Abstract
Recent work shows that cytokinesis and other cellular morphogenesis events are tuned by an interplay among biochemical signals, cell shape, and cellular mechanics. In cytokinesis, this includes cross-talk between the cortical cytoskeleton and the mitotic spindle in coordination with cell cycle control, resulting in characteristic changes in cellular morphology and mechanics through metaphase and cytokinesis. The changes in cellular mechanics affect not just overall cell shape, but also mitotic spindle morphology and function. This review will address how these principles apply to oocytes undergoing the asymmetric cell divisions of meiosis I and II. The biochemical signals that regulate cell cycle timing during meiotic maturation and egg activation are crucial for temporal control of meiosis. Spatial control of the meiotic divisions is also important, ensuring that the chromosomes are segregated evenly and that meiotic division is clearly asymmetric, yielding two daughter cells – oocyte and polar body – with enormous volume differences. In contrast to mitotic cells, the oocyte does not undergo overt changes in cell shape with its progression through meiosis, but instead maintains a relatively round morphology with the exception of very localized changes at the time of polar body emission. Placement of the metaphase-I and -II spindles at the oocyte periphery is clearly important for normal polar body emission, although this is likely not the only control element. Here, consideration is given to how cellular mechanics could contribute to successful mammalian female meiosis, ultimately affecting egg quality and competence to form a healthy embryo.
Keywords: oocyte, fertilization, meiotic maturation, cytokinesis, cell cortex, cytoskeleton, spindle, myosin II, ezrin-radixin-moesin
In all cells, one of most fundamental functions of the cortex (i.e., the cortical region of the cell, the region underlying the plasma membrane) is the regulation of the cell’s shape and mechanical properties. These regulatory events in the cell are central to nearly all aspects of cellular function, and are relevant to multicellular structures, such as during contraction of muscles, wound healing, gastrulation, and organogenesis, and to individual cells, in processes such as cell migration and cell division. A key function of the cortex comes into play specifically during cytokinesis, which requires the coordinated actions of the cortical cytoskeleton and the spindle. The spindle provides cues for its own localization as well the subsequent localization of the cleavage machinery, and the cortex provides cues to the spindle as well. Recent work shows that cytokinesis and other cellular morphogenesis events are tuned by an intricate interplay between three linked elements: (a) biochemical signals, (b) cell shape and morphology, and (c) cellular mechanics (Fig. 1A, B). Biochemical signals include events that are typically considered components of cellular signal transduction cascades, such as a post-translational modification that activates or inactivates a substrate, the generation of cAMP by adenylate cyclase, or an increase in cytosolic calcium ions through influx from the extracellular space or release from intracellular calcium stores. The intersection of these three elements is apparent in a variety of cellular processes. For example, tissue injury triggers a dramatic response of cell proliferation and wound healing, both of which involve coordinated intracellular signaling, cell shape changes, and modulation of cellular mechanics. This tripartite control system has been characterized in studies of Dictyostelium cell shape and cytokinesis, showing that these interacting biochemical and mechanical modules impact cell morphology, and these three elements form a series of feedback loops (Surcel et al. 2010) (addressed in more detail below). Features of this control system are now readily recognizable in other cells types and organisms, suggesting that this may be a universal, fundamental principle for cytokinesis regulation (Reichl et al. 2005; Ren et al. 2009; Zhang and Robinson 2005). In this review, we will address how these principles apply to oocytes.
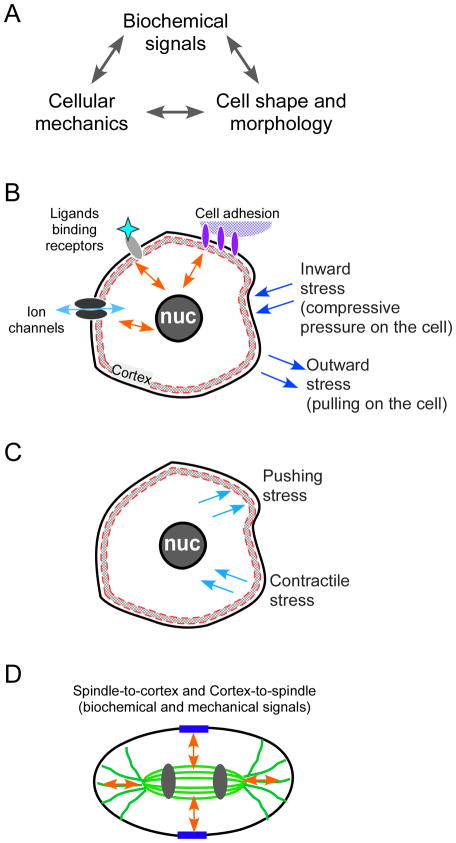
Figure 1. Principles governing cell shape change.
The core principle being addressed here is that cellular morphogenesis (including cell shape in oocytes during their progression through meiosis) is governed by three inextricably linked elements: biochemical signals and pathways, cell shape, and cell mechanics (Panel A). The schematic diagrams in Panels B–D illustrate these signals in a cellular context. There are a myriad of extrinsic signals to the typical cell that govern cellular behavior (Panel B), including biochemical inputs from receptor-mediated signaling upon ligand binding, signals generated by cell adhesion (cell-to-extracellular matrix, illustrated here, or cell-to-cell), and ion transport. Perhaps less appreciated and addressed in cell biology textbooks, but no less important, is the collection of cellular responses to internal and externally imposed stress (which can be thought of as a force per area). Cellular sensing of mechanical cues (also known as mechanosensing) can occur through (a) stretch-activated channels (Martinac 2004), (b) adhesion-associated proteins (Geiger et al. 2009; Moore et al. 2010), and (c) through myosin motors, which are force-transmitting enzymes (Kee and Robinson 2008; Ren et al. 2009). Moreover, there also can be intrinsic mechanical stresses generated by the cytoskeleton in the cortex and throughout the cytoplasm (Panel C). Finally, there are specialized cues occurring in a mitotic cell, with the mitotic spindle in metaphase and the central spindle in anaphase (illustrated in Panel D) sending biochemical and mechanical signals to the cortex, as well as the cortex signaling to the spindle.
