Abstract
Zinc is a micronutrient important in several biological processes including growth and development. We have limited knowledge on the impact of maternal zinc deficiency on zinc and zinc regulatory mechanisms in the developing embryo due to a lack of in vivo experimental models that allow us to directly study the effects of maternal zinc on embryonic development following implantation. To overcome this barrier, we have proposed to use zebrafish as a model organism to study the impact of zinc during development. The goal of the current study was to profile the mRNA expression of all the known zinc transporter genes in the zebrafish across embryonic and larval development and to quantify the embryonic zinc concentrations at these corresponding developmental time points. The SLC30A zinc transporter family (ZnT) and SCL39A family, Zir-,Irt-like protein (ZIP) zinc transporter proteins were profiled in zebrafish embryos at 0, 2, 6, 12, 24, 48 and 120 hours post fertilization to capture expression patterns from a single cell through full development. We observed consistent embryonic zinc levels, but differential expression of several zinc transporters across development. These results suggest that zebrafish is an effective model organism to study the effects of zinc deficiency and further investigation is underway to identify possible molecular pathways that are dysregulated with maternal zinc deficiency.
Introduction
The role of zinc in a wide range of cellular processes, including cell proliferation, reproduction, immune function, and defense against free radicals, has been well established. Zinc is considered to be the most abundant trace intracellular element, and there exists increasing evidence that zinc plays an important role in fetal growth and development. It is estimated that 82% of women worldwide have inadequate intakes of zinc and may be at risk for zinc deficiency (Caulfield et al. 1998). In humans, suboptimal zinc intake is associated with poor pregnancy outcomes, increased premature birth, low birthweights and increased congential malformations (Meadows et al. 1983; Garg et al. 1993; Scholl et al. 1993; Shah et al. 2006; Hess et al. 2009). In mammalian experimental models, maternal zinc deficiency also results in increased embryonic cell death and increases in numerous developmental defects in the offspring (Oteiza et al. 1990; Peters et al. 1991; Liu et al. 1992; Keen et al. 1993; Jankowski et al. 1995; Lopez et al. 2008). In addition to these acute effects, maternal marginal zinc deficiency is also associated with longer term health consequences including impaired glucose tolerance (Padmavathi et al. 2009), increased susceptibility to diabetic stress (Uriu-Hare et al. 1989), impaired learning and memory (Halas et al. 1986) and compromised immune system (Vruwink et al. 1991).
Zinc homeostasis is maintained by the activities of a family of zinc transporters in the cell plasma membrane and intracellular organelles. The SLC30A zinc transporter family (ZnT) act to decrease intracellular zinc levels through transport of zinc from the cytoplasm to the extracellular space or into organelles. In contrast the second SCL39A family, Zir-,Irt-like protein (ZIP) act in a opposing manner to increase intracellular zinc levels. At least ten ZnT and fourteen Zip family members have been identified in mammals, and their tissue expression, cellular localization and regulation are very different (see (Lichten et al. 2009) for detailed review). The critical role of zinc and zinc transporters during developmental processes has been clearly been established with molecular and genetic approaches in model systems. For example, knockdown of ZIP6, a LIV1 family zinc transporter, results in early embryonic malformations in zebrafish and is critical for epithelial-mesenchymal transition (EMT) during gastrulation (Yamashita et al. 2004). In rodents, knockdown of ZnT1 results in early embryonic lethality (Andrews et al. 2004). Zinc also plays an important role in cell meiosis, and in during oocyte maturation (Bernhardt et al. 2010; Kim et al. 2010). Thus there is clear evidence supporting a key role of zinc and its regulatory proteins during development.
Although there has been intense study of zinc regulatory proteins, such as zinc transporters, to control zinc homeostasis, their function and regulation at the organism level is far less well understood. In particular the impact of maternal zinc status on fetal zinc homeostasis in vivo is virtually unknown because zinc depletion during pregnancy usually causes severe embryonic deformities (which are often lethal) or causes early fetal resorption. The use of zebrafish (Danio rerio) offers a unique model that allows us to directly study the effects of maternal zinc on embryonic development following implantation and gain an understanding of the mechanistic function and regulation of zinc during development at the organism level. During development, zinc levels rapidly increase after fertilization through 512-cell stage (~2.75 h post fertilization (hpf)) and plateaus at the mid-gastrula state (~ 6 hpf) (Riggio et al. 2003). The dynamics of zinc regulatory protein expression during the early stages of development are unknown. Orthologs for the zinc regulatory proteins, and many of the zinc transporters have also been identified in zebrafish (Chen et al. 2002; Chen et al. 2007; Zheng et al. 2008). The goal of the current study was to mRNA profile the family of zinc transporters in zebrafish across developmental time (from single cell at 0–2 h post fertilization through full development at 5 days of age) and establish the use of zebrafish as a model organism for the study of zinc metabolism and function.
Materials and Methods
Zebrafish Husbandry and Embryo Collection
Embryonic zebrafish (Danio rerio) were reared at Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University from adult AB strain fish 33 weeks of age. Adults were maintained at 29°C with a light/dark cycle of 14/10 hours in reverse osmosis water supplemented with 0.6% Instant Ocean® salt solution. Zebrafish were spawned and embryos were collected at 0, 2, 6, 12, 24, 48 and 120 hpf. Samples were collected in triplicate, with each replicate consisting of 30 pooled embryos. Embryos collected for RNA extraction were stored in 500 μL RNAlater® and kept at −20°C. Embryos collected for ICP analysis were rinsed twice with Chelex-treated water, then stored dry at −20°C.
