Abstract
Background
Congenital myasthenic syndromes (CMS) are disabling but treatable disorders. Anticholinesterase therapy is effective in most, but is contraindicated in endplate (EP) acetylcholinesterase (AChE) deficiency, the slow-channel syndrome, Dok-7 myasthenia, β2-laminin deficiency, and is not useful in CMS due to defects in MuSK, agrin, and plectin. EP AChE, Dok-7 and β2-laminin deficiencies respond favorably to ephedrine but ephedrine can no longer be prescribed in the US.
Methods
We used albuterol, another sympathomimetic agent, to treat three patients with EP AChE deficiency and 15 with Dok-7 myasthenia. Response to therapy was evaluated by a 9-point questionnaire pertaining to activities of daily life.
Results
Comparison of the pre- and post-treatment responses indicated a beneficial response to albuterol (p values <0.001) in both patient groups. The adverse effects of therapy were like those of ephedrine.
Discussion
Our observations should spur controlled prospective clinical trials of albuterol in these as well as other CMS.
Keywords: Congenital myasthenic syndrome, Dok-7 myasthenia, Endplate AChE deficiency, Albuterol
Introduction
Congenital myasthenic syndromes (CMS) are clinically and genetically heterogenous disorders in which the safety margin of neuromuscular transmission is compromised by one or more specific mechanisms.1 Because drugs that benefit one type of CMS can be harmful in another type, treatment is guided by the identified defect of neuromuscular transmission2 and by empiric observations. Acetylcholinesterase (AChE) inhibitors, which augment the synaptic response to ACh, are usually effective when the synaptic response to ACh is attenuated, as in patients with primary endplate (EP) acetylcholine receptor (AChR) deficiency. However, they are ineffective and potentially harmful in the slow-channel CMS, EP AChE deficiency, Dok-7 myasthenia2 and the CMS caused by mutations in β-2 laminin,3 and are of no or limited use in the CMS caused by defects in MuSK,4,5 agrin,6 and plectin7–9.
Ephedrine, a sympathomimetic amine with α- and β-adrenergic effects,10 has been used in asthma and as a decongestant. In 1930, Dr. Harriet Edgeworth, a physician, a biochemist and a patient with adult-onset myasthenia gravis (MG), reported improved muscle strength and decreased fatigability while taking a medication containing ephedrine for dysmenorrhea.11 She subsequently subjected herself to a single-blind experiment with a placebo, which confirmed her initial hypothesis.11 After her seminal observation, ephedrine was widely used in MG but was eventually replaced by anticholinesterase and immunosuppressive medications. More recently, ephedrine was shown to be beneficial treatment of the CMS caused by defects in EP AChE, 12,13β2-laminin,3 and Dok-714–16 but its mode of action is unknown.
In 2004, an FDA regulation prohibited prescribing ephedrine in the United States and thereby deprived CMS patients with EP AChE, 12,13β2-laminin, and Dok-7 deficiency from the mainstay of their therapy. For this reason we evaluated the use of albuterol, a selective β2 adrenergic agonist10 commonly used as a bronchodilator, in the treatment of patients with EP AChE deficiency and Dok-7 myasthenia.
METHODS
This study was initiated as an open label study with intent to treat. After reviewing the results of the study based on questionnaires completed by patients, we obtained written consent from each patient (or their parents if the patient was a minor) to publish their responses. The human studies described here were reviewed and approved by the Institutional Review Board of the Mayo Clinic.
Fifteen of the 18 patients in this study had been initially examined at the Mayo Clinic. In 5 of these the defect in neuromuscular transmission and EP ultrastructure were further investigated by intercostal muscle biopsies. Histories of 3 patients were available from other medical centers. The generic diagnosis of a CMS was based on the history, examination, and compatible EMG studies. A specific diagnosis of AChE deficiency was suggested by a repetitive compound muscle action potential unaffected by edrophonium and refractoriness to pyridostigmine; that of Dok-7 myasthenia was suggested by a predominantly limb-girdle distribution of the muscle weakness and refractoriness or worsening of the symptoms on exposure to pyridostigmine. Patients 1,17 2,18 and Patients 9, 10, 11 and 1214 were previously reported. The genetic diagnosis was established by capillary sequencing of DNA isolated from blood or muscle from each patient and their nuclear family members, and by absence of the identified mutation from 100 to 200 control subjects.