Introduction to cellular mechanics during mitosis
A key metric of cellular mechanics is cortical tension, defined as the force in the cortex and overlying plasma membrane that serves to minimize the surface area to volume ratio, and is comprised of all the mechanical stresses, generated intrinsically as well as extrinsically, at the surface of the cell (Derganc et al. 2000; Evans and Yeung 1989) (Fig. 1B, C). The study of cellular mechanics during mitosis in early embryos dates to the 1930s–1950s with investigations of echinoderm and amphibian zygotes (Cole 1932; Cole and Michaelis 1932; Mitchison and Swann 1954; Mitchison and Swann 1955; Selman and Waddington 1955), and more recently has been extended to multiple types of mitotic cells. Cytokinesis, along with the spindle functions associated with cytokinesis, is a fascinating model for examining the intersections between these three elements noted above (Fig. 1A), as a mitotic cell undergoes coordinated changes in cell shape, mechanical properties, and biochemistry with progression through the cell cycle. These early studies identified an increase in tension occurring at the beginning of embryonic cleavage in zygotes (Mitchison and Swann 1955; Selman and Waddington 1955), which is similarly observed in numerous other cell types, with a characteristic rounding and cortical stiffening occurring with entry into M-phase (D’Avino et al. 2005; Effler et al. 2007; Kunda et al. 2008; Matzke et al. 2001; Reichl et al. 2005; Stewart et al. 2011) (Fig. 2A). Cell rounding is accompanied by entry into metaphase, when the cell elongates, which includes the orientation of the mitotic spindle parallel to the cell’s long axis (Gibson et al. 2011; Minc et al. 2011) (Fig. 2A). In anaphase and telophase, cues from the central spindle aid in cleavage furrow organization (Fig. 1D), which is accompanied by an increase in the apparent cortical stiffness in the furrow region and ultimately leads to myosin II-mediated contraction during cytokinesis (Reichl et al. 2008) (Fig. 1D, 2A). Thus, mitosis is characterized by dramatic changes in cellular mechanics and cell shape, and these changes in cellular mechanics affect not just overall cell shape, but also mitotic spindle morphology and function.
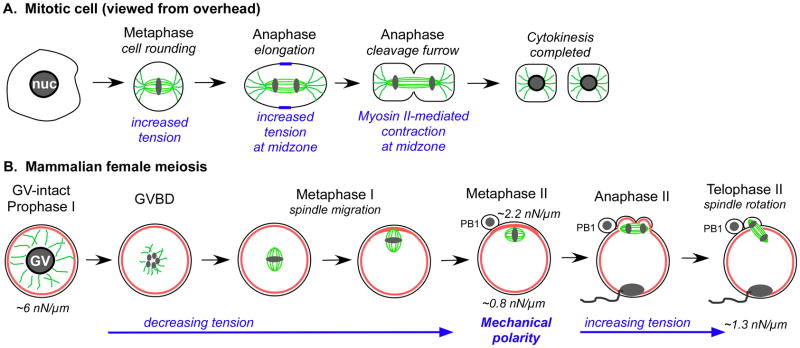
Figure 2. Comparisons of changes occurring during mitosis and mammalian female meiosis.
Schematic diagrams showing cell shape and mechanics changes occurring in a mitotic cell (A) and in a mouse oocyte going through meiosis (B), illustrating the changes that occur through these transitions. Panel A depicts the basic morphological changes and associated mechanical events of a mitotic cell, while Panel B diagram depicts the basic morphological events and mechanical transitions that occur during mouse female meiosis. Tubulin is shown in green, and actin is red.
The mitotic cell system is governed by a system of feedback loops that are characterized by cross-talk between the cortex and the mitotic spindle. Initially, it was observed in Drosophila spermatocytes that perturbations of the proteins associated with the central spindle microtubules disrupted the formation of the contractile ring network. Conversely, disruption of proteins thought to be involved in contractile ring assembly led to central spindle defects (Giansanti et al. 1998). These observations were interpreted to mean that the spindle microtubules and the contractile ring interact cooperatively. Further, cortical myosin II helps the mitotic spindle elongate during anaphase of mammalian cell culture cells (Rosenblatt et al. 2004). More recently in Dictyostelium, a 14-3-3 protein, a cortically enriched, small acidic protein that interacts with many proteins in the cell, was shown to integrate the microtubule network and cortical Rac-family small GTPase to regulate cortical myosin II (Zhou et al. 2010). Further, the 14-3-3 protein was similarly required to maintain normal microtubule structure, indicating that 14-3-3 acts at a nexus between the microtubule and cortical networks. Finally, because the myosin II distribution is exquisitely sensitive to mechanical stress and given these various modes of cross-talk, the entire contractile and microtubule networks are poised to be governed through feedback loops (Surcel et al. 2010).