RNA extraction
RNA extractions were carried out according to the TRIzol method. Briefly, RNAlater® was removed from the embryonic samples and 1.0 mL of TRIzol was added to the sample. Embryos were homogenized for 30 seconds, incubated at room temperature for 5 minutes, and then 200 μL of chloroform was added. After another incubation at room temperature for 2–3 minutes, samples were centrifuged at max speed for 15 minutes at 4°C. The upper aqueous layer was transferred to a new tube and 500 μL isopropyl alcohol was added. After a 10 minute incubation at room temperature, samples were centrifuged at max speed for 10 minutes at 4°C. Supernatent was decanted and following an ethanol wash and spin step, the RNA pellet was resuspended in DEPC treated water. cDNA synthesis was conducted using Superscript® III First Strand Synthesis according to the manufacturer’s suggestions.
qRT-PCR Analysis
Plasmid standards of the qRT-PCR gene targets were cloned using TOPO-TA cloning kit®. Ornithine decarboxylase 1 (odc1) was chosen as a reference gene for normalization. Primers for experimental gene targets and odc1 are listed in Table 1. Each primer set has a melting temperature at or near 60°C and amplifies a 100–300 bp target. qRT-PCR reactions were set up as follows: 50 ng cDNA, 0.5 μM each primer, 10 μL 2X DyNAmo HS SYBR green ENZ mix and DEPC water to a final volume of 20 μL. Using a MJ Research thermocycler, the reaction proceeded as follows: 95°C for 10 minutes, 40 cycles of 94°C for 10 seconds, 58°C for 20 seconds and 72°C for 20 seconds. A final extension at 72°C for 10 minutes was used. A melting curve analysis was also conducted from 60–95°C, read every 0.5°C and held for 1 second. Opticon Monitor version 2.0 software was used to analyze qRT-PCR data following the standard curve method.
Table 1.
primers for qRT-PCR analysis.
| Gene | Forward Primer (5’-3’) | Reverse Primer (5’-3’) | Product Size (bp) |
|---|---|---|---|
| odc1 | GTGGGCGACTGGCTGCTGTT | CCGCAGTGGGATGGCACGTT | 200 |
| ZIP1 | GGTGAGAGTTGGAGCTCTGG | AGTGGGAAGCCATCATCAAG | 243 |
| ZIP3 | CGTATACGGCTGATGTGGTG | AGGCCTGCTGTAAACCACTG | 299 |
| ZIP4 | CAGACATGCTTCCTACGCTG | GCCCGATCTGGTCTTCATAA | 132 |
| ZIP6 | GTCATCATGGGAGACGGACT | GGCAAAATCACCGAGTTCAT | 141 |
| ZIP7 | AAGAAAGTTGTGGAAGCAGGCA | CCACCAGATGCAAAACTCAAGAG | 176 |
| ZIP8 | TCCCCGCCTGCCCTTACACTT | AGTGTCCCGATGGCCAGTCCAA | 199 |
| ZIP9 | TCGGAATGTGACGAGCCTTCGC | ACATGTATCCTCGGAGATCGCGTG | 226 |
| ZIP10 | TCACCTGCACATGGTGTTCT | ACATCCAAACCCATCCTGAA | 223 |
| ZIP11 | TCAGGCCCTGCTGGGGACTC | GCCCACAGCCACTGGGAGGA | 226 |
| ZIP13 | GGAGACCAACCCAAGGAACT | GTCTTTGGGAGGGTGACAAA | 220 |
| ZnT1 | GAAGGCTGCCGATATGTGTC | AGGACATGCAGGAAAACACC | 132 |
| ZnT2 | TCGGCTGGCACAGATCAGAGATT | ACCGTGGCCCACAGGACTCA | 230 |
| ZnT4 | CATCCTGCTGGAGGGTGTA | CTGCAGTTGTACCGTGCAGT | 247 |
| ZnT5 | TATCTCCAGTGGGAAGCTGG | ATCACTGCACACCCCATTTT | 180 |
| ZnT6 | CCATCGCTCCGTCCTGGGGA | ACCGCCAGCACCTCGAAACG | 282 |
| ZnT7 | CCCTTCCTGAATGCTACCAA | CACCGACCTGTGTGAAGATG | 180 |
| ZnT8 | ATCGTCTTGATGGAAGGCAC | TTTCTCGAAGCACCTCCTGT | 187 |
| ZnT9 | CCTGTTTTGGTTGGCAAAGT | GAATGCTCTCTGCCTTCGTC | 240 |
Zinc analysis
Embyronic zinc concentrations were determined by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES; Teledyne Leeman Labs) with small modification of a previous described method (Verbanac et al. 1997). Samples were digested in 69%–70% OmniTrace nitric acid (VWR) overnight. Following digestion, samples were diluted 10 times with water treated with chelex 100 resin (Bio-rad) and analyzed by ICP-OES against known standards (Bruno et al. 2007).
Statistics
Statistical analysis was performed with the use of PRISM (version 4.0; GraphPad Software). Significantly differences between means across time were analyzed by one-way ANOVA followed by Dunnett’s post-hoc test when appropriate.
