Abstract
Lipid peroxidation of polyunsaturated fatty acids generates bioactive aldehydes, which exhibit pro- and anti-inflammatory effects in cells and tissues. Accumulating evidence indicates that 4-hydroxynonenal (4-HNE), a major aldehyde derived from lipid peroxidation of n-6 polyunsaturated fatty acids trigger signals that modulates focal adhesion and adherens junction proteins thereby inducing endothelial barrier dysfunction. Similarly, oxidized phospholipids (Ox-PLs) generated by lipid peroxidation of phospholipids with polyunsaturated fatty acids have been implicated in atherogenesis, inflammation and gene expression. Interestingly, physiological concentration of Ox-PLs are anti-inflammatory and protect against endotoxin- and ventilator-associated acute lung injury. Thus, excess generation of bioactive hydroxyalkenals and Ox-PLs during oxidative stress contributes to pathophysiology of various diseases by modulating signaling pathways that regulate pro- and anti-inflammatory responses and barrier regulation. This review summarizes the role of 4-HNE and Ox-PLs affecting cell signaling pathways and endothelial barrier dysfunction through modulation of the activities of proteins/enzymes by Michael adducts formation, enhancing the level of protein tyrosine phosphorylation of the target proteins, and by reorganization of cytoskeletal, focal adhesion, and adherens junction proteins. A better understanding of molecular mechanisms of hydroxyalkenals- and Ox-PLs-mediated pro-and anti-inflammatory responses and barrier function may lead to development of novel therapies to ameliorate oxidative stress related cardio-pulmonary disorders.
Keywords: Lipid Peroxides, 4-Hydroxynonenal, Oxidized Phospholipids, Cytoskeleton, Endothelial Permeability, Focal adhesions
1. INTRODUCTION
Living systems constantly encounter free radicals that are generated by either enzymatic or non-enzymatic mechanisms which if not detoxified, lead to oxidative stress. Peroxidation of membrane lipids results in the generation of several highly reactive aldehydes, and oxidized phospholipids. 4-Hydroxy-2-nonenal (4-HNE), one of the major biologically active aldehydes, is formed during inflammation and oxidative stress and can accumulate in certain tissues from μM to mM concentrations (Esterbauer et al., 1991). Increased levels of 4-HNE invoke a wide range of biological activities and activation of stress signaling pathways. Several in vivo and in vitro studies have demonstrated that 4-HNE is a key player in cardiovascular and lung disorders (Kaminski et al., 2008; Negre-Salvayre et al., 2010; Parola et al., 1999; Poli et al., 2008; Uchida, 2003; Uchida et al., 1999; Yang et al., 2003b). The formation and accumulation 4-HNE in tissues have been shown under different pathophysiological conditions. Enhanced levels of 4-HNE-modified proteins have been described in ischemic rat hearts or airway and alveolar epithelial cells, and in endothelial cells of subjects with chronic obstructive pulmonary disease (COPD) (Chaudhary et al., 2010; Compton et al., 1998; Eaton et al., 1999; Gutierrez et al., 2006; Herbst et al., 1999; Kaminski et al., 2008; Mertsch et al., 2001; Rahman et al., 2002; Uchida et al., 1999). In human, ozone exposure causes formation of protein adducts, induction of stress proteins and apoptosis in airway and lung cells (Hamilton et al., 1998). It has been proposed that 4-HNE formed during shock, sepsis, and ischemia/reperfusion causally related to lung injury (Compton et al., 1998; Herbst et al., 1999; Negre-Salvayre et al., 2010; Poli et al., 2008). Interestingly, 4-HNE applied exogenously elicits similar effects as that of the endogenous form. Infusion of low doses of 4-HNE into isolated rat lungs results in peri-vascular edema with vascular compression and early endothelial cell (EC) barrier disruption (Herbst et al., 1999). 4-HNE increases paracellular transport of albumin across human umbilical EC monolayer (Herbst et al., 1999) and increases blood-brain barrier permeability (Mertsch et al., 2001). Similarly, peroxidation of membrane associated polyunsaturated phosphatidylcholine (PC) to oxidized PC with a short chain valeraldehyde at sn-2 position has been shown to exhibit potent signaling properties and modulate cellular functions in vascular endothelium (Berliner and Gharavi, 2008; Bochkov et al., 2010; Catala, 2009; Fu and Birukov, 2009). In recent years, a number of excellent reviews have described the pathophysiological roles of 4-HNE and oxidized PC in mammalian systems (Awasthi et al., 2003; Berliner and Gharavi, 2008; Bochkov et al., 2010; Compton et al., 1998; Dubinina and Dadali, 2010; Esterbauer et al., 1991; Forman, 2010; Gutierrez et al., 2006; Leonarduzzi et al., 2004; Negre-Salvayre et al., 2010; Parola et al., 1999; Poli et al., 2008; Uchida, 2003; Yang et al., 2003b). This article focuses on recent advances in 4-HNE and oxidized PC as modulators of endothelial signal transduction, reorganization of cytoskeletal proteins and barrier function.