The perspective of the oocyte
In contrast to what is known about the mechanical properties of mitotic cells, there has been only limited information about mechanical transitions during female meiosis. Nevertheless, female meiosis is of considerable interest because of the challenges of this distinctive cell cycle and its accompanying cell divisions (although meiosis is actually not a cycle per se, and instead is a “one-way trip” to create a haploid cell), as well as because of the importance of meiosis to overall reproductive success.
The female gamete is a fascinating case to study when it comes to cell divisions, due to the unique requirements of female meiosis and the temporal and spatial regulation of the meiotic divisions. Oocytes in all species arrest during prophase of meiosis I; this arrest, which is crucial for oocyte growth, can last for days to years, depending on the species. The timing of progression out of this prophase I arrest must be carefully regulated, both in terms of the oocyte itself, dependent on whether or not the oocyte has reached the appropriate stage of growth, and in terms of overall reproductive physiology (e.g., environmental cues for reproductive season, spawning behavior, or the presence of an appropriate mate; hormonal cues regulating the estrus/menstrual cycle). Species also differ in whether their oocytes progress through two successive meiotic divisions from a single cue or arrest a second time. Some species’ oocytes exit from prophase-I arrest in response to fertilization (e.g., surf clam Spisula and the marine worm Urechis). Most vertebrate oocytes exit from prophase-I arrest upon release from the ovarian follicle, and progress to a second meiotic arrest at metaphase II, remaining at this stage until fertilization by sperm. Other species’ oocytes have a second arrest in metaphase I (ascidian, dog, fox); in some of these species, the oocytes maintain arrest until fertilization, while in other species, the oocytes are typically fertilized at metaphase I, but are also able to progress past metaphase I even in the absence of fertilization (starfish, C. elegans, Drosophila).
No matter what the timing or regulation of progression through meiosis, there are very strict demands on meiotic divisions in all oocytes. The chromosomes must be segregated evenly between the daughter cells during the meiotic divisions, whereas the other cellular contents must be distributed very asymmetrically, so that the egg cytoplasm retains the materials that were stockpiled during oogenesis to support early embryogenesis. Thus, as shown in Figure 2B, the meiotic divisions create a large egg and small polar bodies. This also makes the oocyte’s cell divisions an interesting model for asymmetric cell divisions in general, relevant to numerous developmental and differentiation events (Grill 2010; Gönczy 2008). While the fates of the polar bodies differ among species (Schmerler and Wessel 2011), a conserved aspect of polar body function is the elimination of one set of chromosomes, leaving the haploid maternal genome component in the oocyte to merge with the haploid paternal contribution. These phenomena are crucial for reproductive success, as defects in processes during meiosis I or II (e.g., polar body emission, organization and stability of the metaphase I or II spindles) can compromise egg quality and egg competence to form a healthy embryo. The process of polar body formation is part of this, as defects in this process of segregation of oocyte cytoplasmic components have been observed in numerous instances, some of which are associated with female infertility or subfertility (e.g., De Santis et al. 2005; Levi et al. 2010; Luo et al. 2010; Sharan et al. 2004; Ubaldi and Rienzi 2008).
How to make a polar body
Taking all these demands on the meiotic divisions into account, how does the oocyte complete cytokinesis so that the chromosomes are distributed correctly while undergoing the necessary asymmetric cell divisions to create the egg and the polar bodies? The answer to this fundamental question is not as simple as might be assumed. Spindle positioning certainly is one component of this. During meiosis I in mammalian oocytes, the spindle organizes around the DNA, approximately in the center of the oocyte, then moves to the cell cortex (Brunet and Maro 2005). The actin cytoskeleton, along with Mos kinase but not microtubules, is required for the cortical migration of the spindle (Brunet and Maro 2005). Interestingly, in mos-null oocytes, the first polar body formed around one pole of a symmetrically placed spindle, producing a much larger than normal polar body (Verlhac et al. 2000). Multiple proteins, including formin-2 and the small GTPases Rac, Cdc42, and Ran, have now been implicated in spindle localization, spindle migration, and/or the remodeling of the oocyte cortex in response to underlying chromatin (Deng et al. 2007; Dumont et al. 2007; Halet and Carroll 2007; Leader et al. 2002; Na and Zernicka-Goetz 2006). In meiosis II, the metaphase-II spindle in the arrested egg needs to be maintained in this peripheral localization, sequestered in a portion of the oocyte cytoplasm adjacent to the cortex (Fig. 2B). While the placement of the metaphase-I and -II spindles at the oocyte periphery is clearly important for polar body emission, this is far from a complete picture. As illustrated in Figure 3A, from a cellular mechanics standpoint, it is rather remarkable that this cell division occurs at all. The radius of a mouse egg (r1) is ~34–38 μm whereas the radius of the polar body (r2) is ~10 μm (Barrett and Albertini 2007). The cortical tension of the oocyte (T1) and the cortical tension of the polar body (T2) combined with the average local membrane curvature (κ = 2/r) would be predicted, if T1 and T2 are similar, to yield a pressure differential (P = κ T) across the neck of the polar body that would disfavor the emergence of the polar body. The principles of fluid dynamics predict that the polar body would likely collapse into the oocyte.
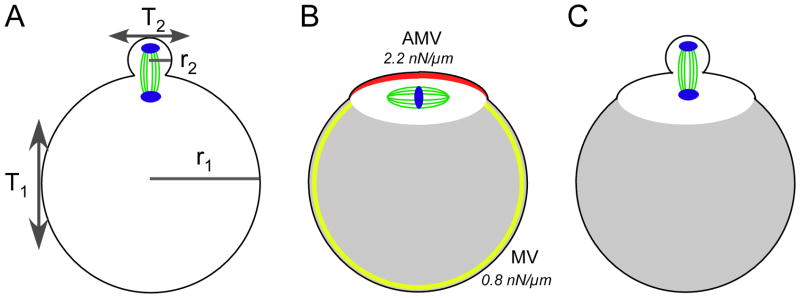
Figure 3. Mechanical and cytoskeletal considerations for polar body emission.