Results
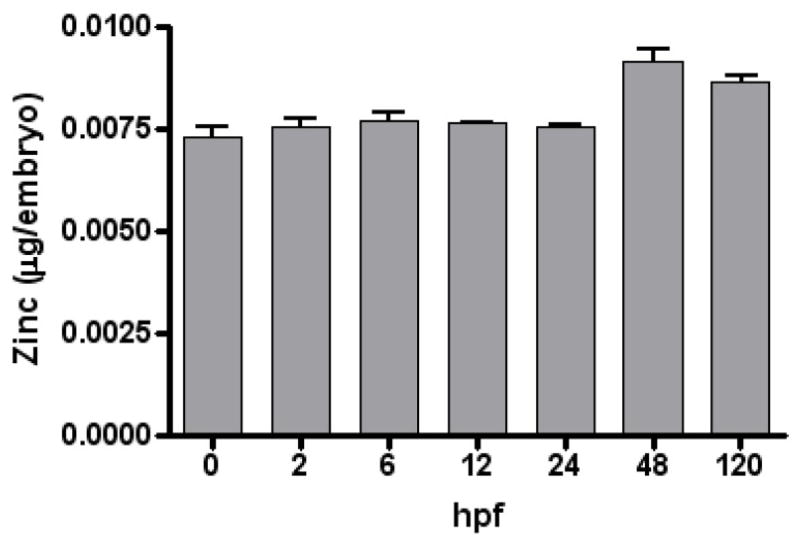
Zinc concentrations and all known zinc transporters from both the ZIP family and the ZnT family were analyzed over developmental time in Danio rerio embryos. Figure 1 depicts ICP data for zinc content in 30 pooled D. rerio embryos over developmental time. Zinc levels were relatively constant over developmental time (0 to 120 hpf).
Figure 1.
Embryonic zinc levels across development. Zinc levels were determined by inductively-coupled plasma spectroscopy. Bars represent means ± SEM (n= 3, 30 pooled embryo samples). Zinc levels were calculated per embryo.
Figure 2 illustrates expression patterns of ZIP transporters normalized to odc1 mRNA transcript through 120 hpf. ZIP3, ZIP4, ZIP7, ZIP8 and ZIP10 showed a pattern of increasing transcript level, with maximal expression at 120 hpf compared to earlier time points. ZIP6, ZIP9, ZIP11 and ZIP13 transcript levels dropped following 0 hpf time point, with lowest expression level at 6 hpf. Transcript levels then increased again at 12 hpf with highest expression at 120 hpf. In contrast, there was a bimodal expression pattern for ZIP1 expression, a rise expression level at 6 hpf and 120 hpf. Table 2 shows ZIP and ZnT expression as a fold change from 0 hpf for each respective gene, after normalization to odc1. Relative transcript levels for ZIPs 1, 6, 9, 10, 11 and 13 was less than 5 fold for all time points. ZIPs 3 and 4 showed a greater increase relative to 0 hpf, with ZIP3 reaching 60 fold and 80 fold difference at 120hpf respectively. Among the ZIPs tested, ZIP8 exhibited the greatest fold change from 0hpf, culminating with an approximately 460 fold change.
Figure 2.
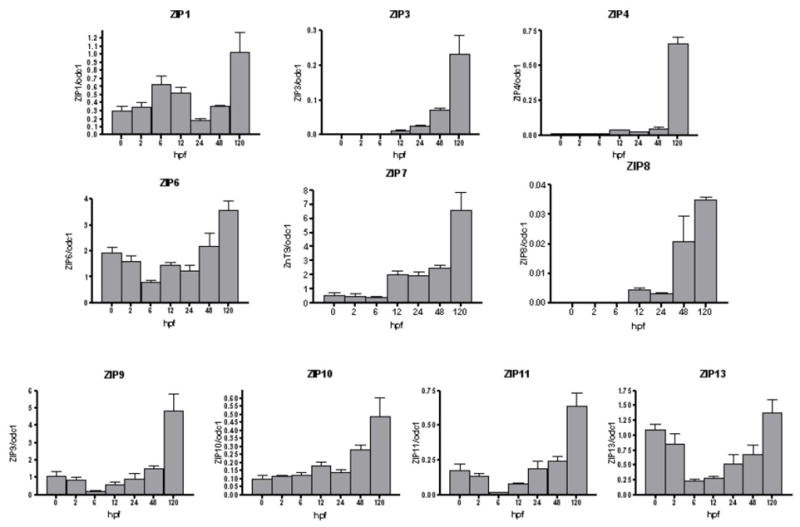
ZIP (SLC39A) mRNA abundance profiles in zebrafish embryos across development. mRNA levels were measured as described in Material and Methods. Results are transcripts copy number normalized to ornithine decarboxylase (ODC1) transcripts. Values are means ± SEM (n= 3).
Table 2.
Normalized ZIP and ZnT gene copies expressed as a fold change with respect to 0hpf for each gene.
