Conspectus
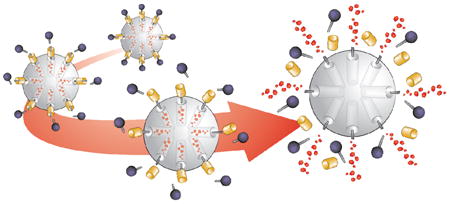
Nanotechnology has been cited as a response to the most challenging issues facing society as a whole today. With nanoscale assemblies promising to improve on previously established therapeutic and diagnostic motifs, medicine stands to benefit significantly from advances in nanotechnology. To this end, the use of delivery platforms has attracted attention during the past decade, with researchers shifting their focus towards devising ways to deliver therapeutic and / or diagnostic agents, and away from developing new drug candidates. Metaphorically, the use of delivery platforms in medicine can be viewed as the “bow-and-arrow” approach, where the drugs are the arrows and the delivery vehicles are the bows. Even if one possesses the best arrows that money can buy, the arrows are not going to be useful if one does not have the appropriate bow to deliver the arrows to a desired location. The same can be said of drugs.
Currently, a variety of strategies for delivering bioactive agents within living tissue exists. Dendrimers, polymers, micelles, vesicles, and nanoparticles have all been investigated for their use as possible delivery vehicles. With the growth of nanomedicine, one can then envisage the possibility in theranostic medicine of fabricating a vector that is capable of releasing simultaneously powerful therapeutics and diagnostic markers selectively to diseased tissue. In our design of new theranostic delivery systems, we have focused our attention on using mesoporous silica nanoparticles (SNPs). It is possible to store a payload of “cargo” molecules within such a robust platform that is stable to a wide range of chemical conditions. This stability allows SNPs to be functionalized with responsive mechanically interlocked molecules (MIMs) in the shape of bistable rotaxanes and psuedorotaxanes to yield mechanized silica nanoparticles (MSNPs). These MIMs can be designed in such a way that they either change shape or shed off some of their parts in response to a specific stimulus, allowing a theranostic payload to be released from the nanopores to a precise location at the most ideal time.
In this Account, we chronicle the evolution of various MSNPs which came about as a result of our decade-long collaboration, and discuss advances that have been made in synthesizing novel hybrid mesoporous silica nanoparticles, and the various MIMs which have been attached to their surfaces. Recognizing the theranostics of the future, we aim to start moving out of the chemical domain and into the biological one, with some MSNPs already being subjected to biological testing.
Introduction
Nanoscale devices are becoming more common in the field of medicine, particularly as they hold promise or advances in the field of drug delivery and controlled release. In the event, a library of mechanized silica nanoparticles (MSNPs) has been fabricated1–8 during the past decade. Their operation has been demonstrated in both organic and aqueous solutions. Their evolution is summarized in the timeline illustrated in Figure 1. All MSNPs have three primary components: they are – (i) a solid support, (ii) a payload of cargo, and (iii) external machinery. Typically, mesoporous silica nanoparticles (SNPs) – MCM-41 in particular – are chosen9 as the solid support for MSNPs (Figure 2), since they are rigid, robust, chemically inert, and relatively easy to fabricate.10 The cargo can be drugs or imaging agents that can be contained within the pores of the SNPs. Highly fluorescent molecules are often chosen as a cargo since release from the nanopores can be tracked by fluorescence spectroscopy. Typically, the external machinery consists of a monolayer (Figure 3) of mechanically interlocked molecules (MIMs) usually in the form of rotaxanes which consist of the following components – (a) linear stalks anchoring the rotaxanes to the surfaces of the SNPs, (b) gating rings, in the form of macrocycles which encircle the stalks and trap the cargo – usually delivered to the MSNPs under a concentration gradient – within the pores of the MSNPs, (c) an alternative ring binding site or weak, cleavable point along all the stalks that are susceptible to some specific stimulus to force the rings to distance themselves from the pores, so releasing the cargo, and (d) stoppers at the ends of the stalks. The individual components employed in the fabrication of MSNPs are highly modular, a situation which means that their customization is straightforward – a major advantage of these integrated systems over other delivery vehicles.
Figure 1.
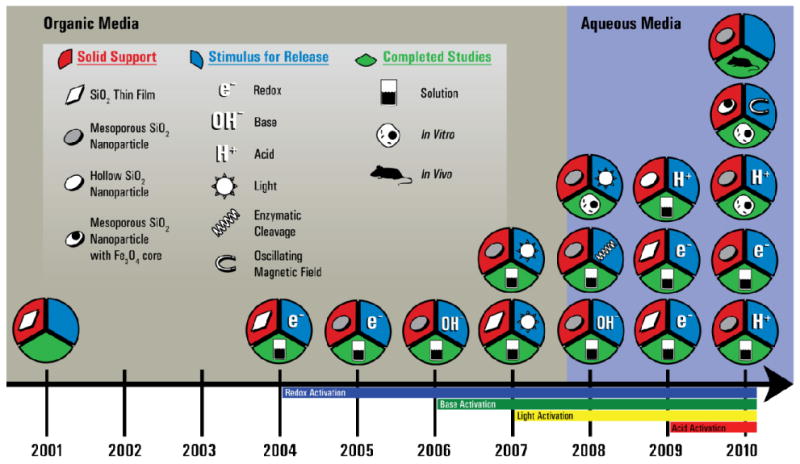
Timeline showing the evolution of MSNPs, where each circle represents a landmark MSNP with the solid support and stimulus used to release the cargo, and which studies the MSNPs were subjected to. The research began in 2001 with the demonstration that supramolecular machines operate on and within glass in a manner similar to that in solution, and progressed to incorporating a variety of machines both on and in SNPs. Several kinds of stimuli have been used to release cargo molecules from MSNPs, including redox-activation (2004), increasing the basicity (2006), irradiating (2007), and increasing the acidity (2009). All MSNPs have been subjected to release experiments in solution to confirm their operation, and some have progressed to in vitro (2008) and in vivo (2010) testing.
Figure 2.

(A) SEM image of MCM-41 SNPs. (B) TEM image of MCM-41 SNPs. (C) TEM image of hollow SNPs. (D) TEM image of magnetic-core SNPs (MCSNPs).
Figure 3.