Panel A: Cytokinesis during mammalian female meiosis yields a daughter polar body that is ~2–4% of the volume of the oocyte. The radius of a mouse egg (r1) is ~34–38 μm whereas the radius of the polar body (r2) is ~10 μm (Barrett and Albertini 2007). The cortical tension of the oocyte (T1) and the cortical tension of the polar body (T2) combined with the average local membrane curvature (κ= 2/r) would be predicted, if T1 and T2 are similar, to yield a pressure differential (P = κ T) across the neck of the polar body that would disfavor the emergence of the polar body. The metaphase-II mouse egg, however, sets up a mechanical polarity (Larson et al. 2010), creating a microdomain for sequestration of the metaphase-II spindle. This mechanical polarity is contributed to by actin (which is present throughout the cortex and enriched in the amicrovillar domain; shown in red in Panel B), combined with the activity of myosin-II, and by ERM proteins (enriched in the microvillar domain; shown in yellow in Panel B). This microdomain surrounding the spindle (shown in white opposite the gray shading in Panels B and C) likely leads to contractile stresses pulling towards the polar body, thereby overcoming the inward pressure from the polar body (Panel C).
This sets up a consideration of the three interacting cues for cellular morphogenesis (i.e., the triumvirate of biochemistry, cell shape, and mechanics (Fig. 1A)). Mitotic cells manage their cell divisions in part through regulation of cell shape, through the coordination of biochemical and mechanical signals (Figs. 1, 2). On the other hand, the mammalian oocyte does not undergo overt changes in cell shape with its progression through meiosis, consistently maintaining a relatively simple, round morphology with the exception of very localized changes at the time of polar body emission. It should be pointed out here that in Xenopus oocytes, the astral microtubules near the cortex define an inner zone of activated cdc42, which leads to new actin assembly, while activated Rho is found in a flanking, outer ring, where myosin II and stable actin filaments are found (Ma et al. 2006; Zhang et al. 2008). While these localizations unquestionably shed light on the biochemical underpinnings of polar body formation, a fundamental question is how do these molecular scale dynamics lead to the relevant changes in cellular mechanics that drive the polar body to form. In other words, how do the mechanics, which ultimately form the polar body, fit in? Little is known about the mechanical properties of a meiotic cell. The original work on cellular mechanics during meiosis was performed on starfish oocytes, which enter and complete oocyte meiotic maturation (and the two cell divisions) in response to application of 1-methyladenine. These studies revealed transient increases in global tension immediately preceding cytokinesis to produce the two polar bodies (Hiramoto 1976; Ikeda et al. 1976; Shôji et al. 1978). However, there was no information on possible asymmetries in tension, which could be important considering how dramatically asymmetric cytokinesis is during female meiosis. Furthermore, as noted above, mammalian female meiosis has more complex temporal regulation than that of echinoderms. In contrast to starfish oocytes, mammalian oocytes progress through meiosis in a very staggered fashion — exit from prophase-I arrest leads to an extended prometaphase/metaphase I (lasting much longer than metaphase for the typical mitotic cell or metaphase in a meiotic starfish oocyte, both of which last ~10–20 min), to an arrest at metaphase II; exit from metaphase II is triggered by fertilization.
Our own recent work has characterized cortical tension in mouse oocytes (Larson et al. 2010). Although oocytes do not undergo striking changes in cell shape during meiosis, we find that mouse oocytes do undergo dramatic changes in cortical mechanics during the key meiotic transitions (Larson et al. 2010). There is a ~6-fold decrease in cortical tension during meiotic maturation from prophase I to metaphase II, and then a ~1.6-fold increase upon fertilization and egg activation from metaphase II to embryonic interphase (Fig. 2B). Interestingly, the tension level in prophase-I oocytes, ~5–6 nN/μm, makes them among the more rigid cell types studied (Hochmuth 2000). At metaphase II arrest, there is a nearly 2.5-fold mechanical polarity in the egg, with higher tension in the spindle-sequestering amicrovillar domain as compared to the microvillar domain, which supports sperm interaction (Fig. 2B, 3C).
The molecular players in oocyte cortical tension as well as the consequences of aberrant tension have started to be uncovered as well. Perturbation of the function of actin, myosin-II, or the family of actin-to-membrane tethering proteins known as ERMs (for the three family members, ezrin, radixin, and moesin) reduces cortical tension in mouse eggs and also causes significant defects in spindle function during exit from metaphase II arrest upon fertilization, with failures in spindle rotation and polar body emission (Larson et al. 2010).