After the diagnosis of Dok-7 myasthenia or EP AChE deficiency was established by combined clinical, electrophysiological and/or molecular genetic methods, the patients (or their parents) were explained possible benefits and risks of albuterol therapy and advised to discontinue the medication should they experience undue palpitation, irregular pulse, or any other unusual symptoms. The prescribed dose of albuterol typically ranged from 4 mg from once to three times a day for adults, 2 mg two to three times a day for children 6 to 12 years of age, and 0.1 mg/kg/day (maximum 2 mg) three times daily for children 2 to 6 years of age.
Because most patients were unable to return for reevaluation, they (or their parents) were requested to complete an evaluation form regarding when the medication was started, the dose employed, and respond to a 9-question survey related to disease-specific symptoms (see Table 1). The participants were asked to return the questionnaire after treatment with albuterol for at least one month and thereafter if they experienced further changes. Some patients continued to inform us even if their clinical status had stabilized. Four of the nine questions concerned limb and axial muscles weakness (difficulty sitting up from supine position, difficulty rising from sitting position, weakness of arm or hand muscles, and weakness of leg or foot muscles), two assessed respiratory function (shortness of breath on exertion and shortness of breath at night), and one pertained to oropharyngeal weakness (difficulty speaking or swallowing). For these questions, the participants were asked to rate their symptoms as normal, mild, moderate or severe. The last two questions aimed to evaluate abnormal fatigability; these inquired about how far patients were able to walk and how many steps they were able to climb without having to rest. The participants were also encouraged to describe any other changes in activities of daily living during therapy and report any adverse reactions. Age at onset, duration of disease, and additional relevant information were obtained from the patients’ medical records. In some cases, additional information came from follow-up letters from referring physicians or patients.
Table 1.
Evaluation of response to Albuterol
| Name: Date of this report: (dd/mm/yyyy): | Before taking Albuterol | On Albuterol (date of this report) |
|---|---|---|
| -- | Current daily dose of albuterol: | |
| Dates when started (d/m/year) | ||
| Difficult to sit up from lying on back* | ||
| Difficult to rise from sitting* | ||
| Difficult to speak or swallow* | ||
| Shortness of breath on exertion* | ||
| Shortness of breath at night* | ||
| Weakness of arm or hand muscles * | ||
| Weakness of leg or foot muscles* | ||
| Distance walked without stopping to rest | ||
| Number of steps climbed without stopping to rest |
Rate as normal, mild, moderate, severe
Describe below any additional changes in your condition such as arm elevation time, number of deep knee bends before having to stop, or in activities of daily living relevant to the effects of the treatment. Also indicate any unwanted side effects of the medication. Continue on other side or separate page if necessary. Return this questionnaire after treatment with albuterol for 1 month and then if you note further changes.
Data Analysis
For the first 7 questions, the responses of normal, mild, moderate, and severe were assigned scores of 0, 1, 2, and 3 respectively. The scale was modified by adding 0.5 to the score when the severity of a symptom was rated between two established ranks. The responses before and after treatment to these 7 questions were evaluated by the Wilcoxon signed rank test. The numerical responses to the distance walked and the steps climbed were not normally distributed and were therefore also evaluated by the Wilcoxon signed rank test.