| Gene | 0 hpf | 2 hpf | 6 hpf | 12 hpf | 24 hpf | 48 hpf | 120 hpf |
|---|---|---|---|---|---|---|---|
| ZIP1 | 1.0 ± 0.2 | 1.17 ± 0.17 | 2.12 ± 0.34 * | 1.75 ± 0.22 | 0.59 ± 0.1 | 1.21 ± 0.02 | 3.44 ± 0.85 * |
| ZIP3 | 1.0 ± 0.04 | 1.08 ± 0.11 | 1.05 ± 0.17 | 3.16 ± 0.56 * | 6.79 ± 0.29 * | 18.58 ± 1.42 * | 60.07 ± 14.3 * |
| ZIP4 | 1.0 ± 0.16 | 1.38 ± 0.16 | 0.79 ± 0.21 | 4.26 ± 0.47 * | 2.68 ± 0.04 * | 5.60 ± 1.31 * | 80.7 ± 6.02 * |
| ZIP6 | 1.0 ± 0.13 | 0.83 ± 0.13 | 0.41 ± 0.04 * | 0.76 ± 0.06 | 0.64 ±0.12 | 1.14 ± 0.27 | 1.86 ± 0.20 |
| ZIP7 | 1.0 ± 0.3 | 0.87 ± 0.35 | 0.74 ± 0.09 | 3.63 ± 0.55 * | 3.53 ± 0.50 * | 4.54 ± 0.27 * | 11.91 ± 2.41 * |
| ZIP8 | 1.0 ± 0.26 | 1.84 ± 0.94 | 4.51 ± 0.15 * | 60.0 ± 6.61 * | 40.4 ± 4.81 * | 273.3 ± 115.0 * | 456.29 ± 14.50 * |
| ZIP9 | 1.0 ± 0.26 | 0.80 ± 0.12 | 0.19 ± 0.02 * | 0.53 ± 0.14 | 0.82 ± 0.33 | 1.38 ± 0.16 | 4.49 ± 0.92 * |
| ZIP10 | 1 ± 0.27 | 1.22 ± 0.046 | 1.24 ± 0.20 | 1.89 ± 0.23 * | 1.47 ± 0.13 | 2.89 ± 0.34 * | 5.04 ± 1.23 * |
| ZIP11 | 1.0 ± 0.24 | 0.76 ± 0.13 | 0.10 ± 0.01 * | 0.46 ± 0.02 * | 1.08 ± 0.28 | 1.39 ± 0.17 | 3.64 ± 0.52 * |
| ZIP13 | 1.0 ± 0.09 | 0.79 ± 0.16 | 0.22 ± 0.02 * | 0.26 ± 0.04 * | 0.48 ± 0.16 * | 0.62 ± 0.15 | 1.27 ± 0.21 |
| ZnT1 | 1.0 ± 0.32 | 1.38 ± 0.15 | 4.73 ± 0.46 * | 6.65 ± 0.038 * | 5.88 ± 0.72 * | 14.78 ± 3.18 * | 82.47 ± 13.27 * |
| ZnT2 | 1.0 ± 0.30 | 2.037 ± 0.62 | 0.78 ± 0.04 | 1.07 ± 0.18 | 50.16 ± 6.24 * | 247.67 ± 68.88 * | 614.52 ± 32.02 * |
| ZnT4 | 1.0 ± 0.30 | 0.58 ± 0.10 | 0.15 ± 0.01 * | 0.24 ± 0.09 * | 0.70 ± 0.23 | 1.17 ± 0.21 | 4.63 ± 0.43 * |
| ZnT5 | 1.0± 0.18 | 1.33 ± 0.36 | 0.17 ± 0.04 | 0.35 ± 0.16 | 0.36 ± 0.21 | 1.61 ± 0.74 | 11.72 ± 1.48 * |
| ZnT6 | 1.0 ± 0.16 | 0.88 ± 0.24 | 0.41 ± 0.04 | 1.01 ± 0.25 | 0.91 ± 0.11 | 2.11 ± 0.52 | 4.92 ± 0.74 * |
| ZnT7 | 1.0 ± 0.17 | 0.87 ± 0.08 | 0.99 ± 0.14 | 1.12 ± 0.13 | 1.72 ± 0.51 | 3.04 ± 0.75 * | 6.34 ± 0.41 * |
| ZnT8 | 1.0 ± 0.61 | 1.20 ± 0.13 | 646.18 ± 103.45 * | 1047.62 ± 111.58 * | 1681.94 ± 401.69 * | 4957.78 ± 787.02 * | 2097.04 ± 716.99 * |
| ZnT9 | 1.0 ± 0.20 | 0.83 ± 0.21 | 0.06 ± 0.01 * | 0.16 ± 0.03 * | 0.34 ± 0.12 * | 1.57 ± 0.26 | 4.47 ± 0.96 * |
P<0.05 versus 0 hpf (1-way ANOVA, Dunnett’s multiple comparison test)
Figure 3 illustrates ZnT transcript levels normalized to odc1 mRNA transcript through 120 hpf. ZnT1, ZnT7 transcript levels increased over time, with highest expression at 120 hpf. ZnT2 expression was not detectable until 24 hpf with highest expression at 120hpf. ZnT4 and ZnT6 showed a similar trend as ZIP13, with a decrease in expression from 0–6 hpf followed by steady increase in expression through 120 hpf. Interestingly, ZnT8 expression was not detectable until 6 hpf and had its highest transcript levels at 48 hpf, and an insignificant but marked decrease in transcript levels at 120 hpf. As shown in Table 2, among the ZnTs analyzed, ZnTs 4, 6, 7 and 9 did not surpass a 7 fold change for all time points. nT5 maintained a fairly constant relative transcript level around 1 fold or less until 120 hpf, when relative expression reached nearly 12 fold that of 0hpf. ZnTs 1 and 2 reached even higher relative fold changes of roughly 80 and 615 at 120 hpf respectively. Lastly, ZnT8 had the greatest overall relative fold change from 0 hpf, reaching a nearly 5000 fold change at 48 hpf and dropping to roughly 2100 fold change at 120 hpf.
Figure 3.