This function of myosin II (Fig. 3B) in mouse oocyte mechanics is consistent with myosin-II function in cortical tension and cell shape changes during mitosis in other cell types (Carreno et al. 2008; Kunda et al. 2008; Lucero et al. 2006; Pasternak and Elson 1985; Pasternak et al. 1989). The members of the ERM family mediate actin-membrane interactions (Bretscher et al. 2002; Fehon et al. 2010), although their roles in cell polarity and cellular mechanics are also starting to be appreciated. Flies deficient in Moesin (the only Drosophila ERM protein) have abnormalities in oocyte cell shape, oocyte polarity, actin organization, and localization of certain maternal determinants such as Oskar and Staufen (Jankovics et al. 2002; Polesello et al. 2002). RNAi studies of Drosophila S2 cells and of Dictyostelium cells show that ERM proteins contribute to cortical mechanics (moesin in Drosophila (Carreno et al. 2008; Kunda et al. 2008), and enlazin, the closest ERM relative in Dictyostelium (Octtaviani et al. 2006)). It is interesting to note that radixin is among the most abundant mRNAs detected in mouse oocyte transcriptomes (e.g., Evsikov et al. 2006; Unigene library IDs 18552, 10029, 14142), and also appears to be an abundant protein, ~8-fold more abundant in oocytes than in liver, which is the tissue where phenotypic abnormalities were reported for the Radixin-null mouse (Kikuchi et al. 2002). Although no fertility defects have been reported for Radixin-null females, it should be noted that (a) no extensive analysis of reproductive function was reported for Radixin-null animals, and (b) mouse eggs also express ezrin and moesin and the three ERM knockouts suggest that there is significant functional overlap between ezrin, radixin and moesin, with each of these knockouts having very tissue-specific phenotypes in the tissues where an individual family appears to be solely or prominently expressed (Doi et al. 1999; Kikuchi et al. 2002; Saotome et al. 2004).
The discovery of the nearly 2.5-fold mechanical differential between the microvillar and amicrovillar domains in mouse eggs (Fig. 3C) sheds important light on a paradox of cytokinesis biology. Mitotic cytokinesis that is producing symmetrically sized daughter cells appears to be exquisitely sensitive to pressure imbalances induced by mechanical stress. Studies of mitotic Dictyostelium cells using micropipette aspiration to induce mechanical stress show that the cell responds to the mechanical stress by triggering a local accumulation of a network of contractile proteins at the aspiration site; this myosin-II-enriched region then retracts back into the cell (Effler et al. 2006; Ren et al. 2009) (see Figure 4 in Effler et al. 2006). In a similar fashion, myosin-II accumulates in the polar cortex of the polar body with progression into telophase (Simerly et al. 1998), which makes it all the more intriguing that the asymmetric cell division of polar body emission occurs rather than have the polar body retract back into the egg, as the myosin-II-enriched region in a mitotic cell does (Effler et al. 2006; Reichl et al. 2008; Ren et al. 2009). Our recent results (Larson et al. 2010) suggest that the egg may deal with this issue in advance of cytokinesis, during metaphase II arrest, by establishing the amicrovillar domain at metaphase II that serves to isolate the spindle and thus the developing polar body from the rest of the egg to allow myosin-II-mediated contraction to locally deform the cortex, facilitating asymmetric cell division (Fig. 3C, D). Similar actin-rich cortical domains are found overlying the spindle in oocytes of other organisms such as the surf clam (Pielak et al. 2004), suggesting that the mechanical differential may prove to be a principle of polar body formation that extends beyond mammalian cells.
Other possible implications of mechanics in oocytes
An additional finding of this recent work on mammalian oocyte mechanics (Larson et al. 2010) was that the mechanical characteristics of metaphase II eggs matured in vivo (i.e., ovulated eggs collected from the oviducts) differ from those of metaphase II eggs matured in vitro (i.e., prophase I oocytes collected from ovaries, and then cultured in conditions that do not maintain high protein kinase A activity, leading to exit from prophase-I arrest (Cho et al. 1974)). Specifically, the effective tension in the amicrovillar domain in in vitro matured metaphase-II eggs is significantly lower than the effective tension in the amicrovillar domain in in vivo matured (ovulated) metaphase-II eggs (2.0 nN/μm and 2.3 nN/μm, respectively (Larson et al. 2010)). This is especially interesting in light of the body of work showing morphological differences between in vivo- and in vitro-matured eggs, including differences in egg and polar body sizes, and differences in the metaphase-II spindles (Barrett and Albertini 2007; Sanfins et al. 2003; Sanfins et al. 2004). Spindles in ovulated eggs tend to be shorter and often with narrower poles as compared to the spindles in in vitro matured eggs, which are longer with broader poles (Barrett and Albertini 2007; Sanfins et al. 2003; Sanfins et al. 2004). This raises the possibility that amicrovillar tension may be a contributing factor associated with these differences in spindle morphology, along with the difference in γ-tubulin that has also been characterized (Barrett and Albertini 2007). This will be an interesting area for future study with implications for cell biology as well as potentially for clinical practice in assisted reproductive technology clinics that use in vitro oocyte maturation methods.
A question to be considered is if cortical tension in the egg could impact not just cytokinesis and polar body emission, but fertilization. At the very least, the discovery of mechanical polarity in the metaphase-II mouse egg brings a new appreciation of cell polarity in this cell type. The microvillar and amicrovillar domains have well-characterized differences in molecular composition and functionality, with polarized distributions of a number of proteins (actin being just one of them), cortical granules, and microvilli (Azoury et al. 2008; Brunet and Maro 2005; Longo and Chen 1984; Longo and Chen 1985; Nicosia et al. 1977). Most notably, sperm-egg fusion occurs preferentially on the microvillar domain (Yanagamachi 1978; Yanagimachi 1988). Also of interest is the membrane block to polyspermy, the post-fertilization decrease in egg membrane receptivity to sperm resulting in a membrane that does not support sperm interactions (Gardner and Evans 2006). As noted, our work showed that the microvillar domain has lower tension than does the amicrovillar domain, and also that the early zygote has higher tension level than does the unfertilized egg (Larson et al. 2010), raising the question of whether or not the level of cortical tension contributes to the spatial and temporal regulation of sperm-egg interaction. But prophase-I oocytes have even higher tension levels than do early embryos or the amicrovillar domain of eggs, and prophase-I oocytes can be fertilized. Based on this, high tension does not appear to be sufficient to make a membrane, either of the zygote or in the amicrovillar domain of an egg, impenetrable by sperm. However, it is possible that cortical tension plays a role in the ability of the oocyte membrane and cortex to respond to sperm; for example, we have found that zona pellucida-free, prophase-I oocytes become highly polyspermic when inseminated due, at least in part, to deficiencies in membrane block establishment (Klein, Moraine, and Evans, unpublished data). A putative function of cortical tension in the ability of oocytes to respond to sperm (known as “activation competence,” a component of developmental competence (Ducibella 1998; Swain and Pool 2008)), remains to be fully characterized, but could be a fruitful area considering that (a) many events of the egg-to-embryo transition initiate in the egg cortex and (b) both cortical tension and activation competence differ dramatically between prophase I and metaphase II.