2. HYDROXYALKENALS AND OXIDIZED PHOSPHOLIPIDS
2.1 GENERATION OF HYDROXYALKENALS AND OXIDIZED PHOSPHOLIPIDS VIA LIPID PEROXIDATION
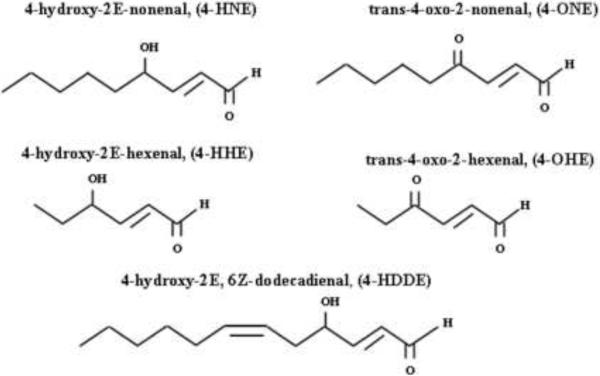
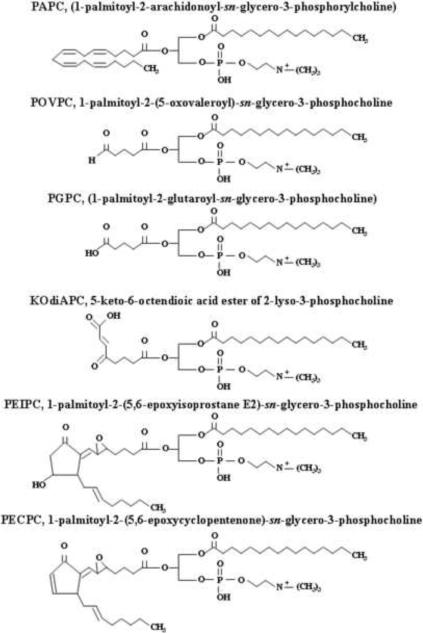
Peroxidation of oxidation products of polyunsaturated fatty acids (PUFAs) by enzymatic- and non-enzymatic mechanisms generate hydroxyalkenals and Ox-PLs (Berliner and Gharavi, 2008; Bochkov et al., 2010; Catala, 2009; Schneider et al., 2001). Non-enzymatic peroxidation of n-3- (18:3 alpha-linolenic acid, 20:5 eicosapentaenoic acid, and 22:6, docosahexaenoic acid) and n-6- (18:2 linoleic acid, 18:3 gamma linoleic acid, 22:4 adrenic acid) PUFAs generates 4-hydroxy-2E-hexenal (4-HHE) and 4-hydroxy-2E-nonenal (4-HNE) (types of 4-hydroxyalkenals), respectively in biological systems (Fig. 1). In the non-enzymatic pathway, the initial step of oxidation is mediated by hydroxyl- and lipid peroxyl-radicals generated by oxidized metabolites that undergo intramolecular rearrangement and carbon-carbon cleavage reactions to form different types of saturated and unsaturated aldehydes. Unlike the non-enzymatic pathway that can utilize both n-3- and n-6- PUFAs (Guichardant et al., 2006; Schneider et al., 2001), the enzymatically regulated peroxidation pathway predominantly converts n-6-PUFAs to generate 4-HNE and 4-hydroxy-2E, 6Z-dodecadienal (4-HDDE) (Guichardant et al., 2002). Additionally, n-3- and n-6- PUFA oxidation leads to the generation of volatile keto aldehydes such as trans-4-oxo-2-hexenal (4-OHE), and trans-4-oxo-2-nonenal (4-ONE), respectively. In cells, PUFAs are esterified to phospholipids and cholesterol; and peroxidation, initiated by formation of hydroperoxides or peroxyradicals, generates a mixture of oxidized phospholipids and cholesterol (Bochkov et al., 2010; Catala, 2009). Oxidative fragmentation of phospholipids produces γ-hydroxy-α,β-unsaturated aldehyde, γ-keto-α,β-unsaturated aldehyde, γ-hydroxy-α,β-unsaturated carboxylic and γ-keto-α,β-unsaturated