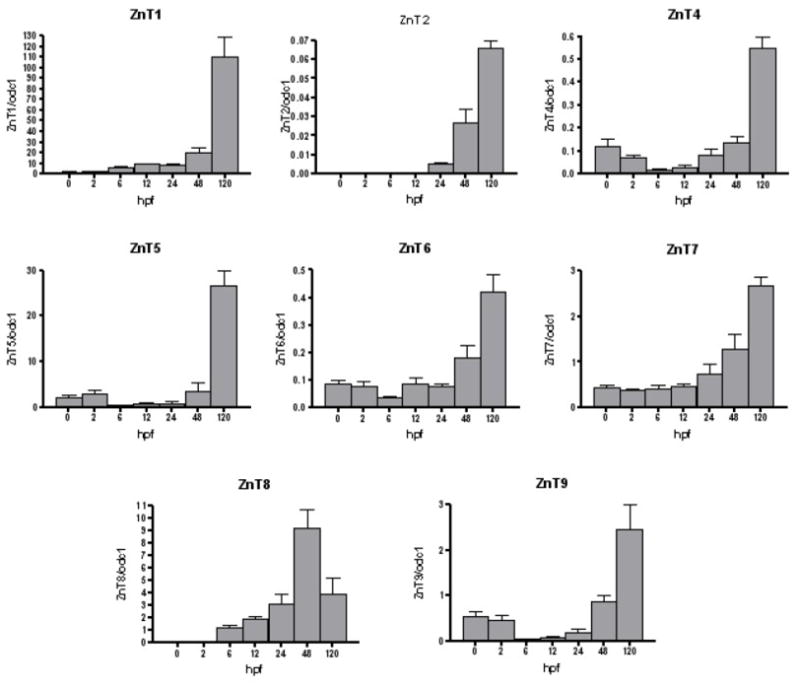
ZnT (SLC30A) mRNA abundance profiles in zebrafish embryos across development. mRNA levels were measured as described in Material and Methods. Results are transcripts copy number normalized to ornithine decarboxylase (ODC1) transcripts. Values are means ± SEM (n= 3).
Discussion
Zinc and its transporters play key roles during embryonic development and many biological processes. Ablation of ZIP6 and ZnT1 results in developmental abnormalities in zebrafish and rodents, respectively (Andrews et al. 2004; Yamashita et al. 2004). ZIP6 also plays a role in the maturation of immune cells, including dendritic cells and the regulation of T-cell receptor signaling (Kitamura et al. 2006; Yu et al. 2011). ZIP13 knockout mice exhibit defects in connective tissue development, via dysregulation of bone morphogenic protein and TGF-β signaling (Fukada et al. 2008). ZIP14 appears to be required for systemic growth via control of G-protein coupled receptor-mediated signaling (Hojyo et al. 2011). ZnT3 is essential for pre-synaptic MAP kinase signaling and the development of hippocampus-dependent memory (Sindreu et al. 2011). Although a key role of zinc during growth and developmental processes has been clearly established in experimental models, in human studies, the data linking zinc to adverse fetal growth and developmental outcomes is conflicting (Hess et al. 2009). However, interpretation of human studies is hampered by the lack of specific and sensitive biomarkers for zinc status, especially during pregnancy and development (Shah et al. 2006). This is coupled with a lack of knowledge of the precise mechanisms that regulate zinc delivery from maternal stores to the fetus, especially at early stages of development, and a lack of understanding the mechanistic consequences of zinc deficiency on fetal outcomes. The development of an in vivo model, such as zebrafish, to study the zinc metabolism during embryo development would significantly aid in filling these knowledge gaps and aid in our understanding of the relationship between zinc & its regulatory proteins and developmental processes at a whole organism level.
The ZnT family of transporters functions in zinc efflux from the cytoplasm to either the extracellular space and/or intracellular organelles. The ZIP family of protein functions in zinc uptake from the extracellular matrix and/or intracellular organelles into the cytoplasm (Lichten et al. 2009). These zinc transporters are expressed in a tissue-specific manner, and respond differentially to dietary zinc levels and physiological conditions. Therefore, a loss of function or dysregulation of certain zinc transporters would result in an impairment of zinc homeostasis and predispose the body to zinc-imbalance-related diseases, such as cancer, asthma, diabetes, and Alzheimer’s disease (Lichten et al. 2009). For example, in humans, mutations of Zip4 gene is the cause of human acrodermatitis enteropathica, an autosomal recessively inherited disease (Kury et al. 2002), and polymorphisms in ZnT8 is associated with type 2 diabetes (Sladek et al. 2007). Dysregulation of ZnT1 has been associated with the genetic disorder epidermodysplasia verruciformia, where patients are highly susceptible to human papillomavirus (HPV) infection (Orth 2008). Mutations in ZnT2 has been linked to an inability for the mammary gland to secrete zinc during lactation (Seo et al. 2010). Over-expression of zinc transporters, such as ZIP6 and ZIP4 has also been associated with cancer cell growth in breast, liver and pancreatic cancers (Li et al. 2007; Shen et al. 2009; Weaver et al. 2010). We observed trends for differential zinc transporter expression patterns that were affected by developmental age. This data highlights our ability to monitor zinc transporter expression during zebrafish development and supports studies using this model organism for zinc metabolism and/or functional zinc transporter studies.
The tissue expression, cellular localization and regulation among each zinc transporter are very different. For example, ZnT1 is ubiquitously expressed, but highly expressed in tissues involved in zinc transfer, such as the basolateral side of enterocytes and kidney tubules (McMahon et al. 1998). ZnT1 in particular appears to play a critical role in dietary zinc absorption, controlling the efflux of zinc out of the enterocyte across the basolateral membrane (Andrews et al. 2004). It also localizes to the yolk sac membrane in rodents and may facilitate transfer of zinc between mother and fetus (Liuzzi et al. 2003). The severity lethality of ZnT1 knockdown in mammals also renders it more difficult to identify the precise role of ZnT1 and zinc during development. Relative to the other zinc transporters, ZnT1 and ZnT5 transcript copy number was most abundant at 0 hpf in zebrafish.