It is starting to be appreciated (or re-appreciated) that mechanical cues as well as mechanical responses can play important roles in cellular physiology, and a mechanical stress can be just as significant as a biochemical event like signaling from a ligand binding to a receptor, the increase in concentration of a cytosolic second messenger like Ca2+ or cAMP, or the activation of a kinase. The sensing of a mechanical stress is known as mechanosensing, and mechanosensing, along with mechanotransduction, comprise the processes by which cells sense and respond to mechanical cues (and these responses could be mechanical or biochemical) and/or undergo a mechanical change in response to a cue (and the cue could be mechanical or biochemical). Known modes of mechanosensing include stretch-activated channels (Martinac 2004), mechanosensitive adhesion-associated proteins (Geiger et al. 2009; Moore et al. 2010), and force transmission through myosin motors (Kee and Robinson 2008; Ren et al. 2009). This first mode of mechanosensing appears to be important in Drosophila oocytes, as mechanical stimulation during ovulation and passage of the oocyte through the narrow female reproductive tract likely is part of the pathway that triggers the oocyte-to-embryo transition, possibly by allowing influx of extracellular Ca2+ into the oocyte (Horner and Wolfner 2008). Our work implicates myosin-II-based contractility in mouse oocyte mechanics (Larson et al. 2010), and this third mode of myosin-II-based mechanosensing, may be functioning in oocytes during progression through meiosis.
In summary, cellular mechanics is returning to the forefront as a crucial subdiscipline in biology. This is underscored by the rich history of studying mechanics in early invertebrate embryos starting in the first part of the 20th century, which in turn provided a foundation for the more recent studies of mouse oocytes noted here (Larson et al. 2010). Taken together, the work suggests that the mechanical properties of the oocyte are critical for successful progression through cytokinesis, polar body emission, and spindle morphology, and more broadly raises the possibility that oocyte mechanics may prove to be a marker linked with egg health and quality.
Acknowledgments
Funding: This work was supported by NIH grants HD045671 and GM066817.
This work was supported by NIH grants HD045671 to J.P.E. and GM066817 to D.N.R.
References
- Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol. 2008;18:1514–1519. doi: 10.1016/j.cub.2008.08.044. [DOI] [PubMed] [Google Scholar]
- Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–957. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Brunet S, Maro B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction. 2005;130:801–811. doi: 10.1530/rep.1.00364. [DOI] [PubMed] [Google Scholar]
- Carreno S, Kouranti I, Glusman ES, Fuller MT, Echard A, Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J Cell Biol. 2008;180:739–746. doi: 10.1083/jcb.200709161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WK, Stern S, Biggers JD. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool. 1974;187:383–386. doi: 10.1002/jez.1401870307. [DOI] [PubMed] [Google Scholar]
- Cole KS. Surface forces of the Arbacia egg. J Cell Comp Physiol. 1932;1:1–9. [Google Scholar]
- Cole KS, Michaelis EM. Surface forces of fertilized Arbacia eggs. J Cell Comp Physiol. 1932;2:121–126. [Google Scholar]
- D’Avino PP, Savoian MS, Glover DM. Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J Cell Sci. 2005;118:1549–1558. doi: 10.1242/jcs.02335. [DOI] [PubMed] [Google Scholar]
- De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Boroni A, Coticchio G. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005;11:36–42. doi: 10.1016/s1472-6483(10)61296-5. [DOI] [PubMed] [Google Scholar]
- Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev Cell. 2007;12:301–308. doi: 10.1016/j.devcel.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Derganc J, Božic B, Sventina S, Žekš B. Stability analysis of micropipette aspriation of neutrophils. Biophys J. 2000;79:153–162. doi: 10.1016/S0006-3495(00)76280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y, Itoh M, Yonemura S, Ishihara S, Takano H, Noda T, Tsukita S. Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J Biol Chem. 1999;274:2315–2321. doi: 10.1074/jbc.274.4.2315. [DOI] [PubMed] [Google Scholar]
- Ducibella T. Biochemical and cellular insights into the temporal window of normal fertilization. Theriogenology. 1998;49:53–65. doi: 10.1016/s0093-691x(97)00402-0. [DOI] [PubMed] [Google Scholar]