carboxylic phospholipids (Bochkov et al., 2010; Catala, 2009). Oxidation of 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine (PAPC), a major phospholipid present in cell membranes and minimally modified low density lipoproteins (LDL), generates several products with truncated oxidized residues at sn-2 position of the glycerol backbone (Groeneweg et al., 2008). Some of the well characterized oxidized PAPC include (Fig. 2): 1-palmitoyl-2-(5-oxovalleroyl)-sn-glycero-3-phosphorylcholine (POVPC),1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine (PGPC), and 5-keto-6-octenoic acid ester of 2-lysophosphatidylcholine (KOdiAPC) (Groeneweg et al., 2008). Addition of oxygen atom to PUFAs at sn-2 position generates another set of oxygenated Ox-PL such as 1-palmitoyl-2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphocholine (PEIPC) and 1-palmitoyl-2-(5,6-epoxycyclopentenone)-sn-glycero-3-phosphocholine (PECPC). A recent review on generation of oxidized phospholipids and their biological activities provides a comprehensive view with well illustrated structures of the hydroxyalkenals and Ox-PLs derived from PAPC (Bochkov et al., 2010; Catala, 2009; Compton et al., 1998; Dubinina and Dadali, 2010; Esterbauer et al., 1991; Forman, 2010; Fu and Birukov, 2009; Leonarduzzi et al., 2004; Parola et al., 1999; Poli et al., 2008).
Fig. 1. Hydroxyalkenals generated by lipid peroxidation under oxidative stress.
Peroxidation of polyunsaturated fatty acids by enzymatic- and non-enzymatic mechanisms generate bioactive hydroxy- and keto-aldehydes such as 4-hydroxy-2E-nonenal (4-HNE), trans-4-oxo-2-nonenal (4-ONE), 4-hydroxy-2E-hexenal (4-HHE), trans-4-oxo-2-hexenal (4-OHE), and 4-hydroxy-2E-, 6Z-dodecadienal (4-HDDE).
Fig. 2. Oxidized phospholipids generated under oxidative stress.
Oxidation of 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphoryllcholine (PAPC) generates several products with truncated oxidized residues at sn-2 position of the glycerol backbone.