RESULTS
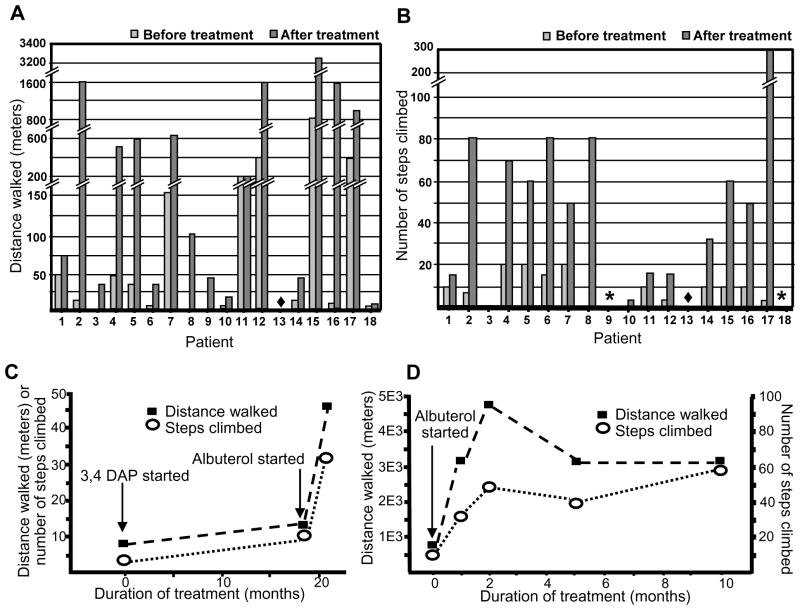
Three patients with EP AChE deficiency and 15 with Dok7-CMS were enrolled in the study. Patients 1, 7, 9, 12, 14, 15, and 17 had previously been treated with pyridostigmine; 5 patients (1, 9, 14, 15, and 17) did not respond, and 2 patients (7 and 12) became weaker. Patient 14 was also treated with 3,4-DAP before treatment with albuterol (see text). Exposure to albuterol ranged from 1 to 25 months (median 4 months) except for Patient 14 whose treatment was stopped after 2 weeks due to atrial flutter. Table 2 summarizes the clinical data, the identified mutations, as well as the dose, duration, and adverse effects of therapy. Table 3 indicates the sum of disability scores rated from 0 to 3, the distance walked without having to rest and the number of steps climbed without having to stop before the start of therapy and at the time of the last evaluation.. Figure 1A and B show the distance walked and the number of steps climbed by each patient before albuterol therapy and at the last evaluation. Comparison of each category of response before and after treatment revealed a beneficial effect of albuterol (P values <0.001).
Table 2.
Clinical data
| Patient number, sex | Gene | Mutations | Age at onset (years) | Age when albuterol started | Albuterol dose | Duration of treatment (month) |
|---|---|---|---|---|---|---|
| 1, M | COLQ | p.S169X p.S169X |
Birth | 50 | 4 mg BID | 6 # |
| 2, M | COLQ | p.R282X c.1082delC |
Birth | 17 | 4 mg (ER) BID | 25 |
| 3, M | COLQ | p.R410W c.1082delC |
Infancy | 27 | 4 mg (ER) TID | 4 |
| 4, M | DOK7 | c.54+14_28del c.1124_1127dupTGCC |
5 | 22 | 6 mg QAM and 4 mg QPM | 3 |
| 5, F | DOK7 | c.54+14_28del c.1124_1127dupTGCC |
3 | 17 | 4 mg BID | 3 |
| 6, F | DOK7 | c.54+14_28del c.1124_1127dupTGCC |
Birth | 36 | 4 mg (ER) TID | 3 |
| 7, M | DOK7 | c.54+14_28del c.1124_1127dupTGCC |
Birth | 41 | 6 mg TID | 2.5 |
| 8, M | DOK7 | c.55-1G>T c.1124_1127dupTGCC |
Birth | 5 | 2 mg BID | 23 |
| 9, F | DOK7 | c.55-2A>C c.1124_1127dupTGCC |
Birth | 16 | 4 mg BID | 1 |
| 10, M | DOK7 | c.55-2A>C c.1124_1127dupTGCC |
Birth | 11 | 4 mg BID | 1 |
| 11, F | DOK7 | c.1124_1127dupTGCC c.1124_1127dupTGCC |
Early childhood | 58 | 4 mg (ER) BID | 2 |
| 12, F | DOK7 | c.1124_1127dupTGCC c.1263insC |
1.5 | 33 | 2 mg QID | 7 |
| 13, M | DOK7 | c.1124_1127dupTGCC c.1263insC |
7 | 37 | 4 mg (ER) TID | 12 |
| 14, M | DOK7 | c.1124_1127dupTGCC c.1263insC |
Birth | 52 | 4 mg OD | 0.5# |
| 15, F | DOK7 | c.1124_1127dupTGCC c.1263insC |
Infancy | 27 | 4 mg (ER) BID | 10 |
| 16, F | DOK7 | c.1124_1127dupTGCC c.1263insC |
4 | 27 | 4 mg (ER) BID | 8 |
| 17, M | DOK7 | c.1124_1127dupTGCC c.1378insC |
Early childhood | 29 | 4 mg (ER) OD | 6 |
| 18, F | DOK7 | c.1124_1127dupTGCC * | Infancy | 32 | 4 mg TID | 2 |
-, none. ER, extended release.
Only a single mutation was identified; no mRNA was available for mutation analysis. ** Patients 4 and 5 and 9 and 10 are siblings.
Patient 1 also received prednisone, and Patient 14 also received 3,4-DAP before and during therapy with albuterol.
