of interest
The authors confirm that no actual, or potential, conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresini G, Morganti S, Rebecchi I, Freddi M, Ceda GP, Banchini A, Solerte SB, Ferrari E, Ablondi F, Valenti G. Evaluation of the circadian profiles of serum dehydroepiandrosterone (DHEA), cortisol, and cortisol/DHEA molar ratio after a single oral administration of DHEA in elderly subjects. Metabolism. 2000;49:548–551. doi: 10.1016/s0026-0495(00)80024-4. [DOI] [PubMed] [Google Scholar]
- Cheblowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Chehab O, Ouertani M, Chaieb K, Haouala F, Mahdouani K. Hormonal status of cortisol and dehydroepiandrosterone sulfate in an elderly Tunisian population. C R Biol. 2007;330:755–763. doi: 10.1016/j.crvi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Uezu K, Gaskin FS, Morley JE. DHEAS improves learning and memory in aged SAMP8 mice but not in diabetic mice. Life Sci. 2004;75:2775–2785. doi: 10.1016/j.lfs.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Ferrari E, Magri F. Role of neuroendocrine pathways in cognitive decline during aging. Ageing Res Rev. 2008;7:225–233. doi: 10.1016/j.arr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, Zabriskie J. Observations in a preliminary open trial of estradiol therapy for Senile Dementia-Alzheimer’s Type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- Flood JF, Farr SA, Johnson DA, Li PK, Morley JE. Peripheral steroid sulfatase inhibition potentiates improvement of memory retention for hippocampally administered dehydroepiandrosterone sulfate but not pregnenolone sulfate. Psychoneuroendocrinology. 1999;24:799–811. doi: 10.1016/s0306-4530(99)00030-x. [DOI] [PubMed] [Google Scholar]
- Flood JF, Roberts E. Dehydroepiandrosterone improves memory in aging mice. Brain Res. 1988;448:178–181. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- Flood JF, Smith GE, Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988;447:269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- George O, Vallée M, Le Moal M, Mayo W. Neurosteroids and cholinergic systems: implications for sleep and cognitive processes and potential role of age-related changes. Psychopharmacology (Berl) 2006;186:402–413. doi: 10.1007/s00213-005-0254-6. [DOI] [PubMed] [Google Scholar]
- Grimley-Evans J, Malouf R, Huppert F, van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 2006;18:CD006221. doi: 10.1002/14651858.CD006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. J Clin Endocrinol Metab. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- Haley GE, Landauer N, Renner L, Weiss A, Hooper K, Urbanski HF, Kohama SG, Neuringer M, Raber J. Circadian activity associated with spatial learning and memory in aging rhesus monkeys. Exp Neurol. 2009;217:55–62. doi: 10.1016/j.expneurol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Maclusky NJ, Leranth C. Dehydroepiandrosterone increases hippocampal spine density in ovariectomized female rats. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Lacreuse A, Ladinsky E, Killiany RG, Rosene DL, Moss MB. Age-related decline in DHEAS is not related to cognitive impairment in aged monkeys. Neuroreport. 1999;10:3507–3511. doi: 10.1097/00001756-199911260-00008. [DOI] [PubMed] [Google Scholar]
- Hirshman E, Merritt P, Wang CC, Wierman M, Budescu DV, Kohrt W, Templin JL, Bhasin S. Evidence that androgenic and estrogenic metabolites contribute to the effects of dehydroepiandrosterone on cognition in postmenopausal women. Horm Behav. 2004;45:144–155. doi: 10.1016/j.yhbeh.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Juhász-Vedre G, Rózsa E, Rákos G, Dobszay MB, Kis Z, Wölfling J, Toldi J, Párducz A, Farkas T. Dehydroepiandrosterone sulfate is neuroprotective when administered either before or after injury in a focal cortical cold lesion model. Endocrinology. 2006;147:683–686. doi: 10.1210/en.2005-0693. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, Launer LJ, Stolk RP, de Jong FH, Pols HA, Hofman A, Breteler MM, Lamberts SW. A prospective study on cortisol, dehydroepiandrosterone sulfate, and cognitive function in the elderly. J Clin Endocrinol Metab. 1998;83:3487–3492. doi: 10.1210/jcem.83.10.5164. [DOI] [PubMed] [Google Scholar]