2.2 INTERACTIONS AND ADDUCT FORMATION OF 4-HYDROXYALKENALS AND OXIDIZED PHOSPHOLIPIDS
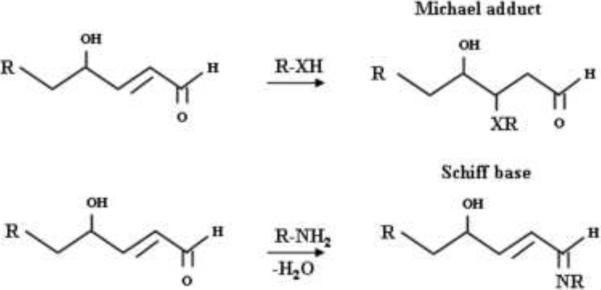
The presence of electrophilic α, β-unsaturated aldehydes in 4-hydroxyalkenals promotes Michael adduct formation at C3 position with biomolecules containing nucleophiles such as thiols, and amines and Schiff base adducts (Fig. 3) with proteins, phospholipids and DNA. To date, both 4-HNE and 4-HHE have been demonstrated to form adducts with DNA, proteins, glutathione, and phosphatidylethanolamine (Bacot et al., 2003; Dickinson and Forman, 2002; Guichardant et al., 2002; Negre-Salvayre et al., 2008; Roede et al., 2008; Spite et al., 2007). The high lipophilicity of 4-HNE and 4-HDDE favors their accumulation and generation of adducts with membrane phospholipids and proteins (Esterbauer et al., 1991). 4-Hydroxyalkenal-induced modification of membrane phospholipids and proteins can modulate membrane fluidity, structure and function. 4-Hydroxyalkenals can also interact with DNA forming etheno (ε) adducts with deoxyribonucleotide moieties (Esterbauer et al., 1991). Ox-PLs containing reactive aldehyde group or α, β-unsaturated carbonyl groups form Schiff bases or Michael adducts with NH2- and SH-groups of proteins and free thiols such as glutathione (Dickinson and Forman, 2002; Dubinina and Dadali, 2010; Esterbauer et al., 1991; Forman, 2010). Thus, interaction of 4-hydroxyalkenals and Ox-PLs with NH2- group(s) in proteins can inactivate amino acid residues and reduce protein activity or induce conformational changes in proteins resulting in protein dysfunction and membrane fluidity. Generation and accumulation of 4-hydroxyalkenals vary in different cell types, which dictate differences in cytotoxicity and biological functions among tissues.
Fig. 3. Modification of macromolecules by hydroxyalkenals.
Hydroxyalkenals form Michael adducts and Schiff base with proteins, DNA and lipids.
2.3. DETOXIFICATION OF 4-HYDROXYALKENAL AND OXIDIZED PHOSPHOLIPIDS
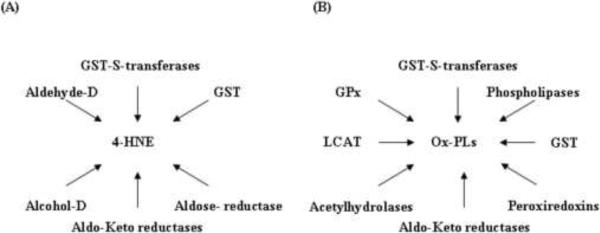
Under physiological conditions the cellular concentrations of 4-hydroxyalkenals range from 0.1–1.0 μM (Esterbauer et al., 1991). However, under conditions of oxidative stress, 4-HNE accumulates in tissues up to concentration of 10 μM to 5 mM (Esterbauer et al., 1991). Post-hypoxic re-oxygenation condition or post-ischemic organ reperfusion results in enhanced 4-HNE level in different tissues and organs such as liver (hepatocytes), heart and small intestine (Compton et al., 1998; Eaton et al., 1999; Gutierrez et al., 2006; Hamilton et al., 1998; Kaminski et al., 2008; Mertsch et al., 2001; Nakamura et al., 2009; Rahman et al., 2002; Renner et al., 2005). 