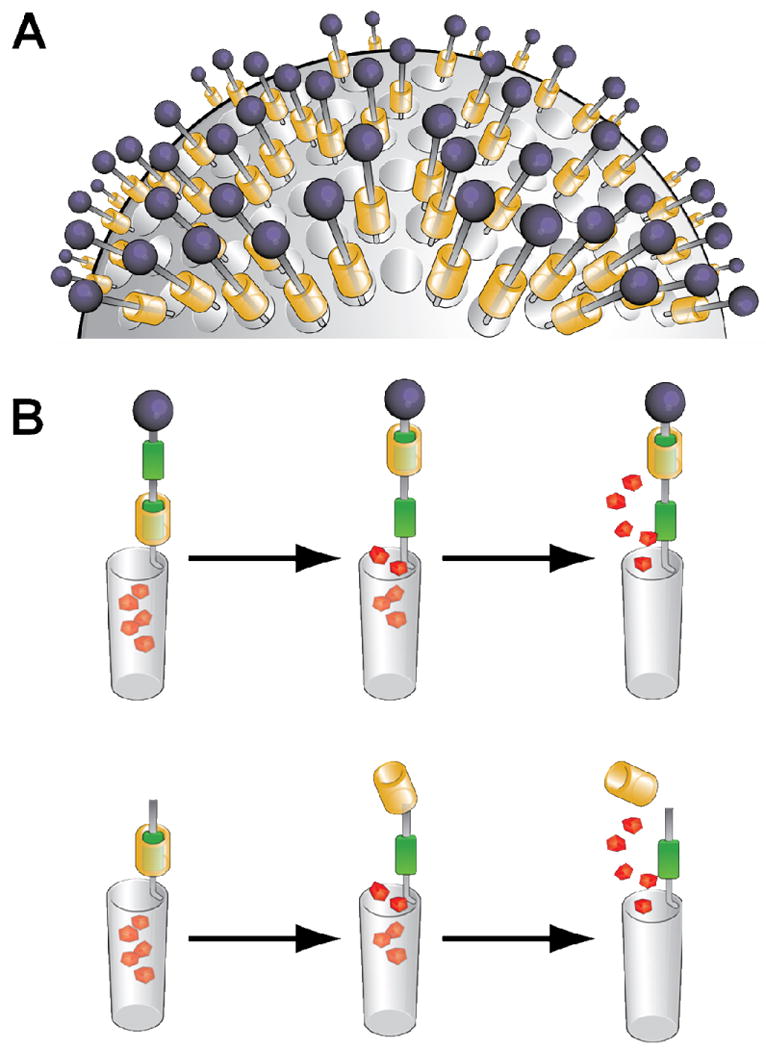
(A) A monolayer of rotaxanes covering the surface of an SNP. (B) Schematic representation of cargo being released from the nanopores of an MSNP using rotaxanes (top) or psuedorotaxanes (bottom) as the external machinery.
Building the Foundation
The collaboration, which is the bedrock upon which this Account is being written, began with the demonstration11 that supramolecular machines can be either trapped within a silica monolith or mounted on a silica film and yet continue to operate as if they were in solution. The supramolecular machines studied in this seminal investigation consisted of the electron-deficient, tetracationic cyclophane, cyclobis(paraquat-p-phenylene) (CBPQT4+), which has a high affinity for the electron-rich stalk, 1,5-bis[2-(2-hydroxyethoxy)ethoxy]naphthalene (BHEEN), resulting in the BHEEN stalks threading themselves through CBPQT4+ rings to give complexes we refer to as pseudorotaxanes. The decomplexation can be controlled by reduction of CBPQT4+ and it was demonstrated that this (de)threading process can also occur when the pseudorotaxanes are in or on a silica solid support. The silica films12-14 that provided the solid supports were also used to develop methods for deliberately attaching multiple types of functional molecules in specific regions of the mesoporous silica materials. This research laid the groundwork for attaching increasingly sophisticated machinery that can release a payload of cargos in a controlled manner in response to a wide variety of chemical or biological triggers. These early studies, carried out in nonaqueous solvents, enabled the development of sophisticated machines but needed to be redesigned for use in biological systems.
Redox Activation
Nanovalves which can be opened and closed reversibly were the next major development.5 In this integrated system, bistable [2]rotaxanes are arranged as a monolayer on the surfaces of the SNPs. The dual function stalks (dumbbells) carry CBPQT4+ rings which operate as gating rings in these [2]rotaxanes. The CBPQT4+ rings have extremely high affinities for the tetrathiofulvalene (TTF) units, the “green stations” on the stalks. In the ground state, the CBPQT4+ rings sit on the green stations, thus setting the MSNPs in an open state and allowing cargos to diffuse into and out of the pores of the nanoparticles. Upon the addition of the chemical oxidant, Fe(ClO4)3 • 6H2O, TTF is oxidized to TTF2+, forcing the CBPQT4+ rings to move down to the “red stations”, or 1,5-dioxynaphthalene (DNP) units. When the CBPQT4+ rings sit on the red stations, the MSNPs remain in the closed state until they are exposed to ascorbic acid, which reduces TFF2+ back to TTF, causing the CBPQT4+ rings to leave the red stations and return to the green ones, thus opening the pores of the MSNPs and leading to the controlled release of the cargo.
As part of the early proof-of-principle, another integrated system of MSNPs was fabricated15,16 that consists of rotaxanes containing stalks with –NH2+– binding sites, which have a high affinity, by dint of hydrogen bonding, for macrocyclic polyethers, such as dibenzo-24-crown-8 (DB24C8). Rings of DB24C8 encircle secondary dialkylammonium (R2NH2+) centers on the surface of the SNPs, retaining a cargo within the nanopores. The DB24C8 rings can then be expelled from the stalks, either (i) by abstracting15,16 a proton from the –CH2NH2+CH2– centers with a base – e.g., NEt3, EtNiPr2, or P(NMe2)3 – or (ii) by competitive binding16 upon exposure to a competing cation, which can be a metal ion, such as Cs+, K+, Li+, or Ca2+, or an R2NH2+ ion. Thus, cargos can be released from these MSNPs, either by raising the pH, or through competitive binding.
Light Activation
At the outset of our investigations, we also sought to use light as a “remote control” stimulus for the controlled release17,18 of cargos from MSNPs. Our initial studies modified the CBPQT4+ ring and DNP system. A separate photosensitizer, such as 9-anthracenecarboxylic acid or [Ru(bpy)2(bpy(CH2OH)2)]2+ (bpy = 2,2′-bipyridine), was also tethered to the surfaces of the SNPs such that, upon excitation, it causes photoinduced electron transfer to the CBPQT4+ rings, inducing them to dissociate from the stalks and release the cargo.
Light-stimulated MSNPs witnessed further development18,19 in the same year (2007) when photoactive azobenzene “nanoimpeller” molecules were anchored inside the pores of SNPs. The azobenzene molecules, which exist in the trans configuration in their ground state photoisomerize to the cis configuration upon irradiation with UV light and revert back to the trans configuration upon exposure to visible light. Because both the trans and cis configurations of the azobenzene units absorb light at 406 nm, excitation at this wavelength results in continuous photoisomerization between the two configurations, causing a wagging motion that expels cargos from the pores of the MSNPs into solution. This particular design does not require an additional photosensitizer to be attached to the MSNPs.
Working in Water
At the outset, we demonstrated that appropriately designed MSNPs trap and release cargo in organic media in response to external stimuli, such as light and changes in pH or redox potentials. Since our goal is to deploy MSNPs in biological environments, our research efforts in more recent times have been directed towards preparing biocompatible MSNPs which operate in aqueous solution. Since both cyclodextrins (CDs) and cucurbit[6]uril (CB[6]) have the propensity to form inclusion complexes – which can be dissociated reversibly in response to external stimuli – with a variety of guest molecules in aqueous solution, we have commandeered these biologically benign polymacrocycles to serve as the rings on the nanovalves, thus rendering the MSNPs biocompatible.14 They also confer other attributes on MSNPs on account of (i) their low toxicities, (ii) their targeted recognition of organic substrates, (iii) their ability to solubilize organic compounds in water, and (iv) their protection of the drug molecules from physical, chemical, and enzymatic degradation.