Table 3.
Evaluations of clinical status before treatment and at the time of the last evaluation
| Patient number | Gene | Sums of disability scores scaled from 0 to 3 | Walking distance, meters | Number of steps climbed | |||
|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||
| 1 | COLQ | 11 | 6.5 | 46 | 73 | 10 | 15 |
| 2 | COLQ | 15 | 7 | 11 | 1600 | 7 | 80 |
| 3 | COLQ | 17 | 10 | 0 | 30 | 0 | 0 |
| 4 | DOK7 | 6 | 0 | 50 | 500 | 20 | 70 |
| 5 | DOK7 | 4 | 0 | 30 | 600 | 20 | 60 |
| 6 | DOK7 | 19.5 | 9.5 | 3 | 30 | 15 | 80 |
| 7 | DOK7 | 12 | 4 | 152 | 610 | 20 | 50 |
| 8 | DOK7 | 13 | 7.5 | 0 | 110 | 1 | 80 |
| 9 | DOK7 | 18 | 5.5 | 0 | 41 | 0 | Did not try |
| 10 | DOK7 | 12 | 7 | 1 | 18 | 0 | 5 |
| 11 | DOK7 | 13 | 12 | 200 | 200 | 10 | 15 |
| 12 | DOK7 | 18 | 8 | 400 | 1600 | 5 | 15 |
| 13 | DOK7 | 5 | 2 | No difficulty walking | No difficulty walking | No difficulty climbing stairs | No difficulty climbing stairs |
| 14 | DOK7 | 12 | 4.5 | 12 | 46 | 10 | 32 |
| 15 | DOK7 | 14 | 1 | 804 | 3218 | 10 | 60 |
| 16 | DOK7 | 12 | 1 | 14 | 1600 | 10 | 48 |
| 17 | DOK7 | 10 | 0 | 400 | 1000 | 5 | 300 |
| 18 | DOK7 | 18.5 | 1.5 | 3 | 9 | 0 | Did not try |
| P<0.001 | P<0.001 | P<0.001 | |||||
Beneficial effect is indicated by decrease of the disability scores and increase in the distance walked and steps climbed without having to rest.
Figure 1.
Distance walked (A) and steps climbed (B) without having to rest before and after albuterol therapy. Asterisks indicate patients who were unable to climb steps before therapy and have not tried to do so after therapy. (C) Effect of 3,4-DAP, and of 3,4-DAP plus albuterol, on walking distance and steps climbed by Patient 14. (D) Walking distance and steps climbed by Patient 15 before and at 1, 2, 5, and 10 months after the treatment with albuterol.
The effects of the short acting medication lasted 4–6 hours and that of the extended release medication lasted 8–12 hours. None of the patients reported that the effectiveness of the medication wore off with continued use. In addition, Patients 2, 3 and 15 reported a progressive increase of baseline strength in the course of therapy. Some patients reported albuterol therapy was a life-changing experience. Patient 11 was the only poor responder with essentially unchanged pre- and post-treatment scores. All except Patient 11 reported an improved quality of life. For example, Patients 2 and 18 became independent in all daily activities at home and were able to return to school. Patients 2, 3 and 18 were no longer wheelchair bound, and Patient 2 no longer needed nocturnal ventilation. Patient 15 was able to return to work full time, and Patient 16 became nearly symptom free.
Patients with combined therapy
Patient 1 has taken prednisone before and during treatment with albuterol. Prednisone was initially thought to improve his weakness but he remained severely disabled. Patient 14 was first treated with 3,4-diaminopyridine (3,4-DAP) for 19 months and then with 3,4-DAP plus albuterol for 2 weeks. Before any treatment, the sum of the 7 disability scores was 19; after therapy with 3,4-DAP it was 12; and after combined therapy with 3,4-DAP plus albuterol it was 4.5. Figure 1C shows the distance walked and the number of steps climbed before treatment, after 3,4-DAP treatment, and after treatment with 3,4-DAP plus albuterol by Patient 14.
Patients with multiple responses to the questionnaire
Patient 15 completed questionnaires at 1, 2, 5, and 10 months after taking albuterol. Figure 1D shows the distance walked and the number of steps climbed at each time point. Using the MRC scale, before taking albuterol, she had grade 2 weakness of iliopsoas and quadriceps muscles, grade 4 weaknesses of the hamstring, biceps, triceps, deltoid, neck flexor and bulbar muscles as well as moderately severe ptosis. After taking albuterol for 2 months, her limb muscles were of normal strength, but her ptosis and bulbar weakness remained unchanged.