4-HNE accumulation is regulated by multiple pathways involved in its detoxification and mediated (Fig. 4 A) by glutathione S-transferases, aldehyde dehydrogenase, alcohol dehydrogenase, aldose reductase, and aldo-keto reductase (Awasthi et al., 2004; Cheng et al., 2001a; Cheng et al., 2001b; Dickinson and Forman, 2002; Forman, 2010; He et al., 1996; Raza and John, 2006; Sharma et al., 2004; Sharma et al., 2003; Srivastava et al., 2004; Tjalkens et al., 1998; Yang et al., 2004). GSH is a vital and protective intracellular antioxidant; and changes in cellular GSH or GSH/GSSG ratio may be a key factor in the development and progression of many cardiovascular diseases (Dickinson and Forman, 2002; Sharma et al., 2003). Glutathione S-transferase, the main enzyme that detoxifies cellular 4-HNE by conjugation with GSH reduces accumulation of lipid hydroperoxides including reactive aldehydes (Awasthi et al., 2004). In addition to acting via the constitutively expressed glutathione S-transferase, oxidants such as H2O2, 4-HNE, lipid hydroperoxides or UV-light treatment can induce glutathione S-transferase to detoxify the excess oxidants generated in mammalian cells (Awasthi et al., 2003; Raza and John, 2006; Tjalkens et al., 1998; Yang et al., 2003a). Similar to 4-hydroxyalkenal mediated signaling pathways, several other systems also contribute to detoxification of Ox-PLs (Fig. 4 B). Reactive peroxide groups in Ox-PLs are reduced by phospholipid glutathione peroxidase (GPx) as well as peroxiredoxin VI and glutathione transferase to corresponding hydroxides (Berliner and Gharavi, 2008; Bochkov et al., 2010; Catala, 2009; Manevich and Fisher, 2005; Negre-Salvayre et al., 2008; Roede et al., 2008). Further, reactive carbonyl groups in Ox-PLs are detoxified by aldo-keto reductases that transform the carbonyl groups to hydroxyl groups. Additionally, several phospholipases and platelet activating factor acetylhydrolase cleave the oxidized residues at sn-2 position of the Ox-PL to generate lyso phospholipids and oxidized fatty aldehydes/acids. Similarly, lecithin: cholesterol acyltransferase (LCAT) can catalyze the transfer of the oxidized fatty acid from Ox-PLs to cholesterol generating lyso phospholipids and cholesterol esters (Goyal et al., 1997). Ox-PLs also form covalent adducts with proteins via their aldehyde or α, β-unsaturated carbonyl groups forming Michael adducts or Schiff bases (Forman, 2010; Leonarduzzi et al., 2004; Poli et al., 2008; Uchida, 2003).
Fig. 4. Detoxification pathways of hydroxyalkenals and oxidized phospholipids.
(A) 4-HNE accumulation is regulated by glutathione S-transferases (GST-S-transferases), glutathione (GST), aldehyde dehydrogenase (Aldehyde-D), alcohol dehydrogenase (Alcohol-D), aldose reductase, and aldo-keto reductase (Aldo-Keto-reductases). (B) Ox-PLs accumulation is regulated by glutathione S-transferases (GST-S-transferases), glutathione (GST), phospholipid glutathione peroxidase (GPx), peroxiredoxins, aldo-keto reductases (Aldo-Keto-reductases), phospholipases, acetylhydrolase and lecithin: cholesterol acyltransferase (LCAT).