During this same time, multifunctional SNPs enhanced for drug delivery platforms were developed. The particles which are small (∼100 nm spheres) and uniform, have highly ordered pores of ca. 2 nm in diameter. Various functionalities, including the attachment of phosphonate groups for decreased aggregation in water and biological media, folate for specific cell targeting, the addition of fluorescent molecules for optical imaging, and the inclusion of magnetic materials to enable the particles to be used for T2 magnetic resonance imaging (MRI), were incorporated into or onto the MSNPs to create multifunctional systems. When further derivatized with nanomachines, these multifunctional nanoparticles have the ability to carry and deliver drugs into cells, function with stimulated release by MSNP activation, and act as imaging agents in diagnostic tests.
pH Activation in Water
In our initial investigations carried out on biocompatible integrated systems, we elected20 to study pH-responsive MSNPs based on CB[6]-containing [2]pseudorotaxanes. The pH-dependent association/dissociation of the CB[6] ring with diaminoalkanes enables the formation of complexes whose dynamic behavior can be controlled by pH. By taking advantage of the ability21 of CB[6] rings to catalyze 1,3-dipolar cycloadditions, [2]pseudorotaxane-functionalized SNPs were prepared by the reaction between azide-substituted dialkylammonium ions and alkyne-containing dialkylammonium ions, affording disubstituted 1,2,3-triazoles encircled by a CB[6] ring. The pH-dependent binding of the CB[6] rings with the bisammonium stalks has been employed to control the release of cargo molecules (rhodamine B) from the pores of SNPs in aqueous solution. At neutral pH and acidic pH, the CB[6] rings encircle the bisammonium stalks tightly, trapping cargos inside the pores of the MSNPs. Deprotonation of the stalks with base leads to spontaneous dethreading of the CB[6] rings, unblocking of the nanopores, and release of the cargo from the nanopores.
In order to fabricate tunable pH-operable MSNPs in which biologically relevant pH changes are employed to trigger the release of cargo molecules, we have redesigned22 the CB[6] pseudorotaxane-functionalized MSNPs to remain closed at neutral pH (i.e., the bloodstream), but open under mildly acidic conditions (i.e., in lysosomes), releasing their contents autonomously upon cell uptake. The new design utilizes trisammonium stalks that contain one anilinium and two –CH2NH2+CH2– centers. The anilinium nitrogen atom is approximately 106-fold less basic than the other two nitrogen atoms and so is not protonated at neutral pH. Thus, at neutral pH, the CB[6] rings resides on the –NH2+(CH2)4NH2+–recognition units, allowing both portals of the CB[6] ring to become engaged in ion-dipole binding interactions, thus blocking the nanopore orifices and encapsulating the cargo inside the SNPs. The stability constant for the complexation of –NH2+(CH2)6NH2+– units with CB[6] ring is an order of magnitude greater23 than that of – NH2+(CH2)4NH2+– units. Thus, when the pH is lowered and the anilinium nitrogen atoms become protonated, the CB[6] rings shuttle to the distal –NH2+(CH2)6NH2+– recognition units, leading to the unblocking of the nanopore orifices and the release of the encapsulated cargo. On tuning the pKa of the anilinium nitrogen by varying the para-substituent on the aryl rings, the release rate, and the pH at which the MSNPs are opened, can be adjusted very precisely. It follows that these MSNPs present an excellent opportunity to transport therapeutic molecules into various types of human cancer cells with varying lysosomal pH levels.
In 2009, we introduced24 a new category of MSNPs consisting of hollow mesoporous SNPs capped by supramolecular machines based on cyclodextrin. We prepared pH-responsive nanovalves controlled by a supramolecular system containing α-CD rings on stalks that contain aniline residues. When the α-CD rings are complexed with the stalks at neutral pH, the α-CD rings are located near the nanopore openings, thereby blocking departure of the cargo, loaded beforehand through the nanopores into the hollow interiors of the SNPs. When the nitrogen atoms on the aniline residues are protonated at lower pH, the binding affinities between the α-CD rings and the stalks are decreased, leading to the escape of the α-CD rings and allowing the cargo to be released. The ease of synthesis, straightforward operation, functional versatility, and simplicity of design are key attributes of these pH-responsive hollow particles.
In all of the valves discussed so far, the rings were movable and the stalks of the [2]pseudorotaxanes and [2]rotaxanes were immobilized on the surfaces of the MSNPs. In some of our more recent work,25 we have investigated the opposite recognition: the rings (β-CD) are immobilized and the stalks are movable. In this case, the movable rhodamine B/benzidine stalks function as nanopistons and move in and out of the cylindrical cavities provided by the β-CD rings in response to changes in pH. The fabrication process of the nanopiston system attaches the β-CD rings at the orifices of the pores and the rings become essentially an extension of the orifices, i.e., the ring openings are aligned with those of the pores. It is thus now possible to store small cargo molecules – e.g., 2,6-naphthalenedisulfonic acid dianions – within their nanopores at neutral pH and then release them by passage through the cavities of the β-CD rings. In a further investigation, the seven linkers attaching the β-CD rings to the nanopore orifices contain cleavable imine double bonds. When the imine bonds are hydrolyzed under acidic conditions (pH ≤ 6), the β-CD rings are severed from the surfaces of the SNPs, leading to the release of the larger cargo. This system has the properties of being able to release selectively small and then large molecules under pH control and provides a significant step towards the development of pH-responsive dual drug delivery.
Light Activation in Water
Since activating processes by light are rapid and directional,7 light-operated MSNPs allow for low invasiveness in biological systems. The azobenzene impellers were studied in aqueous systems and shown to deliver dye and drug molecules. The next goal was to develop a light-operated nanovalve.
Previous investigations26,27 had demonstrated a high binding affinity in aqueous solution between β-CD and trans-azobenzene derivatives and low, if any, binding between β-CD and cis-azobenzene derivatives. Based on these observations, we have functionalized28 the surfaces of the mesoporous SNPs with azobenzene-containing stalks which are complexed by β-CD rings. In the case of the SNPs carrying azobenzene-containing stalks, either β-CD rings or pyrene-modified β-CD rings can be threaded onto the stalks and bind to trans-azobenzene units, thus sealing the nanopores and stopping release of the cargo from SNPs which have already been loaded with cargo. Upon irradiation (λ = 351 nm), the isomerization of trans-to-cis azobenzene units leads to the dissociation of β-CD or pyrene-modified β-CD rings from the stalks, thus opening the gates to the nanopores and releasing the cargo. The hydrophilic character of the β-CD/azobenzene-containing MSNPs and their ability to release cargo in response to an external light source make these kinds of MSNPs potentially useful for light-operated intracellular drug delivery.