- Dumont J, Million K, Sunderland K, Rassinier P, Lim H, Leader B, Verlhac MH. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev Biol. 2007;301:254–265. doi: 10.1016/j.ydbio.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Effler JC, Iglesias PA, Robinson DN. A mechanosensory system controls cell shape changes during mitosis. Cell Cycle. 2007;6:30–35. doi: 10.4161/cc.6.1.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effler JC, Kee YS, Berk JM, Tran MN, Iglesias PA, Robinson DN. Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr Biol. 2006;16:1962–1967. doi: 10.1016/j.cub.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56:151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evsikov AV, Graber JH, Brockman JM, Hampl A, Holbrook AE, Singh P, Eppig JJ, Solter D, Knowles BB. Cracking the egg: molecular dynamics and evolutionary aspects of the transition from the fully grown oocyte to embryo. Genes Dev. 2006;20:2713–2727. doi: 10.1101/gad.1471006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AJ, Evans JP. Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil Dev. 2006;18:53–61. doi: 10.1071/rd05122. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Giansanti, Bonaccorsi S, Williams B, Williams EV, Santolamazza C, Goldberg ML, Gatti M. Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev. 1998;12:396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson WT, Veldhuis JH, Rubinstein B, Cartwright HN, Perrimon N, Brodland GW, Nagpal R, Gibson MC. Control of the mitotic cleavage plane by local epithelial topology. Cell. 2011;144:427–428. doi: 10.1016/j.cell.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill SW. Cell biology: forced to be unequal. Science. 2010;330:597–598. doi: 10.1126/science.1198343. [DOI] [PubMed] [Google Scholar]
- Gönczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9:355–366. doi: 10.1038/nrm2388. [DOI] [PubMed] [Google Scholar]
- Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–317. doi: 10.1016/j.devcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y. Mechanical properties of starfish oocytes. Dev Growth Differ. 1976;18:205–209. doi: 10.1111/j.1440-169X.1976.00205.x. [DOI] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipet aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF. Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Dev Biol. 2008;316:100–109. doi: 10.1016/j.ydbio.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Nemoto S, Yoneda M. Periodic changes in the content of protein bound sulfhydryl groups and tension at the surface of starfish oocyts in correlation with the meiotic division cycle. Dev Growth Differ. 1976;18:221–225. doi: 10.1111/j.1440-169X.1976.00221.x. [DOI] [PubMed] [Google Scholar]
- Jankovics F, Sinka R, Lukacsovich T, Erdelyi M. MOESIN crosslinks actin and cell membrane in Drosophila oocytes and is required for OSKAR anchoring. Curr Biol. 2002;12:2060–2065. doi: 10.1016/s0960-9822(02)01256-3. [DOI] [PubMed] [Google Scholar]
- Kee YS, Robinson DN. Motor proteins: myosin mechanosensors. Curr Biol. 2008;18:R860–R862. doi: 10.1016/j.cub.2008.07.071. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, Yonemura S, Yamagishi H, Keppler D, Tsukita S. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet. 2002;31:320–325. doi: 10.1038/ng905. [DOI] [PubMed] [Google Scholar]
- Kunda P, Pelling AE, Liu T, Baum B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr Biol. 2008;18:91–101. doi: 10.1016/j.cub.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Larson SM, Lee HJ, Hung PH, Matthews LM, Robinson DN, Evans JP. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and Ezrin-Radixin-Moesin (ERM) proteins. Mol Biol Cell. 2010;21:3182–3192. doi: 10.1091/mbc.E10-01-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, Maas R, Leder P. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol. 2002;4:921–928. doi: 10.1038/ncb880. [DOI] [PubMed] [Google Scholar]
- Levi M, Maro B, Shalgi R. The involvement of Fyn kinase in resumption of the first meiotic division in mouse oocytes. Cell Cycle. 2010;9:1577–1589. doi: 10.4161/cc.9.8.11299. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Chen DY. Development of surface polarity in mouse eggs. Scan Electron Microsc. 1984;(Pt 2):703–716. [PubMed] [Google Scholar]
- Longo FJ, Chen DY. Development of cortical polarity in mouse eggs: Involvement of the meiotic apparatus. Dev Biol. 1985;107:382–394. doi: 10.1016/0012-1606(85)90320-3. [DOI] [PubMed] [Google Scholar]
- Lucero A, Stack C, Bresnick AR, Shuster CB. A global, myosin light chain kinase-dependent increase in myosin II contractility accompanies the metaphase-anaphase transition in sea urchin eggs. Mol Biol Cell. 2006;17:4093–4104. doi: 10.1091/mbc.E06-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, McGinnis LK, Kinsey WH. Role of Fyn kinase in oocyte developmental potential. Reprod Fertil Dev. 2010;22:966–976. doi: 10.1071/RD09311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Benink HA, Cheng D, Montplaisir V, Wang L, Xi Y, Zheng PP, Bement WM, Liu XJ. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr Biol. 2006;16:214–220. doi: 10.1016/j.cub.2005.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117:2449–2460. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- Matzke R, Jacobson K, Radmacher M. Direct, high-resolution measurement of furrow stiffening during division of adherent cells. Nat Cell Biol. 2001;3:607–610. doi: 10.1038/35078583. [DOI] [PubMed] [Google Scholar]
- Minc N, Burgess DR, Chang F. Influence of cell geometry on division-plan positioning. Cell. 2011;144:414–426. doi: 10.1016/j.cell.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM, Swann MM. The mechanical properties of the cell surface: II. The unfertilized sea-urchin egg. J Exp Biol. 1954;31:461–472. [Google Scholar]
- Mitchison JM, Swann MM. The mechanical properties of the cell surface: III. The sea-urchin egg from fertilization to cleavage. J Exp Biol. 1955;32:734–750. [Google Scholar]
- Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Zernicka-Goetz M. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr Biol. 2006;16:1249–1254. doi: 10.1016/j.cub.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Nicosia SV, Wolf DP, Inoue M. Cortical granule distribution and cell surface characteristics in mouse eggs. Dev Biol. 1977;57:56–74. doi: 10.1016/0012-1606(77)90354-2. [DOI] [PubMed] [Google Scholar]
- Octtaviani E, Effler JC, Robinson DN. Enlazin, a natural fusion of two classes of canonical cytoskeletal proteins, contributes to cytokinesis dynamics. Mol Biol Cell. 2006;17:5275–5286. doi: 10.1091/mbc.E06-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C, Elson EL. Lymphocyte mechanical response triggered by cross-linking surface receptors. J Cell Biol. 1985;100:860–872. doi: 10.1083/jcb.100.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989;341:549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Pielak RM, Gaysinskaya VA, Cohen WD. Formation and function of the polar body contractile ring in Spisula. Dev Biol. 2004;269:421–432. doi: 10.1016/j.ydbio.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Polesello C, Delon I, Valenti P, Ferrer P, Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat Cell Biol. 2002;4:782–789. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- Reichl EM, Effler JC, Robinson DN. The stress and strain of cytokinesis. Trends Cell Biol. 2005;15:200–206. doi: 10.1016/j.tcb.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichl EM, Ren Y, Morphew MK, Delannoy M, Effler JC, Girard KD, Divi S, Iglesias PA, Kuo SC, Robinson DN. Interactions between myosin and actin crosslinkers control cytokinesis contractility dynamics and mechanics. Curr Biol. 2008;18:471–480. doi: 10.1016/j.cub.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Effler JC, Norstrome M, Luo T, Firtel RA, Iglesias PA, Rock RS, Robinson DN. Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin. Curr Biol. 2009;19:1421–1428. doi: 10.1016/j.cub.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–372. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- Sanfins A, Lee GY, Plancha CE, Overstrom EW, Albertini DF. Distinctions in meiotic spindle structure and assembly during in vitro and in vivo maturation of mouse oocytes. Biol Reprod. 2003;69:2059–2067. doi: 10.1095/biolreprod.103.020537. [DOI] [PubMed] [Google Scholar]
- Sanfins A, Plancha CE, Overstrom EW, Albertini DF. Meiotic spindle morphogenesis in in vivo and in vitro matured mouse oocytes: insights into the relationship between nuclear and cytoplasmic quality. Hum Reprod. 2004;19:2889–2899. doi: 10.1093/humrep/deh528. [DOI] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Schmerler S, Wessel GM. Polar bodies -- more a lack of understanding than a lack of respect. Mol Reprod Dev. 2011;78:3–8. doi: 10.1002/mrd.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman GG, Waddington CH. The mechanism of cell division in the cleavage of the newt’s egg. J Exp Biol. 1955;32:700–733. [Google Scholar]
- Sharan SK, Pyle A, Coppola V, Babus J, Swaminathan S, Bendict J, Swing D, Martin BK, Tessarollo L, Evans JP, Flaws JA, Handel MA. BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development. 2004;131:131–142. doi: 10.1242/dev.00888. [DOI] [PubMed] [Google Scholar]
- Shôji Y, Hamaguchi MS, Hiramoto Y. Mechanical properties of the endoplasm in starfish oocytes. Exp Cell Res. 1978;117:79–87. doi: 10.1016/0014-4827(78)90429-9. [DOI] [PubMed] [Google Scholar]
- Simerly C, Nowak G, de Lanerolle P, Schatten G. Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos. Mol Biol Cell. 1998;9:2509–2525. doi: 10.1091/mbc.9.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, Hyman AA. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 2011;469:226–230. doi: 10.1038/nature09642. [DOI] [PubMed] [Google Scholar]
- Surcel A, Kee YS, Luo T, Robinson DN. Cytokinesis through biochemical-mechanical feedback loops. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2010.08.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Pool TB. ART failure: oocyte contributions to unsuccessful fertilization. Human Reprod Update. 2008;14:431–446. doi: 10.1093/humupd/dmn025. [DOI] [PubMed] [Google Scholar]
- Ubaldi FM, Rienzi L. Morphological selection of gametes. Placenta. 2008;29(Suppl B):115–120. doi: 10.1016/j.placenta.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Guillaud P, Rassinier P, Maro B. Asymmetric division in mouse oocytes: with or without Mos. Curr Biol. 2000;10:1303–1306. doi: 10.1016/s0960-9822(00)00753-3. [DOI] [PubMed] [Google Scholar]
- Yanagamachi R. Sperm-egg association in mammals. Curr Top Dev Biol. 1978;12:83–105. [PubMed] [Google Scholar]
- Yanagimachi R. Sperm-egg fusion. Curr Top Membr Transp. 1988;32:3–43. [Google Scholar]
- Zhang W, Robinson DN. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proc Natl Acad Sci U S A. 2005;102:7186–7191. doi: 10.1073/pnas.0502545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ma C, Miller AL, Katbi HA, Bement WM, Liu XJ. Polar body emission requries a RhoA contractile ring and Cdc42-mediated membrane protrusion. Dev Cell. 2008;15:386–400. doi: 10.1016/j.devcel.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Kee YS, Poirier CC, Jelinek C, Osborne J, Divi S, Surcel A, Will ME, Eggert US, Muller-Taudenberger A, Iglesias PA, Cotter RJ, Robinson DN. 14-3-3 coordinates microtubules, Rac, and myosin-II to control cell mechanics and cytokinesis. Curr Biol. 2010;20:1881–1889. doi: 10.1016/j.cub.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]