3. PATHOPHYSIOLOGICAL AND SIGNALING EFFECTS OF HYDROXYALKENALS AND OXIDIZED PHOSPHOLIPIDS
3.1 PATHOPHYSIOLOGICAL ASPECTS OF HYDROXYNONENALS
Hydroxyalkenals and Ox-PLs generated in vivo during various pathological conditions such as atherosclerosis, ischemia/reperfusion injury, diabetes and cancer can exert deleterious effects (Bochkov et al., 2010; Poli et al., 2008). There is evidence in the literature that show an association between increased levels of 4-HNE and other lipid peroxidation products and development of oxidative stress-related human disorders (Berliner and Gharavi, 2008; Bochkov et al., 2010; Eaton et al., 1999; Hamilton et al., 1998; Herbst et al., 1999; Kaminski et al., 2008; Mertsch et al., 2001; Nakamura et al., 2009; Rahman et al., 2002; Renner et al., 2005; Uchida et al., 1999; Yang et al., 2004); however, it is unclear if hydroxyalkenals have a direct role in the pathophysiology or are a consequence. Moreover, hydroxyalkenals and Ox-PLs play a role as intracellular signaling molecules altering cellular responses to stress and toxicity, modulate cellular DNA, RNA, and protein synthesis, cell growth, and differentiation, and apoptosis (Bochkov et al., 2010; Parola et al., 1999; Poli et al., 2008; Uchida, 2003). One very interesting target for 4-HNE is the caspases. Caspases are a family of cysteine proteases responsible for promoting cell death (Awasthi et al., 2003). Present as inactive pro-caspases, most caspases are activated following cleavage at a specific aspartate site by assembly of their active subunit forms - caspase-3, caspase-8 and caspase-9. Caspase-3 may then cleave cellular target proteins including poly-ADP-ribose-polymerase (PARP) (Awasthi et al., 2003). 4-HNE treatment of ECs resulted in a 7 to 9 fold increase in caspase-3 activity at 50 and 100 μM concentrations, respectively. However, at lower doses of 4-HNE (1– 25 μM) there was no significant change in caspase-3 activity confirming the induction of apoptosis at higher, but not at lower doses of 4-HNE (Usatyuk and Natarajan, 2004). Furthermore, PARP cleavage, as assessed by appearance of ~89 kDa protein, was detected by 4 h after 4-HNE (50 and 100 μM) treatment of ECs (Usatyuk and Natarajan, 2004).
Several studies describe pathological aspects of lipid peroxidation in different cell types and organs such as hepatocytes, monocytes, intestinal epithelium, kidney tubular cells, heart and lung. The formation and accumulation of 4-HNE in tissues have been shown under different pathophysiological conditions. Atherosclerotic lesions in experimental animal models and patients exhibited accumulation of oxidatively modified LDL with the enhanced 4-HNE levels in the lesions (Hamilton et al., 1998). Further, accumulation of 4-HNE and 4-HNE-modified proteins have been described in ischemic or dilated cardiomyopathy hearts, airway and alveolar epithelial cells, and in ECs of subjects with chronic obstructive pulmonary disease (COPD (Poli et al., 2008; Rahman et al., 2002; Uchida, 2003). Increased 4-HNE-protein adducts was detected in plasma from patients with acute myocardial infarction and with systemic inflammatory syndrome (Kaminski et al., 2008). In human subjects, ozone exposure induces formation of 4-HNE protein adducts, and apoptosis in airway and lung cells (Hamilton et al., 1998). Infusion of low doses of 4-HNE into isolated rat lungs results in peri-vascular edema with vascular compression and early ECs disruption (Herbst et al., 1999). 4-HNE increases paracellular transport of albumin across human umbilical ECs monolayer (Herbst et al., 1999) and increases blood-brain barrier permeability (Mertsch et al., 2001). 4-HNE, applied exogenously, in a time- and dose-dependent (1–100 μM) fashion, increased EC permeability (Usatyuk and Natarajan, 2004) through the changes in cell-cell adhesion interaction (Usatyuk et al., 2006).
Changes in [Ca2+]i in response to agonists or reactive oxygen species ROS have been implicated in EC barrier function. It has been shown that 4-HNE did not increase intracellular calcium, although it decreased transmembrane electrical resistance (TER) suggesting calcium-independent mechanism of 4-HNE-mediated barrier dysfunction in lung microvascular ECs (Usatyuk and Natarajan, 2004). In contrast to these results, earlier studies have demonstrated that 4-HNE modulated [Ca2+]i in hepatocytes and neurons (Lu et al., 2002; Mark et al., 1997). Indeed, Ox-PLs, but not its non-oxidized precursor PLs, induced rapid and transient elevation of Ca2+ in HUVECs (Bochkov et al., 2002b). 4-HNE-induced generation of ROS was mainly localized to mitochondria (Landar et al., 2006; Roede and Jones, 2008; Usatyuk et al., 2006) and was almost completely abolished by rotenone suggesting involvement of mitochondrial complex I in ROS generation (Usatyuk et al., 2006). Substances containing –SH groups such as mercapto-propionyl-glycine (MPG) and N-acetylcysteine (NAC) confer protective effect against 4-HNE mediated cytotoxic responses (Dickinson and Forman, 2002). NAC blocks 4-HNE-induced depletion in cellular glutathione (Usatyuk et al., 2006), apoptosis, and suppresses activation of stress-activated protein kinases (Usatyuk et al., 2006). Administration of MPG to the heart before ischemia, reduces 4-HNE protein adducts by 75% (Eaton et al., 1999), and there is evidence for beneficial effects of thiol agents against pathological aspects of lipid peroxidation in cardiovascular diseases (Sochman, 2002).