Enzymatic Activation
In parallel with the development of the CB[6]-containing MSNPs, we have designed and fabricated CD-containing MSNPs capable of operating in aqueous solution. In 2008, we described a biocompatible, enzyme-responsive motif we call snap-top covered silica nanocontainers.29 The snap-tops consist of [2]rotaxanes, in which α-CD tori encircle polyethylene glycol stalks and are held in place by cleavable stoppers – in the form of ester-linked adamantyl stoppers – tethered to the surfaces of mesoporous SNPs. When closed, the snap-tops retain the cargo stored within the nanopores, but, following enzymatic cleavage of the stoppers, dethreading of the α-CD rings occur, releasing the cargo. In our first snap-top system, we employed porcine liver esterase to catalyze30 the hydrolysis of adamantyl ester stoppers, leading to dethreading of the α-CD rings, and release of the cargo from the nanopores. This research features biocompatible MSNPs which exploit enzymatic specificity.
Redox Activation Redux
In a motif,31 resembling the MSNPs which use enzymatic activation to release cargo, an integrated snap-top system was created that utilizes mesoporous SNPs, functionalized with disulfide-containing [2]rotaxanes, to release their cargos selectively upon exposure to chemical reductants. The snap-top system relies on the reductive cleavage of disulfide bonds in the stalks, which are encircled by either CB[6] or α-CD rings. When the reduction is performed in aqueous solution by adding dithiothreitol or 2-mercaptoethanol, the snapping of the stalks leads to the cargo being released from the interiors of the SNPs. This particular integrated system is unique since it employs a highly modular approach to the piecing together of the individual building blocks that extend outward from the surfaces of the SNPs and hence is amenable rather easily to customization. It differs from previous redox-activated MSNP systems in that relies on internal biological triggers, e.g., glutathione inside of cells, to carry out the redox chemistry autonomously, and does not require the use of an external chemical reductant to operate.
Progressing into the Biological Domain
While there have been many developments in the field of cancer chemotherapy, many of the most promising drugs have limited water solubility, rendering their delivery in clinical settings tricky. Chemical modification of anti-cancer drugs to make them more water-soluble may alter their chemotherapeutic properties, possibly leading to reduced efficacy.32–34 By taking advantage of MSNPs as delivery systems, we are able to use the tried and tested drugs. Hydrophobic drugs can be loaded in the pores using nonaqueous solvents. Inside cells, hydrophobic regions, perhaps the phospholipid bilayer, allow the drugs to escape from the nanopores into the cytoplasm.35 Although employing SNPs without any external machinery allows us to test the utility of SNPs to deliver drugs intracellularly, it is limited to hydrophobic drugs, since they are contained in the nanopores without the use of molecular machines in aqueous systems. Several therapeutics, including campothecin35 (CPT), paclitaxel,36 have been shown to be capable of intracellular release in this manner (Figure 4), and can be released in response to either an external stimulus or internal biological triggers.
Figure 4.
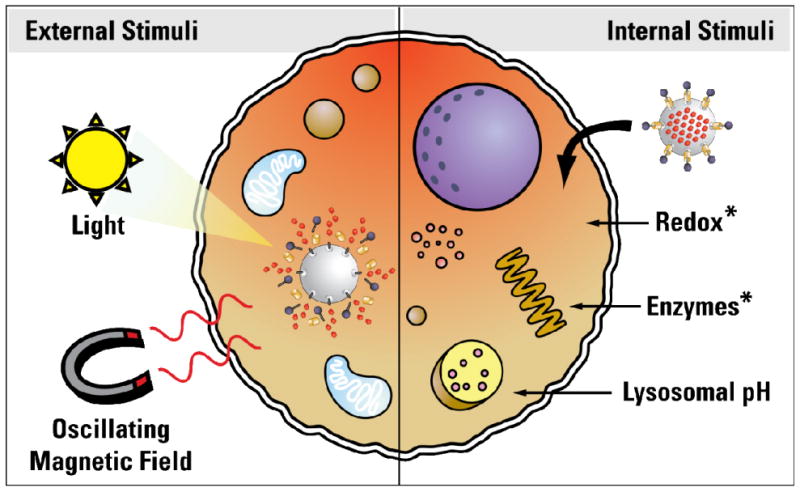
Diagram of the different methods employed for controlled release of cargos in vitro. Cargo can be released in response to external stimuli – such as light or a magnetic field – or by taking advantage of the natural biochemistry inside cells by using redox, enzymes, or a pH change in the cellular compartments to release the cargo. Redox and enzymatic activation has yet to be tested in vitro.
Initial Cell Studies
At the start, the influence of SNPs upon cells was examined by determining the toxicity of empty SNPs in various cell types. Limited toxicity is observed with the empty SNPs, suggesting that they function37–39 as nontoxic vehicles for the delivery of drugs into cells. When the pancreatic cancer cell line PANC-1 is treated with SNPs containing the hydrophobic drug, CPT, significant cell death occurs.35 In the first demonstration of MSNPs successfully operating in cells,40 MSNPs incorporating azobenzene machines (Figure 5) are loaded with CPT and irradiated with 413 nm light to activate the azobenzene cis-trans isomerization. The wagging, large-amplitude motion of the azobenzene molecules causes CPT to be expelled from the nanopores. In both the pancreatic cancer cell line PANC-1 and the colon cancer cell line SW480, the MSNPs were taken up by the cells and CPT was released, inducing cell death in both cancer cell lines. Although these light-activated MSNPs allow for an external method of control for release of drugs, any application would be limited to surface treatments because of the tissue penetration limit of the blue light used.
Figure 5.