- Kohama SG, Bethea CL. Steroid regulation of tyrosine hydroxylase messenger ribonucleic acid in dopaminergic subpopulation of monkey hypothalamus. Endocrinology. 1995;136:1790–1800. doi: 10.1210/endo.136.4.7895692. [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Kamat A, Mendelson CR. Estrogen receptor alpha (ERalpha) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta. Mol Endocrinol. 2009;23:784–793. doi: 10.1210/me.2008-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin SX, Simard J, Labrie C, El-Alfy M, Pelletier G, Bélanger A. Intracrinology: role of the family of 17 beta-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol. 2000;25:1–16. doi: 10.1677/jme.0.0250001. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Bélanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocrine Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87:3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Downs JL, Urbanski HF. Twenty-four hour rhythmic gene expression in the rhesus macaque adrenal gland. Molec Endocrinol. 2006;20:1164–1176. doi: 10.1210/me.2005-0361. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Downs JL, Raitiere MN, Urbanski HF. Photoperiodic modulation of adrenal gland function in the rhesus macaque: effect on 24-h plasma cortisol and dehydroepiandrosterone sulfate rhythms and adrenal gland gene expression. J Endocrinol. 2009;201:275–285. doi: 10.1677/JOE-08-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Laughlin GA, Fischer UG, Yen SS. Marked attenuation of ultradian and circadian rhythms of dehydroepiandrosterone in postmenopausal women: evidence for a reduced 17,20-desmolase enzymatic activity. J Clin Endocrinol Metab. 1990;71:900–906. doi: 10.1210/jcem-71-4-900. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Luu-The V, Labrie F. The intracrine sex steroid biosynthesis pathways. Prog Brain Res. 2010;181:177–192. doi: 10.1016/S0079-6123(08)81010-2. [DOI] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Schwartz G, Sharma S, Hauger RL, Meaney MJ, Nair NP. Longitudinal study of basal cortisol levels in healthy elderly subjects: Evidence for subgroups. Neurobiol Aging. 1996;17:95–105. doi: 10.1016/0197-4580(95)02005-5. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M Women’s Health Initiative Investigators. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- Mao X, Barger SA. Neuroprotection by dehydroepiandrosterone-sulfate: role of an NFκB-like factor. Neuroreport. 1998;9:759–763. doi: 10.1097/00001756-199803090-00036. [DOI] [PubMed] [Google Scholar]
- Markowski M, Ungeheuer M, Bitran D, Locurto C. Memory-enhancing effects of DHEAS in aged mice on a win-shift water escape task. Physiol Behav. 2001;72:521–525. doi: 10.1016/s0031-9384(00)00446-7. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Terasawa E. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinizing hormone-releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta) J Neuroendocrinol. 2005;17:238–245. doi: 10.1111/j.1365-2826.2005.01295.x. [DOI] [PubMed] [Google Scholar]
- Moeller G, Adamski J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2009;301:7–19. doi: 10.1016/j.mce.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Noriega NC, Kohama SG, Urbanski HF. Microarray analysis of relative gene expression stability for selection of internal reference genes in the rhesus macaque brain. BMC Molec Biol. 2010;11:47–71. doi: 10.1186/1471-2199-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjari M, Bell RJ, Jane F, Adams J, Morrow C, Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas. 2009;63:240–245. doi: 10.1016/j.maturitas.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Pawlikowski M, Kolomecka M, Wojtczak A, Karasek M. Effects of six months of melatonin treatment on sleep quality and serum concentrations of estradiol, cortisol, dehydroepiandrosterone sulfate, and somatomedin C in elderly women. Neuro Endocrinol Lett. 2002;23(Suppl 1):17–19. [PubMed] [Google Scholar]