Adverse reactions during therapy
Patients 2, 12 and 13 developed variable degrees of exercise-induced muscle cramps or burning sensation of the calf and occasionally of other muscles. Patients 12 and 13 also noted tightness of the jaw muscles on chewing. The cramps were considered mild by Patient 2, moderately severe by Patient 13, and severe by Patient 12. Decreasing the dose of albuterol from 12 to 8 mg per day in Patients 12 and 13 alleviated these adverse effects. Patients 9 and 10 noted mild jitteriness and tremor; Patient 11 noted insomnia; Patient 12 had worsening of hypertension which was corrected by medication. These side effects were tolerable in the context of the beneficial effects of therapy. Patient 14 developed atrial flutter which mandated stopping therapy with albuterol after two weeks.
Discussion
The present study indicates that albuterol, like ephedrine, has a beneficial effect in the treatment of EP AChE deficiency and Dok-7 myasthenia. Although there are several well-established outcome measures for the treatment of autoimmune MG, outcome measures for the different types of CMS have not been defined or standardized. The first seven questions used in this study are similar to questions that evaluate the MG-specific activities of daily living (MG-ADL)19 but include no questions on weakness of the extraocular and facial muscles because, except for ptosis, this is usually absent in Dok-7 myasthenia14,15,20 and is variable in EP AChE deficiency.12
Although not in the questionnaire, Patients 2 and 15 reported no improvement of ptosis, and Patient 11 noted mild worsening of facial weakness during therapy. Previous studies on the use of ephedrine in Dok-7 myasthenia reported similar observations.15,16 In both studies, the positive effects of ephedrine were more pronounced in proximal limb than in other muscles including the levator palpebrae15 and facial muscles.16
Patients typically noticed improvement after the first few days of therapy but in Patient 15 albuterol reached its maximum effect at 2 months (Figure 1D). In Patients 6, 7, 12, and 13, the response to albuterol was dose-dependent. The effective daily dose of albuterol in the current study ranged from a total of 4 to 12 mg per day. The optimal dose of albuterol varied from patient to patient. For example, Patient 4 achieved a post-treatment disability score of 0 taking 4 mg of albuterol per day whereas Patient 5 required 10 mg per day to achieve this effect.
The adverse effects of albuterol noted here have also been observed in patients treated with albuterol for obstructive airway disease, and nearly half of these patients experienced muscle cramps.21–23 Decreasing the dose of albuterol in Patients 12 and 13 alleviated the muscle cramps but also attenuated the beneficial effects of the medication. Patient 13 elected to resume taking the higher dose of albuterol despite his leg and jaw discomfort.
The mechanism by which ephedrine or albuterol improves neuromuscular transmission is not known. In vitro microelectrode studies of canine intercostal muscles showed that 100 μM ephedrine increased the quantal content of the endplate potential by 21%, but reduced the amplitude of miniature endplate potential by 38%, probably by blocking the AChR channel.24 A subsequent single-channel patch-clamp study of rat lumbrical muscles revealed that both ephedrine and albuterol act as short-lived open-channel blockers of the AChR channel.25 In both studies, the effects on neuromuscular transmission occurred only at drug concentrations higher than attainable in clinical practice. A possible explanation of the beneficial effect of sympathomimetic agents would be that they increase muscle strength by an anabolic effect;10,26 however, such an effect would not appear within 2 days after the start of therapy.
Sympathomimetic agents could also affect neuromuscular transmission in other ways.27 Stimulation of presynaptic α1- and β-adrenoceptors facilitates neuromuscular transmission via activation of the diacylglycerol-protein kinase C signaling pathway and the cyclic AMP-protein kinase A (PKA) cascade, respectively. Liganding of the postsynaptic β2-adrenoceptors, the most common adrenoceptor subtypes in skeletal muscle, activates the PKA signaling pathway in the muscle fibers.26–29 Postsynaptic accumulation of PKA is important for synaptic integrity and AChR stability.30 Reduced PKA levels in the juxtajunctional sarcoplasm caused fragmentation and decreased the size of the EPs, and increased the AChR turnover rate.30 After denervation, calcitonin-gene related peptide, a cAMP agonist, or cAMP by itself, prevented fragmentation of the EPs and decreased the AChR degradation rate via PKA.30,31
Although the mechanism by which albuterol or ephedrine improve neuromuscular transmission in EP AChE deficiency or Dok-7 myasthenia is not understood, our observations should spur prospectively designed clinical trial to determine the efficacy of albuterol in the two CMS considered here as well as in other types of CMS. Ideally, such study should be cooperative, multicenter, randomized, and placebo-controlled. However, launching such a study could be hindered by the CMS being rare disorders and because the affected patients are geographically dispersed.