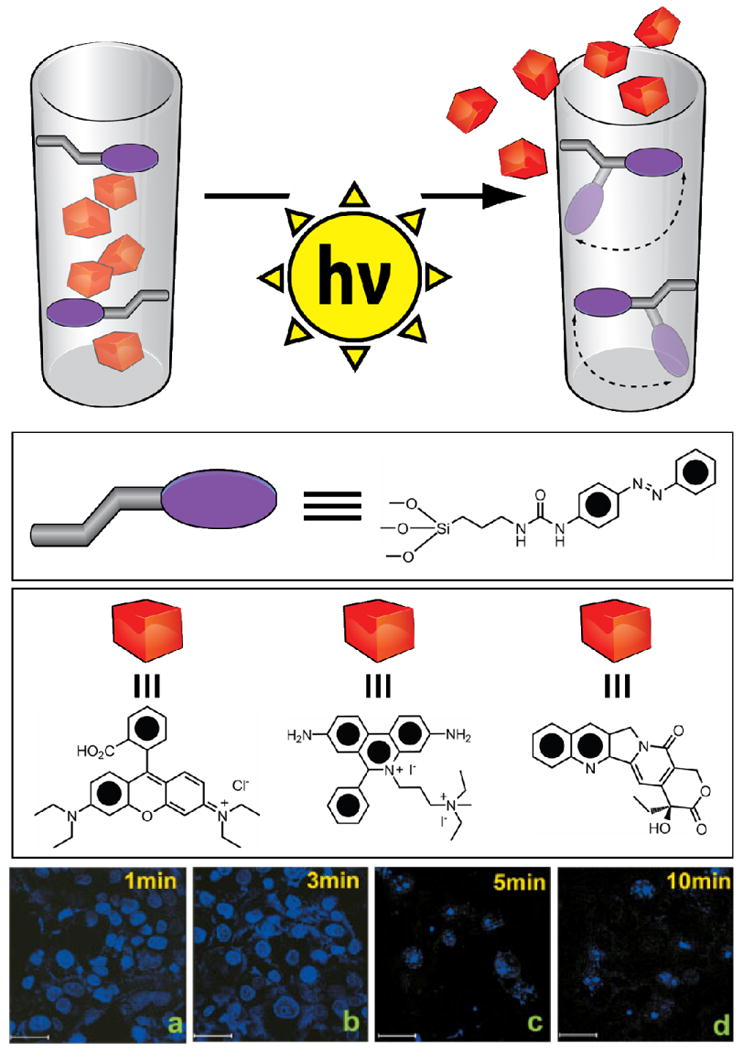
MSNPs with azobenzene-based nanoimpellers attached to the inner surface of the nanopores. When the MSNPs are uptaken into PANC-1 cells, apoptosis is induced by releasing CPT after irradiating for 1 minute (a) 3 minutes (b) 5 minutes (c) or 10 minutes (d).
Autonomous In Vitro Release of Therapeutics
An example of MSNPs, which offer41 autonomous control by acid activation contain benzimidazole in the stalks attached to their surfaces. Under neutral conditions, β-CD rings are bound to the benzimidazoles (Figure 6), but become unbound upon exposure to acidic media. Consequently, when the particles are taken up in the squamous carcinoma cell line KB-31, the lower pH of the lysosomal compartments causes the benzimidazoles to become protonated, freeing the β-CD rings from the stalks and releasing the cargo into the internal compartments of the cells. Since MSNPs can become localized in tumor cells on account of the enhanced permeability and retention (EPR) effect,42,43 drug release results from uptake into the cell compartments of the MSNPs from the bloodstream with its neutral pH. Upon entering the internal compartments of the KB-31 cells, the MSNPs were able to release cargos (Figure 6) of either Hoechst 33342 – used to image the internal compartments of cells – or doxorubicin, a powerful cancer therapeutic drug which induces apoptosis.
Figure 6.
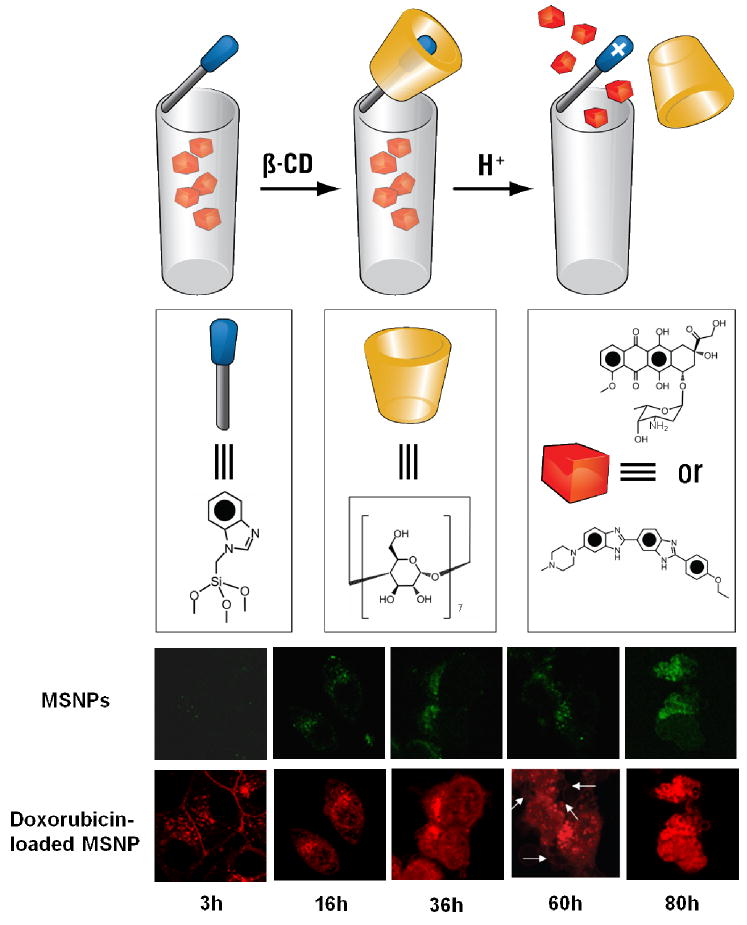
MSNPs in which stalks containing benzimidazoles are attached to the surfaces of SNPs. They are encircled by β-CD rings, and upon the addition of acid, the benzimidazoles are protonated, causing the β-CD rings to dissociate from the stalks, releasing the cargo – either Hoechst 33342 for cell imaging or doxorubicin for inducing apoptosis. KB-31 cancer cells endocytosed the doxorubicin-loaded fluorescein-labeled MSNPs within 3 h. This action is followed by doxorubicin release to the nucleus, induction of cytotoxicity and the appearance of apoptotic bodies after 60 h (indicated by arrows), followed by nuclear fragmentation after 80 h.
Controlled Release In Vitro via Magnetic Activation
MSNPs have been developed44 that incorporate multiple functionalities into a single integrated SNP. These functionalities include the addition of metal or metal oxide nanocrystals at the center of nanoparticles to form core@shell-type particles, while retaining their drug delivery capabilities. Of particular interest are nanoparticles encapsulating iron oxide nanocrystals at their centers. The inclusion of iron oxide allows for multiple uses, including drug delivery and imaging. Magnetic-core silica nanoparticles (MCSNPs) have been fabricated with thermally-responsive machines attached to their surfaces. The water-soluble drug doxorubicin was loaded into the nanopores, and the system was examined in breast cancer cells, MDA-MB-231. When exposed to an oscillating magnetic field, local heat generation induced the function of the molecular machinery (Figure 7), such that doxorubicin stored in the nanopores was released into the cancer cells, causing cell death.45 This design produces a noninvasive method for activating the nanomachines remotely, keeping the drugs contained within the nanopores until the external activation of the oscillating field is applied.
Figure 7.