Large increases in expression of ZIP3,4,8 and ZnT2 and ZnT8 were apparent across development (Table 2). The greatest change in expression levels occurred in ZnT8 mRNA levels across development. We highlight that there is marked increase in expression of ZnT8 at 6 hpf (~646 fold increase) that corresponds with embryonic synthesis of proinsulin (Kinkel et al. 2009) and peaks at 48hpf (~5000 fold increase) that corresponds with organogenesis. ZnT8 appears to play a critical role in the synthesis and transport of zinc into secretory vesicles and formation of zinc-insulin complexes (Chistiakov et al. 2009). Loss of ZnT8 in pancreatic β cells reduces insulin content and loss of insulin release (Fu et al. 2009; Lemaire et al. 2009). Genome-wide association studies have found that a polymorphism variant in ZnT8, rs1226634 [C/T transition; Arg(325)- Trp (325)] is associated with increased risk of Type 2 diabetes (Saxena et al. 2007; Scott et al. 2007; Pound et al. 2009). Interestingly, ZnT8 is also an auto-antigen in Type I diabetes (Wenzlau et al. 2007). The insulin producing pancreatic β-cells contain some of the highest levels of zinc in the body. This high zinc requirement is largely due to the critical function of zinc for insulin synthesis, secretion and signaling (Tallman et al. 1999). High zinc levels in the pancreas may also be necessary to providing protection against oxidative stresses (Ho et al. 2001). Zinc deficiency may predispose individuals to diabetes and its cardiovascular complications (Mocchegiani et al. 2008). Interestingly, maternal zinc deficiency increases her offsprings’ susceptibility to increased body mass, glucose intolerance and impaired insulin secretion (Padmavathi et al. 2009). The use of zebrafish as a model organism for zinc studies is powerful in that for the first time we could quantitatively track zinc transporter expression from the single cell zygote stage through all stages of vertebrate development. Since early stages of vertebrate development are remarkably conserved, these zebrafish studies are highly relevant to human health. Importantly, similar to humans, zebrafish also require zinc and have in place similar zinc regulatory proteins to maintain zinc homeostasis (Feeney et al. 2005). Both family of zinc transports (ZIP and ZnT) and their control via Metal Transcription Factor and Metal Response Elements have been previously established to occur in zebrafish (Zheng et al. 2008). Studies in zebrafish gills, also suggest that zebrafish tissues respond to both zinc supplementation and zinc deficiency exposures (Zheng et al.; Zheng et al.). The zebrafish, especially the developing embryo, is an attractive model system for studies of zinc function. Beneficial attributes include its small size (<1 gram body weight), rapid embryonic development (less than 1 week) and short life cycle (Dodd et al. 2000; Wixon 2000; Udvadia et al. 2003). The zebrafish model enables assessment of integrative, whole animal effects. Importantly, fundamental processes and mechanisms of development are conserved across species (Lein et al. 2005).
Collectively, the current data confirms the expression of zinc transporter proteins in zebrafish, and that they are differentially expression during development. This sets the stage for using zebrafish to aid in defining the specific molecular targets that control zinc homeostasis during development and characterize mechanisms by which zinc status or alterations in transporter expression affects fetal health. Future studies using purified zebrafish diets containing adequate or deficiency levels of zinc will be performed to closely examine the impact of dietary zinc status on embryonic developmental processes. This mechanistic work in zebrafish will provide the framework for translating results to humans and gain a better understanding of importance of zinc during pregnancy and development.
Acknowledgments
We gratefully acknowledge Carrie Barton, Clarissa Buchner and the staff at the Sinnhuber Aquatic Research Laboratory and the WM Keck Collaboratory at Oregon State University for their assistance in conducting these studies. This study was funded by Oregon AES (OR00735), and the Environmental Health Science Center at Oregon State University (NIEHS P30 ES00210).