Acknowledgments
We thank the patients and their families who participated in this study. This study was supported by NIH Grant NS6277 and by a Research Grant by the Muscular Dystrophy Association to AGE.
Abbreviations
- AChE
acetylcholinesterase
- CMS
congenital myasthenic syndrome
- EP
endplate
References
- 1.Engel AG, Shen XM, Selcen D, Sine SM. What have we learned from the congenital myasthenic syndromes. J Mol Neurosci. 2010;40:143–153. doi: 10.1007/s12031-009-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel AG. The therapy of congenital myasthenic syndromes. Neurotherapeutics. 2007;4:252–257. doi: 10.1016/j.nurt.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maselli RA, Ng JJ, Anderson JA, Cagney O, Arredondo J, Williams C, et al. Mutations in LAMB2 causing a severe form of synaptic congenital myasthenic syndrome. J Med Genet. 2009;46:203–208. doi: 10.1136/jmg.2008.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chevessier F, Faraut B, Ravel-Chapuis A, Richard P, Gaudon K, Bauche S, et al. MUSK, a new target for mutations causing congenital myasthenic syndrome. Hum Mol Genet. 2004;13:3229–3240. doi: 10.1093/hmg/ddh333. [DOI] [PubMed] [Google Scholar]
- 5.Mihaylova V, Salih MA, Mukhtar MM, Abuzeid HA, El-Sadig SM, von der Hagen M, Huebner A, et al. Refinement of the clinical phenotype in musk-related congenital myasthenic syndromes. Neurology. 2009;73:1926–1928. doi: 10.1212/WNL.0b013e3181c3fce9. [DOI] [PubMed] [Google Scholar]
- 6.Huze C, Bauche S, Richard P, Chevessier F, Goillot E, Gaudon K, et al. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet. 2009;85:155–167. doi: 10.1016/j.ajhg.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banwell BL, Russel J, Fukudome T, Shen XM, Stilling G, Engel AG. Myopathy, myasthenic syndrome, and epidermolysis bullosa simplex due to plectin deficiency. J Neuropath Exp Neurol. 1999;58:832–846. doi: 10.1097/00005072-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Selcen D, Juel VC, Hobson-Webb LD, Smith EC, Stickler DE, Bite AV, et al. Myasthenic syndrome caused by plectinopathy. Neurology. 2011;76:327–336. doi: 10.1212/WNL.0b013e31820882bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrest K, Mellerio JE, Robb S, Dopping-Hepenstal PJ, McGrath JA, Liu L, et al. Congenital muscular dystrophy, myasthenic symptoms and epidermolysis bullosa simplex (EBS) associated with mutations in the PLEC1 gene encoding plectin. Neuromuscul Disord. 2010;20:709–711. doi: 10.1016/j.nmd.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 11. New York: The McGraw-Hill Companies, Inc; 2006. pp. 237–296. [Google Scholar]
- 11.Edgeworth H. A report of progress on the use of ephedrine in a case of myasthenia gravis. JAMA. 1930;94:1136. [Google Scholar]
- 12.Bestue-Cardiel M, Saenz de Cabezon-Alvarez A, Capablo-Liesa JL, Lopez-Pison J, Pena-Segura JL, Martin-Martinez J, Engel AG. Congenital endplate acetylcholinesterase deficiency responsive to ephedrine. Neurology. 2005;65:144–146. doi: 10.1212/01.wnl.0000167132.35865.31. [DOI] [PubMed] [Google Scholar]
- 13.Mihaylova V, Muller JS, Vilchez JJ, Salih MA, Kabiraj MM, D’Amico A, et al. Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain. 2008;131:747–759. doi: 10.1093/brain/awm325. [DOI] [PubMed] [Google Scholar]