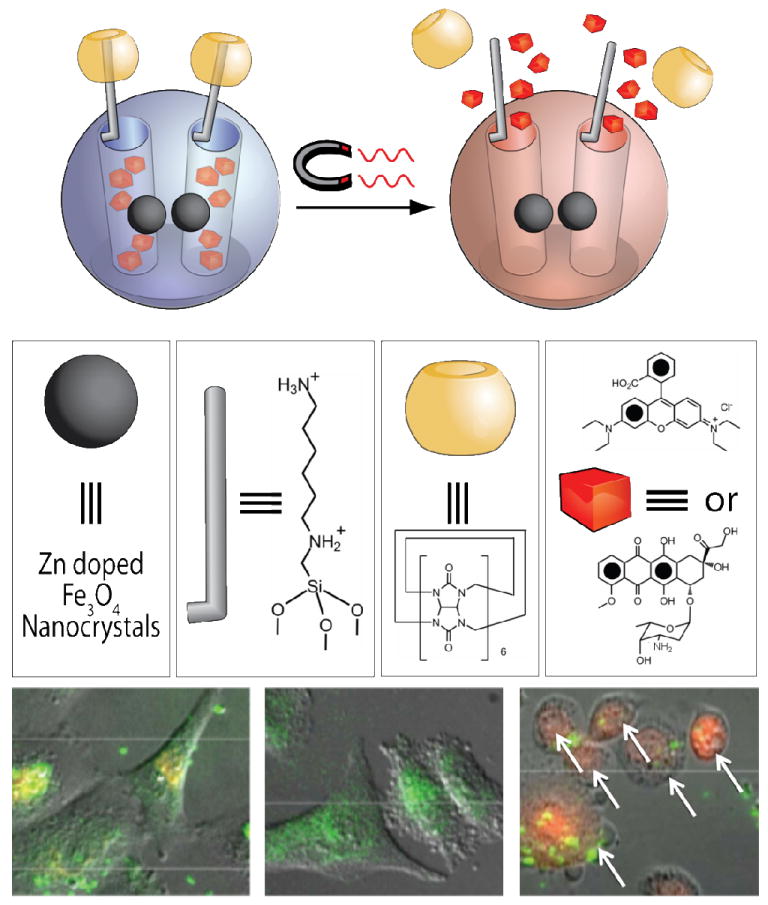
MCSNPs which generate heat upon exposure to an oscillating magnetic field, causing the CB[6] rings to slip off the stalks, thus releasing a cargo of either rhodamine B or doxorubicin. No cell death is observed prior to applying a magnetic field (left panel), while 16% of the cells were killed upon the application of an oscillating magnetic field to MCSNPs without doxorubicin loaded in the pores (center panel). When MCSNPs are loaded with doxorubicin and exposed to an oscillating magnetic field, 37% of the cells are killed, with apoptotic bodies indicated by arrows (right panel).
MCSNPs have also been examined with respect to imaging techniques. Since iron oxide nanocrystals have been shown to improve T2 contrast on MRI, T2-MRI contrast was examined using MSCNPs.44 While the addition of the silica diminishes the contrast slightly, compared with bare iron oxide nanocrystals not encapsulated within silica, the contrast is still observed. This observation indicates that MCSNPs could be used to deliver drugs and monitor tumor size, resulting in a theranostic, imaging and drug delivery system.
In Vitro Delivery of Biomolecules
The MSNP delivery platform can also be envisioned as one that is capable of delivering larger biomolecules such as DNA, RNA, and proteins to the interior of diseased cells. Indeed, it has also been shown46–48 that SNPs are capable of carrying siRNA into cells as a result of electrostatic binding of the siRNA to PEI coated on the surfaces of the SNPs. In an important study, siRNA shut down the mechanism that shuttles drugs from cell interiors and provides drug resistance to cells while simultaneously delivering an anticancer drug to enhance cell killing.48
Initial Animal Studies
SNPs have been shown49,50 to be well tolerated in animal studies, indicating that they are nontoxic and viable for in vivo drug delivery (Figure 8). In this investigation, fluorescent silica nanoparticles (FSNPs) have been used to show that the FSNPs are nontoxic in mice that were given daily doses of 1 mg of FSNPs for 10 days. Additionally, the mice were able to excrete the FSNPs, as evidenced by the elevated levels of silicon in their urine and feces, an observation which was confirmed by inductively coupled plasma optical emission spectroscopy (ICP-OES). FSNPs were also shown to be able to store and deliver a cargo of CPT to tumor tissue in tumor-bearing mice, resulting in a significant reduction of tumor volume compared with administration of CPT alone. FSNPs could also be functionalized with a targeting ligand such as a folate moiety, resulting in a slightly increased reduction in tumor size.
Figure 8.
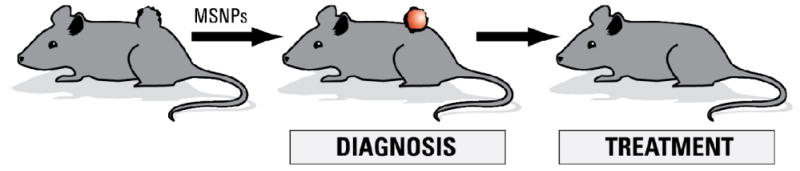
Diagram showing how MSNPs, when injected into an organism with a tumor, are capable of localizing in and around diseased tissue and display a signal (diagnosis), and release therapeutics to eliminate diseased cells (treatment), yielding a true theranostic device.
Conclusion
The field of theranostics, one which is still in the early stages of its development, stands to benefit society greatly with the implementation of nanotechnology. We began humbly by showing in 2001 that molecular machines can operate efficiently, when anchored onto a silica support, to being able to release cargo from MSNPs in solution upon activation by redox, acid / base chemistry, light, or by the application of a magnetic field. We have also shown that MSNPs can operate, not only within cells, but also in live animals in response to pre-existing biological triggers. Thus, MSNPs combine well-established concepts and techniques from a variety of sub-disciplines within physics, chemistry, and biology with the emerging discipline of nanotechnology to yield novel functional materials on the nanoscale which offer a completely fresh approach to the treatment of degenerative diseases including cancer.
Acknowledgments
We acknowledge the National Institute of Health (CA133697) for financial support and Aleksandr Bosoy for generating images for the illustrations.
Biographies
Michael W. Ambrogio was born in 1985 in the suburbs of Cleveland, Ohio. He obtained his B.Sc. in chemistry in 2007 from Miami University in Oxford, Ohio where he did research under Professor Hong-Cai Zhou. In 2007, Michael joined the research group led by Prof. J. Fraser Stoddart at Northwestern University (NU), where he is currently in his fourth year of graduate school.
Courtney R. Thomas was born in 1985 in Cedar Rapids, Iowa and grew up in Columbia, South Carolina. She attended Furman University for her undergraduate studies, and completed her B.Sc. in Chemistry in 2007, where she performed research under the direction of Professor Paul S. Wagenknecht. She is currently a Ph.D. student at the University of California, Los Angeles (UCLA) under the supervision of Prof. Jeffrey I. Zink (since 2007). During her studies, she received the UCLA Chemistry Excellence in Teaching Award, and the UCLA Chemistry Excellence in Academics and Research Award.
Yan-Li Zhao received his B.Sc. degree in Chemistry from Nankai University in 2000 and his Ph.D. degree in Physical Chemistry there in 2005 under the supervision of Professor Yu Liu. He was a postdoctoral scholar with Professor J. Fraser Stoddart at UCLA (October 2005–November 2008) and subsequently at NU (January 2010–August 2010). In between times (December 2008–December 2009), he was a postdoctoral scholar performing research work in the group of Professor Jeffrey I. Zink at UCLA. He is currently a Nanyang Assistant Professor and a National Research Foundation Research Fellow in both the School of Physical and Mathematical Sciences and the School of Materials Science and Engineering at Nanyang Technological University in Singapore.