Footnotes
This paper is based on a presentation given at the 5th Aquatic Annual Models of Human Disease conference: hosted by Oregon State University and Texas State University-San Marcos, and convened at Corvallis, OR, USA September 20–22, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews GK, Wang H, Dey SK, Palmiter RD. Mouse zinc transporter 1 gene provides an essential function during early embryonic development. Genesis. 2004;40:74–81. doi: 10.1002/gene.20067. [DOI] [PubMed] [Google Scholar]
- Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc requirement during meiosis I-meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biol Reprod. 2010;84:526–536. doi: 10.1095/biolreprod.110.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RS, Song Y, Leonard SW, Mustacich DJ, Taylor AW, Traber MG, Ho E. Dietary zinc restriction in rats alters antioxidant status and increases plasma F2 isoprostanes. J Nutr Biochem. 2007;18:509–518. doi: 10.1016/j.jnutbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am J Clin Nutr. 1998;68:499S–508S. doi: 10.1093/ajcn/68.2.499S. [DOI] [PubMed] [Google Scholar]
- Chen WY, John JA, Lin CH, Chang CY. Molecular cloning and developmental expression of zinc finger transcription factor MTF-1 gene in zebrafish, Danio rerio. Biochem Biophys Res Commun. 2002;291:798–805. doi: 10.1006/bbrc.2002.6517. [DOI] [PubMed] [Google Scholar]
- Chen WY, John JA, Lin CH, Chang CY. Expression pattern of metallothionein, MTF-1 nuclear translocation, and its dna-binding activity in zebrafish (Danio rerio) induced by zinc and cadmium. Environ Toxicol Chem. 2007;26:110–117. doi: 10.1897/06-153r.1. [DOI] [PubMed] [Google Scholar]
- Chistiakov DA, Voronova NV. Zn(2+)-transporter-8: a dual role in diabetes. Biofactors. 2009;35:356–363. doi: 10.1002/biof.49. [DOI] [PubMed] [Google Scholar]
- Dodd A, Curtis PM, Williams LC, Love DR. Zebrafish: bridging the gap between development and disease. Hum Mol Genet. 2000;9:2443–2449. doi: 10.1093/hmg/9.16.2443. [DOI] [PubMed] [Google Scholar]
- Feeney GP, Zheng D, Kille P, Hogstrand C. The phylogeny of teleost ZIP and ZnT zinc transporters and their tissue specific expression and response to zinc in zebrafish. Biochim Biophys Acta. 2005;1732:88–95. doi: 10.1016/j.bbaexp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tian W, Pratt EB, Dirling LB, Shyng SL, Meshul CK, Cohen DM. Down-regulation of ZnT8 expression in INS-1 rat pancreatic beta cells reduces insulin content and glucose-inducible insulin secretion. PLoS One. 2009;4:e5679. doi: 10.1371/journal.pone.0005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, Higashiyama H, Idaira Y, Asada Y, Kitamura H, Yamasaki S, Hojyo S, Nakayama M, Ohara O, Koseki H, Dos Santos HG, Bonafe L, Ha-Vinh R, Zankl A, Unger S, Kraenzlin ME, Beckmann JS, Saito I, Rivolta C, Ikegawa S, Superti-Furga A, Hirano T. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS One. 2008;3:e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg HK, Singhal KC, Arshad Z. A study of the effect of oral zinc supplementation during pregnancy on pregnancy outcome. Ind J Physiol Pharmacol. 1993;37:276–284. [PubMed] [Google Scholar]
- Halas ES, Hunt CD, Eberhardt MJ. Learning and memory disabilities in young adult rats from mildly zinc deficient dams. Physiol Behav. 1986;37:451–458. doi: 10.1016/0031-9384(86)90205-2. [DOI] [PubMed] [Google Scholar]
- Hess SY, King JC. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food Nutr Bull. 2009;30:S60–78. doi: 10.1177/15648265090301S105. [DOI] [PubMed] [Google Scholar]
- Ho E, Quan N, Tsai YH, Lai W, Bray TM. Dietary zinc supplementation inhibits NFkappaB activation and protects against chemically induced diabetes in CD1 mice. Exp Biol Med (Maywood) 2001;226:103–111. doi: 10.1177/153537020122600207. [DOI] [PubMed] [Google Scholar]
- Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin BH, Koseki H, Hirano T. The Zinc Transporter SLC39A14/ZIP14 Controls G-Protein Coupled Receptor-Mediated Signaling Required for Systemic Growth. PLoS One. 2011;6:e18059. doi: 10.1371/journal.pone.0018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MA, Uriu-Hare JY, Rucker RB, Rogers JM, Keen CL. Maternal zinc deficiency, but not copper deficiency or diabetes, results in increased embryonic cell death in the rat: implications for mechanisms underlying abnormal development. Teratology. 1995;51:85–93. doi: 10.1002/tera.1420510207. [DOI] [PubMed] [Google Scholar]
- Keen CL, Lonnerdal B, Golub MS, Olin KL, Graham TW, Uriu-Hare JY, Hendrickx AG, Gershwin ME. Effect of the severity of maternal zinc deficiency on pregnancy outcome and infant zinc status in rhesus monkeys. Pediatr Res. 1993;33:233–241. doi: 10.1203/00006450-199303000-00005. [DOI] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkel MD, Prince VE. On the diabetic menu: zebrafish as a model for pancreas development and function. Bioessays. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:239–240. doi: 10.1038/ng913. [DOI] [PubMed] [Google Scholar]
- Lein P, Silbergeld EK, Locke P, Goldberg AM. In vitro and other alternative approaches to developmental neurotoxicity testing (DNT) Environ Toxicol Pharmacol. 2005;19:735–744. doi: 10.1016/j.etap.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA, Gilon P, In't Veld PA, Schuit FC. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- Liu H, Oteiza PI, Gershwin ME, Golub MS, Keen CL. Effects of maternal marginal zinc deficiency on myelin protein profiles in the suckling rat and infant rhesus monkey. Biol Trace Elem Res. 1992;34:55–66. doi: 10.1007/BF02783898. [DOI] [PubMed] [Google Scholar]
- Liuzzi JP, Bobo JA, Cui L, McMahon RJ, Cousins RJ. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J Nutr. 2003;133:342–351. doi: 10.1093/jn/133.2.342. [DOI] [PubMed] [Google Scholar]