- 14.Selcen D, Milone M, Shen XM, Harper CM, Stans AA, Wieben ED, Engel AG. Dok-7 myasthenia: phenotypic and molecular genetic studies in 16 patients. Ann Neurol. 2008;64:71–87. doi: 10.1002/ana.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schara U, Barisic N, Deschauer M, Lindberg C, Straub V, Strigl-Pill N, et al. Ephedrine therapy in eight patients with congenital myasthenic syndrome due to DOK7 mutations. Neuromuscul Disord. 2009;19:828–832. doi: 10.1016/j.nmd.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Lashley D, Palace J, Jayawant S, Robb S, Beeson D. Ephedrine treatment in congenital myasthenic syndrome due to mutations in DOK7. Neurology. 2010;74:1517–1523. doi: 10.1212/WNL.0b013e3181dd43bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engel AG, Lambert EH, Gomez MR. A new myasthenic syndrome with end-plate acetylcholinesterase deficiency, small nerve terminals, and reduced acetylcholine release. Ann Neurol. 1977;1:315–330. doi: 10.1002/ana.410010403. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson DO, Walls TJ, Nakano S, Camp S, Taylor P, Harper CM, et al. Congenital endplate acetylcholinesterase deficiency. Brain. 1993;116:633–653. doi: 10.1093/brain/116.3.633. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487–1489. doi: 10.1212/wnl.52.7.1487. [DOI] [PubMed] [Google Scholar]
- 20.Muller JS, Herczegfalvi A, Vilchez JJ, Colomer J, Bachinski LL, Mihaylova V, et al. Phenotypical spectrum of DOK7 mutations in congenital myasthenic syndromes. Brain. 2007;130:1497–1506. doi: 10.1093/brain/awm068. [DOI] [PubMed] [Google Scholar]
- 21.Palmer KN. Muscle cramp and oral salbutamol. BMJ. 1978;2:833. doi: 10.1136/bmj.2.6140.833-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig TJ, Smits W, Soontornniyomkiu V. Elevation of creatine kinase from skeletal muscle associated with inhaled albuterol. Ann Allergy Asthma Immunol. 1996;77:488–490. doi: 10.1016/S1081-1206(10)63356-X. [DOI] [PubMed] [Google Scholar]
- 23.Hellier JP, Baudrimont M, Dussaule JC, Berenbaum F. Reversible selective beta(2)-adrenoceptor agonist-induced myopathy. Rheumatology (Oxford, England) 2002;41:111–113. doi: 10.1093/rheumatology/41.1.111. [DOI] [PubMed] [Google Scholar]
- 24.Sieb JP, Engel AG. Ephedrine: effects on neuromuscular transmission. Brain Res. 1993;623:167–171. doi: 10.1016/0006-8993(93)90025-i. [DOI] [PubMed] [Google Scholar]
- 25.Milone M, Engel AG. Block of the endplate acetylcholine receptor channel by the sympathomimetic agents ephedrine, pseudoephedrine, and albuterol. Brain Res. 1996;740:346–352. doi: 10.1016/s0006-8993(96)00894-3. [DOI] [PubMed] [Google Scholar]
- 26.Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88:729–767. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- 27.Wessler I. Acetylcholine at motor nerves: storage, release, and presynaptic modulation by autoreceptors and adrenoceptors. Int Rev Neurobiol. 1992;34:283–384. doi: 10.1016/s0074-7742(08)60100-2. [DOI] [PubMed] [Google Scholar]
- 28.Besalduch N, Tomas M, Santafe MM, Garcia N, Tomas J, Lanuza MA. Synaptic activity-related classical protein kinase C isoform localization in the adult rat neuromuscular synapse. J Comp Neurol. 2010;518:211–228. doi: 10.1002/cne.22220. [DOI] [PubMed] [Google Scholar]
- 29.Westfall TC, Westfall DP. Neurotransmission: the autonomic and somatic motor nervous systems. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 11. New York: The McGraw-Hill Companies, Inc; 2006. pp. 137–181. [Google Scholar]
- 30.Roder IV, Choi KR, Reischl M, Petersen Y, Diefenbacher ME, Zaccolo M, et al. Myosin Va cooperates with PKA RIalpha to mediate maintenance of the endplate in vivo. Proc Natl Acad Sci USA. 2010;107:2031–2036. doi: 10.1073/pnas.0914087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu R, Salpeter MM. Protein kinase A regulates the degradation rate of Rs acetylcholine receptors. J Cell Physiol. 1995;165:30–39. doi: 10.1002/jcp.1041650105. [DOI] [PubMed] [Google Scholar]