Jeffrey I. Zink is a Distinguished Professor of Chemistry in the Department of Chemistry and Biochemistry and a member of the California Nanosystems Institute at UCLA. He received his B.Sc. degree at the University of Wisconsin and his Ph.D. degree at the University of Illinois Urbana–Champaign. His research interests include excited-state properties of metal-containing molecules, triboluminescence, and nanostructured mechanized multifunctional nanomaterials.
J. Fraser Stoddart received all (B.Sc., Ph.D., D.Sc.) of his degrees from the University of Edinburgh, UK. Presently, he holds a Board of Trustees Professorship in the Department of Chemistry at NU. His research has opened up a new materials world of mechanically interlocked molecules and, in doing so, has produced a blueprint for the subsequent growth of functional molecular nanotechnology.
References
- 1.Cotí KK, Belowich ME, Liong M, Ambrogio MW, Lau YA, Khatib HA, Zink JI, Khashab NM, Stoddart JF. Mechanised Nanoparticles for Drug Delivery. Nanoscale. 2009;1:16–39. doi: 10.1039/b9nr00162j. [DOI] [PubMed] [Google Scholar]
- 2.Klajn R, Stoddart JF, Grzybowski BA. Nanoparticles Functionalised with Reversible Molecular and Supramolecular Switches. Chem Soc Rev. 2010;39:2203–2237. doi: 10.1039/b920377j. [DOI] [PubMed] [Google Scholar]
- 3.Mal NK, Fujiwara M, Tanaka Y. Photocontrolled Reversible Release of Guest Molecules from Coumarin-Modified Mesoporous Silica. Nature. 2003;421:350–353. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]
- 4.Liu R, Zhao X, Wu T, Feng P. Tunable Redox-Responsive Hybrid Nanogated Ensembles. J Am Chem Soc. 2008;130:14418–14419. doi: 10.1021/ja8060886. [DOI] [PubMed] [Google Scholar]
- 5.Park C, Kim H, Kim S, Kim C. Enzyme Responsive Nanocontainers with Cyclodextrin Gatekeepers and Synergistic Effects in Release of Guests. J Am Chem Soc. 2009;131:16614–16615. doi: 10.1021/ja9061085. [DOI] [PubMed] [Google Scholar]
- 6.Rosenholm JM, Sahlgren C, Linden M. Towards Multifunctional, Targeted Drug Delivery Systems Using Mesoporous Silica Nanoparticles – Opportunities & Challenges. Nanoscale. 2010;2:1870–1883. doi: 10.1039/c0nr00156b. [DOI] [PubMed] [Google Scholar]
- 7.Sauer AM, Schlossbauer A, Ruthardt N, Cauda V, Bein T, Bräuchle C. Role of Endosomal Escape for Disulfide-Based Drug Delivery from Colloidal Mesoporous Silica Evaluated by Live-Cell Imaging. Nano Letters. 2010;10:3684–3691. doi: 10.1021/nl102180s. [DOI] [PubMed] [Google Scholar]
- 8.Climent E, Martínez-Máñez R, Sancenón F, Marcos MD, Soto J, Maquieira A, Amorós P. Controlled Delivery Using Oligonucleotide-Capped Mesoporous Silica Nanoparticles. Angew Chem Int Ed. 2010;49:7281–7283. doi: 10.1002/anie.201001847. [DOI] [PubMed] [Google Scholar]
- 9.Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature. 1992;359:710–712. [Google Scholar]
- 10.Trewyn BG, Slowing II, Giri S, Chen HT, Lin VSY. Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol–Gel Process and Applications in Controlled Release. Acc Chem Res. 2007;40:846–853. doi: 10.1021/ar600032u. [DOI] [PubMed] [Google Scholar]
- 11.Chia S, Cao J, Stoddart JF, Zink JI. Working Supramolecular Machines Trapped in Glass and Mounted on a Film Surface. Angew Chem Int Ed. 2001;40:2447–2451. doi: 10.1002/1521-3773(20010702)40:13<2447::AID-ANIE2447>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Huang MH, Dunn BS, Soyez H, Zink JI. In Situ Probing by Fluorescence Spectroscopy of the Formation of Continuous Highly-Ordered Lamellar-Phase Mesostructured Thin Films. Langmuir. 1998;14:7331–7333. [Google Scholar]
- 13.Hernandez R, Franville AC, Minoofar P, Dunn B, Zink JI. Controlled Placement of Luminescent Molecules and Polymers in Mesostructured Sol–Gel Thin Films. J Am Chem Soc. 2001;123:1248–1249. doi: 10.1021/ja003634e. [DOI] [PubMed] [Google Scholar]
- 14.Minoofar PN, Hernandez R, Chia S, Dunn B, Zink JI, Franville AC. Placement and Characterization of Pairs of Luminescent Molecules in Spatially Separated Regions of Nanostructured Thin Films. J Am Chem Soc. 2002;124:14388–14396. doi: 10.1021/ja020817n. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TD, Leung KCF, Liong M, Pentecost CD, Stoddart JF, Zink JI. Construction of a pH-Driven Supramolecular Nanovalve. Org Lett. 2006;8:3363–3366. doi: 10.1021/ol0612509. [DOI] [PubMed] [Google Scholar]
- 16.Leung KCF, Nguyen TD, Stoddart JF, Zink JI. Supramolecular Nanovalves Controlled by Proton Abstraction and Competitive Binding. Chem Mater. 2006;18:5919–5928. [Google Scholar]
- 17.Nguyen TD, Leung KCF, Liong M, Liu Y, Stoddart JF, Zink JI. Versatile Supramolecular Nanovalves Reconfigured for Light Activation. Adv Func Mater. 2007;17:2101–2110. [Google Scholar]
- 18.Angelos S, Choi E, Vögtle F, De Cola L, Zink JI. Photo-Driven Expulsion of Molecules from Mesostructured Silica Nanoparticles. J Phys Chem C. 2007;111:6589–6592. [Google Scholar]
- 19.Sierocki P, Maas H, Dragut P, Richardt G, Vögtle F, De Cola L, Brouwer F, Zink JI. Photoisomerization of Azobenzene Derivatives in Nanostructured Silica. J Phys Chem B. 2006;110:24390–24398. doi: 10.1021/jp0641334. [DOI] [PubMed] [Google Scholar]
- 20.Angelos S, Yang YW, Patel K, Stoddart JF, Zink JI. pH-Responsive Supramolecular Nanovalves Based on Cucurbit[6]Uril Pseudorotaxanes. Angew Chem Int Ed. 2008;47:2222–2226. doi: 10.1002/anie.200705211. [DOI] [PubMed] [Google Scholar]
- 21.Krasia TC, Steinke JHG. Formation of Oligotriazoles Catalysed by Cucurbituril. Chem Commun. 2002:22–23. doi: 10.1039/b108519k. [DOI] [PubMed] [Google Scholar]