3.2 HYDROXYALKENALS AND OXIDIZED PHOSPHOLIPIDS AS SIGNALING MOLECULES
Hydroxyalkenals and Ox-PLs because of their electrophilic nature, formed adducts with cellular nucleophiles such as proteins and lipids, which provide a rationale for the active aldehydes to modulate signaling pathways regulating cellular functions (Esterbauer et al., 1991). The ability of hydroxyalkenals and Ox-PLs to modulate enzymes and signaling pathways depends on the cell type, detoxification mechanisms and concentrations employed. There is growing evidence that 4-HNE and Ox-POPC, in physiological and pathophysiological concentrations, modulates stress response mechanisms, detoxification pathways, survival and apoptotic mechanisms and inflammatory responses, which are briefly described below.
3.2.1. 4-HYDROXYNONENAL MODULATES PROTEIN KINASE C and RECEPTOR TYROSINE KINASES
There is mounting evidence for 4-HNE and Ox-PLs to modulate a number of protein kinases involved in cell signaling. While higher concentrations of 4-HNE (10–100 μM) modulated both Ca2+ - and Ca2+- independent protein kinase C (PKC) isoforms, activation of PKC-β isoforms at lower concentrations of 4-HNE (0.1–1 μM) was observed that correlated to transport and secretion of glycoproteins in rat hepatocytes (Chiarpotto et al., 1999). Similarly, 4-HNE induced activation of PKC-β isoforms in NT2 cells (Paola et al., 2000) that correlated with increased intracellular PKC-β generation. In addition to PKC-β isoforms, higher concentrations of 4-HNE activated PKC δ in isolated rat hepatocytes that was linked to apoptosis (Paola et al., 2000). Potential mechanism(s) of down-regulation of PKC activity by relatively high doses of 4-HNE could involve excessive alkylation of thiol groups due to PKC-HNE adduct formation (Schwarzer et al., 1996). 4-HNE and other hydroxyalkenals, depending upon incubation time and concentration, stimulated autophosphorylation of receptor tyrosine kinases (RTKs) such as PDGF- and EGFRs via formation of stable adducts with specific amino acid residues in the RTKs (Escargueil-Blanc et al., 2001; Negre-Salvayre et al., 2003). Such a stimulation of RTK by 4-HNE resulted in activation of down-stream signaling cascades such as PI3K/Akt survival pathway and mitogenic responses in SMCs (Auge et al., 2002). In contrast to inhibitory effects of higher concentrations of 4-HNE on PKC-β isoforms, low concentrations of 4-HNE inhibited tyrosine phosphorylation of PDGF-Rβ by PDGF-BB resulting in inhibition of MAPKs and PI3K signaling and decreased DNA synthesis (Robino et al., 2000).
3.2.2. 4-HYDROXYNONENAL MODULATES IkB AND NF-kB SIGNAL TRANSDUCTION
Hydroxyalkenals affect NF-kB signal transduction, a major signaling pathway linked to oxidative stress and inflammation in mammalian cells. Chlamydia pneumonia or LPS-activated IKK/I kappa B-mediated signaling was inhibited by primary exposure to 4-HNE (Donath et al., 2002; Page et al., 1999). This inhibitory effect of 4-HNE seems to involve direct interaction and covalent adduct formation between 4-HNE and IkB proteins with subsequent blockage of phosphorylation and degradation of IkB proteins (Ji et al., 2001). Such an effect on nF-kB signaling by 4-HNE can be relevant to expression of genes regulated by NF-kB and in the pathophysiology of inflammatory diseases (Page et al., 1999).