- Lopez V, Keen CL, Lanoue L. Prenatal zinc deficiency: influence on heart morphology and distribution of key heart proteins in a rat model. Biol Trace Elem Res. 2008;122:238–255. doi: 10.1007/s12011-007-8079-2. [DOI] [PubMed] [Google Scholar]
- McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci U S A. 1998;95:4841–4846. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows N, Ruse W, Keeling PW, Scopes JW, Thompson RP. Peripheral blood leucocyte zinc depletion in babies with intrauterine growth retardation. Arch Dis Child. 1983;58:807–809. doi: 10.1136/adc.58.10.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchegiani E, Giacconi R, Malavolta M. Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol Med. 2008;14:419–428. doi: 10.1016/j.molmed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Orth G. Host defenses against human papillomaviruses: lessons from epidermodysplasia verruciformis. Curr Top Microbiol Immunol. 2008;321:59–83. doi: 10.1007/978-3-540-75203-5_3. [DOI] [PubMed] [Google Scholar]
- Oteiza PI, Hurley LS, Lonnerdal B, Keen CL. Effects of marginal zinc deficiency on microtubule polymerization in the developing rat brain. Biol Trace Elem Res. 1990;24:13–23. doi: 10.1007/BF02789137. [DOI] [PubMed] [Google Scholar]
- Padmavathi IJ, Kishore YD, Venu L, Ganeshan M, Harishankar N, Giridharan NV, Raghunath M. Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. Exp Physiol. 2009;94:761–769. doi: 10.1113/expphysiol.2008.045856. [DOI] [PubMed] [Google Scholar]
- Peters JM, Wiley LM, Zidenberg-Cherr S, Keen CL. Influence of short-term maternal zinc deficiency on the in vitro development of preimplantation mouse embryos. Proc Soc Exp Biol Med. 1991;198:561–568. doi: 10.3181/00379727-198-43289. [DOI] [PubMed] [Google Scholar]
- Pound LD, Sarkar SA, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, McGuinness OP, Hutton JC, Powell DR, O'Brien RM. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggio M, Filosa S, Parisi E, Scudiero R. Changes in zinc, copper and metallothionein contents during oocyte growth and early development of the teleost Danio rerio (zebrafish) Comp Biochem Physiol C Toxicol Pharmacol. 2003;135:191–196. doi: 10.1016/s1532-0456(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Schall JI, Fischer RL, Khoo CS. Low zinc intake during pregnancy: its association with preterm and very preterm delivery. Am J Epidemiol. 1993;137:1115–1124. doi: 10.1093/oxfordjournals.aje.a116615. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YA, Kelleher SL. Functional analysis of two single nucleotide polymorphisms in SLC30A2 (ZnT2): implications for mammary gland function and breast disease in women. Physiol Genomics. 2010;42A:219–227. doi: 10.1152/physiolgenomics.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D, Sachdev HP. Zinc deficiency in pregnancy and fetal outcome. Nutr Rev. 2006;64:15–30. doi: 10.1111/j.1753-4887.2006.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Shen H, Qin H, Guo J. Concordant correlation of LIV-1 and E-cadherin expression in human breast cancer cell MCF-7. Mol Biol Rep. 2009;36:653–659. doi: 10.1007/s11033-008-9225-4. [DOI] [PubMed] [Google Scholar]
- Sindreu C, Palmiter RD, Storm DR. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc Natl Acad Sci USA. 2011;108:3366–3370. doi: 10.1073/pnas.1019166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Tallman DL, Taylor CG. Potential interactions of zinc in the neuroendocrine-endocrine disturbances of diabetes mellitus type 2. Can J Physiol Pharmacol. 1999;77:919–933. [PubMed] [Google Scholar]
- Udvadia AJ, Linney E. Windows into development: historic, current, and future perspectives on transgenic zebrafish. Dev Biol. 2003;256:1–17. doi: 10.1016/s0012-1606(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Uriu-Hare JY, Stern JS, Keen CL. Influence of maternal dietary Zn intake on expression of diabetes-induced teratogenicity in rats. Diabetes. 1989;38:1282–1290. doi: 10.2337/diab.38.10.1282. [DOI] [PubMed] [Google Scholar]
- Verbanac D, Milin C, Domitrovic R, Giacometti J, Pantovic R, Ciganj Z. Determination of standard zinc values in the intact tissues of mice by ICP spectrometry. Biol Trace Elem Res. 1997;57:91–96. doi: 10.1007/BF02803873. [DOI] [PubMed] [Google Scholar]
- Vruwink KG, Fletcher MP, Keen CL, Golub MS, Hendrickx AG, Gershwin ME. Moderate zinc deficiency in rhesus monkeys. An intrinsic defect of neutrophil chemotaxis corrected by zinc repletion. J Immunol. 1991;146:244–249. [PubMed] [Google Scholar]
- Weaver BP, Zhang Y, Hiscox S, Guo GL, Apte U, Taylor KM, Sheline CT, Wang L, Andrews GK. Zip4 (Slc39a4) expression is activated in hepatocellular carcinomas and functions to repress apoptosis, enhance cell cycle and increase migration. PLoS One. 2010:5. doi: 10.1371/journal.pone.0013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixon J. Featured organism: Danio rerio, the zebrafish. Yeast. 2000;17:225–231. doi: 10.1002/1097-0061(20000930)17:3<225::AID-YEA34>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Miyagi C, Fukada T, Kagara N, Che YS, Hirano T. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;429:298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- Yu M, Lee WW, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, Li G, Singh K, Tian L, Weyand CM, Goronzy JJ. Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med. 2011;208:775–785. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Feeney GP, Kille P, Hogstrand C. Regulation of ZIP and ZnT zinc transporters in zebrafish gill: zinc repression of ZIP10 transcription by an intronic MRE cluster. Physiol Genomics. 2008;34:205–214. doi: 10.1152/physiolgenomics.90206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Kille P, Feeney GP, Cunningham P, Handy RD, Hogstrand C. Dynamic transcriptomic profiles of zebrafish gills in response to zinc depletion. BMC Genomics. 2010;11:548. doi: 10.1186/1471-2164-11-548. [DOI] [PMC free article] [PubMed] [Google Scholar]