- 22.Angelos S, Khashab NM, Yang YW, Trabolsi A, Khatib HA, Stoddart JF, Zink JI. pH Clock-Operated Mechanized Nanoparticles. J Am Chem Soc. 2009;131:12912–12914. doi: 10.1021/ja9010157. [DOI] [PubMed] [Google Scholar]
- 23.Mock WL, Pierpont J. A Cucurbituril-Based Molecular Switch. J Chem Soc, Chem Commun. 1990:1509–1511. [Google Scholar]
- 24.Du L, Liao S, Khatib HA, Stoddart JF, Zink JI. Controlled-Access Hollow Mechanized Silica Nanocontainers. J Am Chem Soc. 2009;131:15136–15142. doi: 10.1021/ja904982j. [DOI] [PubMed] [Google Scholar]
- 25.Zhao YL, Li Z, Kabehie S, Botros YY, Stoddart JF, Zink JI. pH-Operated Nanopistons on the Surfaces of Mesoporous Silica Nanoparticles. J Am Chem Soc. 2010;132:13016–13025. doi: 10.1021/ja105371u. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez AM, de Rossi RH. Effect of β-Cyclodextrin on the Thermal Cis-Trans Isomerization of Azobenzenes. J Org Chem. 1996;61:3446–3451. [Google Scholar]
- 27.Zhao YL, Stoddart JF. Azobenzene-Based Light-Responsive Hydrogel System. Langmuir. 2009;25:8442–8446. doi: 10.1021/la804316u. [DOI] [PubMed] [Google Scholar]
- 28.Ferris DP, Zhao YL, Khashab NM, Khatib HA, Stoddart JF, Zink JI. Light-Operated Mechanized Nanoparticles. J Am Chem Soc. 2009;131:1686–1688. doi: 10.1021/ja807798g. [DOI] [PubMed] [Google Scholar]
- 29.Patel K, Angelos S, Dichtel WR, Coskun A, Yang YW, Zink JI, Stoddart JF. Enzyme-Responsive Snap-Top Covered Silica Nanocontainers. J Am Chem Soc. 2008;130:2382–2383. doi: 10.1021/ja0772086. [DOI] [PubMed] [Google Scholar]
- 30.Woodroofe CC, Lippard SJ. A Novel Two-Fluorophore Approach to Ratiometric Sensing of Zn2+ J Am Chem Soc. 2003;125:11458–11459. doi: 10.1021/ja0364930. [DOI] [PubMed] [Google Scholar]
- 31.Ambrogio MW, Pecorelli TA, Patel K, Khashab NM, Trabolsi A, Khatib HA, Botros YY, Zink JI, Stoddart JF. Snap-Top Nanocarriers. Org Lett. 2010;12:3304–3307. doi: 10.1021/ol101286a. [DOI] [PubMed] [Google Scholar]
- 32.Hertzberg RP, Caranfa MJ, Holden KG, Jakas DR, Gallagher G, Mattern MR, Mong SM, Bartus JOL, Johnson RK, Kingsbury WD. Modification of the Hydroxylactone Ring of Camptothecin: Inhibition of Mammalian Topoisomerase I and Biological Activity. J Med Chem. 1989;32:715–720. doi: 10.1021/jm00123a038. [DOI] [PubMed] [Google Scholar]
- 33.Scott DO, Bindra DS, Stella VJ. Plasma Pharmacokinetics of the Lactone and Carboxylate Forms of 20(S)-Camptothecin in Anesthetized Rats. Pharmaceutical Res. 1993;10:1451–1457. doi: 10.1023/a:1018919224450. [DOI] [PubMed] [Google Scholar]
- 34.Guiotto A, Canevari M, Orsolini P, Lavanchy O, Deuschel C, Kaneda N, Kurita A, Matsuzaki T, Yaegashi T, Sawada S, Veronese FM. Synthesis, Characterization, and Preliminary in Vivo Tests of New Poly(ethylene glycol) Conjugates of the Antitumor Agent 10-Amino-7-ethylcamptothecin. J Med Chem. 2004;47:1280–1289. doi: 10.1021/jm031072e. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small. 2007;3:1341–1346. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 36.Lu J, Liong M, Sherman S, Xia T, Kovochich M, Nel A, Zink J, Tamanoi F. Mesoporous Silica Nanoparticles for Cancer Therapy: Energy-Dependent Cellular Uptake and Delivery of Paclitaxel to Cancer Cells. NanoBioTechnol. 2007;3:89–95. doi: 10.1007/s12030-008-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao Z, Toms B, Goodisman J, Asefa T. Mesoporous Silica Microparticles Enhance the Cytotoxicity of Anticancer Platinum Drugs. ACS Nano. 2010;4:789–794. doi: 10.1021/nn9015345. [DOI] [PubMed] [Google Scholar]
- 38.Souris JS, Lee CH, Cheng SH, Chen CT, Yang CS, Ho JA, Mou CY, Lo LW. Surface Charge-Mediated Rapid Hepatobiliary Excretion of Mesoporous Silica Nanoparticles. Biomater. 2010;31:5564–5574. doi: 10.1016/j.biomaterials.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VSY. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small. 2010;6:1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Choi E, Tamanoi F, Zink JI. Light-Activated Nanoimpeller-Controlled Drug Release in Cancer Cells. Small. 2008;4:421–426. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng H, Xue M, Xia T, Zhao YL, Tamanoi F, Stoddart JF, Zink JI, Nel AE. Autonomous in Vitro Anticancer Drug Release from Mesoporous Silica Nanoparticles by pH-Sensitive Nanovalves. J Am Chem Soc. 2010;132:12690–12697. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 43.Muggia FM. Doxorubicin-Polymer Conjugates: Further Demonstration of the Concept of Enhanced Permeability and Retention. Clin Cancer Res. 1999;5:7–8. [PubMed] [Google Scholar]
- 44.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas CR, Ferris DP, Lee JH, Choi E, Cho MH, Kim ES, Stoddart JF, Shin JS, Cheon J, Zink JI. Noninvasive Remote-Controlled Release of Drug Molecules in Vitro Using Magnetic Actuation of Mechanized Nanoparticles. J Am Chem Soc. 2010;132:10623–10625. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- 46.Hom C, Lu J, Liong M, Luo H, Li Z, Zink JI, Tamanoi F. Mesoporous Silica Nanoparticles Facilitate Delivery of siRNA to Shutdown Signaling Pathways in Mammalian Cells. Small. 2010;6:1185–1190. doi: 10.1002/smll.200901966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI, Nel AE. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs. ACS Nano. 2009;3:3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein siRNA to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor KML, Kim JS, Rieter WJ, An H, Lin W, Lin W. Mesoporous Silica Nanospheres as Highly Efficient MRI Contrast Agents. J Am Chem Soc. 2008;130:2154–2155. doi: 10.1021/ja710193c. [DOI] [PubMed] [Google Scholar]
- 50.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small. 2010;6:1794–1805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]