- Phillips AC, Carroll D, Gale CR, Lord JM, Arlt W, Batty GD. Cortisol, DHEA sulphate, their ratio, and all-cause and cause-specific mortality in the Vietnam Experience Study. Eur J Endocrinol. 2010;163:285–292. doi: 10.1530/EJE-10-0299. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Genazzani AD, Bernardi F, Casarosa E, Pieri M, Palumbo M, Picciarelli G, Gabbanini M, Luisi M, Genazzani AR. Tibolone, transdermal estradiol or oral estrogen-progestin therapies: Effects on circulating allopregnanolone, cortisol, and dehydroepiandrosterone levels. Gynecol Endocrinol. 2005;20:144–149. doi: 10.1080/09513590400021169. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Jarry H, Rune GM. Estrus cyclicity of spinogenesis: underlying mechanisms. J Neural Transm. 2009;116:1417–1425. doi: 10.1007/s00702-009-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM. Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J Cell Bio. 2008;180:417–426. doi: 10.1083/jcb.200707043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Li PK, Burke AM, Johnson DA. Enhanced plasma DHEAS, brain acetylcholine and memory mediated by steroid sulfatase inhibition. Brain Res. 1997;773:28–32. doi: 10.1016/s0006-8993(97)00867-6. [DOI] [PubMed] [Google Scholar]
- Robel P, Bourreau E, Corpéchot C, Dang DC, Halberg F, Clarke C, Haug M, Schlegel ML, Synguelakis M, Vourch C. Neuro-steroids: 3β-hydroxy-Δ5-derivatives in rat and monkey brain. J Steroid Biochem. 1987;27:649–655. doi: 10.1016/0022-4731(87)90133-6. [DOI] [PubMed] [Google Scholar]
- Roberts E, Bologa L, Flood JF, Smith GE. Effects of dehydroepiandrosterone and its sulfate on brain tissue in culture and on memory in mice. Brain Res. 1987;406:357–362. doi: 10.1016/0006-8993(87)90807-9. [DOI] [PubMed] [Google Scholar]
- Rosati F, Sturli N, Cungi MC, Morello M, Villanelli F, Bartolucci G, Finocchi C, Peri A, Serio M, Danza G. Gonadotropin-releasing hormone modulates cholesterol synthesis and steroidogenesis in SH-SY-5Y cells. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Rosenfeld RS, Rosenberg BJ, Fukushima DK, Hellman L. 24-hour secretory pattern of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J Clin Endocrinol Metab. 1975;40:850–855. doi: 10.1210/jcem-40-5-850. [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Cappola AR, Arnold AM, Boudreau RM, Chaves PH, Robbins J, Cushman M, Newman AB. Concurrent change in dehydroepiandrosterone sulfate and functional performance in the oldest old: results from the Cardiovascular Health Study All Stars study. J Gerontol A Biol Sci Med Sci. 2010;65:976–981. doi: 10.1093/gerona/glq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age (Dodr) 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi F, Couse JF, Rodriguez KF, Emmen JM, Poirier D, Korach KS. Estrogen receptor-alpha mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17alpha-hydroxylase/17,20 lyase) expression. FASEB J. 2007;21:586–595. doi: 10.1096/fj.06-6681com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Maueux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber J, editor. Animal Models of Behavioral Analysis, Neuromethods. Vol. 50. Springer; New York: 2011. pp. 217–235. [Google Scholar]
- Urbanski HF, Garyfallou VT, Kohama SG, Hess DL. Alpha-adrenergic receptor antagonism and N-methyl-D-aspartate (NMDA) induced luteinizing hormone release in female rhesus macaques. Brain Res. 1997;744:96–104. doi: 10.1016/s0006-8993(96)01083-9. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Noriega NC, Lemos DR, Kohama SG. Gene expression profiling in the rhesus macaque: experimental design considerations. Methods. 2009;49:26–31. doi: 10.1016/j.ymeth.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab Rev. 2006;38:89–116. doi: 10.1080/03602530600569877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and it ssulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Yilmaz MB, Wolfe A, Cheng YH, Glidewell-Kenney C, Jameson JL, Bulun SE. Aromatase promoter I.f is regulated by estrogen receptor alpha (ESR1) in mouse hypothalamic neuronal cell lines. Biol Reprod. 2009;81:956–965. doi: 10.1095/biolreprod.109.077206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwain IH, Yen SCC. DHEA: biosynthesis and metabolism in the brain. Endocrinology. 1999;140:880–887. doi: 10.1210/endo.140.2.6528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hippocampal expression of key steroidogenic genes correlates with age in adult rhesus macaques. Relative mRNA levels were quantified using real-time PCR from males and females and due to small group sizes the sexes were collectively analyzed, using Pearson correlation. Significant age-related decreases were detected in the expression of CYP17A1, STS, and 3BHSD2 (R = −0.454, P = 0.009; R = −0.0583, P < 0.001; and R = −0.514, P = 0.001, respectively). No significant sex differences were observed.