3.2.3. 4-HYDROXYNONENAL MODULATES MITOGEN-ACTIVATED PROTEIN KINASES
Mitogen activated protein kinases (MAPKs) that include extracellular signal-regulated kinase (ERK1/2), p38 mitogen-activated protein kinase (MAPK) and c-Jun NH2-terminal kinase (JNK) play significant role in the cellular stress responses. 4-HNE was shown to markedly up-regulate MAPK activities, which in turn alter other cell signaling pathways (Borbiev et al., 2004; Cheng et al., 2001b; Usatyuk and Natarajan, 2004; Usatyuk et al., 2003; Wang and Doerschuk, 2001). Possible mechanism(s) includes phosphorylation of targets like heat shock protein (HSP) 27 (Guay et al., 1997), cytoskeletal proteins (Usatyuk and Natarajan, 2004; Wang and Doerschuk, 2001), focal adhesion and adherens junction proteins including cadherins and catenins (Usatyuk et al., 2006). Phosphorylation of HSP 27 is mediated by the mitogen activated kinase-activated protein kinase-2, a downstream target of activated p38 MAPK (Stokoe et al., 1992). Earlier studies have shown that 4-HNE-mediated MAPK activation were attenuated by MAPK specific inhibitors, PD 98059 (an inhibitor of MEK1/2), SP600125 (an inhibitor of JNK) or SB 202190 (an inhibitor of p38 MAPK). JNKs were activated in PC12 neuroblastoma cells by 4-HNE, while ERK1/2 and p38 MAPK remained unchanged (Song et al., 2001). Thus, hydroxyalkenals activate MAPKs, which are involved in several cellular functions including proliferation, apoptosis, secretion and endothelial/epithelial barrier function.
3.2.4. 4-HYDROXYNONENAL MODULATES PHOSPHOLIPASE D
Phospholipase D (PLD), a phospholipid phosphohydrolase, catalyzes the hydrolysis of phosphatidylcholine and other membrane phospholipids to phosphatidic acid (PA) and choline. PLD, ubiquitous in mammals, is a critical enzyme in intracellular signal transduction. PA generated by agonist- or ROS-mediated activation of the PLD1 and PLD2 isoforms can be subsequently converted to lyso PA (LPA) or diacylglycerol (DAG) by phospholipase A1/A2 or lipid phosphate phosphatases (Cummings et al., 2002). Treatment of bovine pulmonary artery endothelial cells (BPAECs) labeled with [32P] orthophosphate (5 h for minimal phospholipid labeling) and [3H] myristic acid (24 h) with 4-HNE in the presence of 0.5% ethanol resulted in the formation of [3H] phosphatidylethanol (PEt) and [3H] phosphatidic acid (PA) with very little accumulation of [32P] PEt. The formation of [3H] PEt, as opposed to [32P] PEt, suggests that PEt synthesis was not through de novo pathway but rather through the PLD mechanism. 4-Hydroxynonenal-induced PLD activation was dose- and time-dependent, and the formation of PEt was not affected by chelation of either extracellular Ca2+ with EGTA or intracellular Ca2+ with BAPTA-AM. PLD activation by 4-HNE was independent of PKC activity (Natarajan et al., 1993). Furthermore, vanadate, phenylarsine oxide, and diamide, inhibitors of protein tyrosine phosphatases (PTPases), markedly increased the 4-HNE-induced PLD activation (Natarajan et al., 1997). The effects of tyrosine kinase and PTPase inhibitors were specific towards the 4-HNE, as these agents had no effect on the agonist- or TPA-induced PLD activation. In addition to 4-HNE, 4-hydroxyoctenal and 4-hydroxyhexenal also stimulated PLD (Natarajan et al., 1993). Among the various nonylaldehydes examined, only trans-2-nonenal and trans-2-cis 6-nonadienal exhibited PLD activation, suggesting the requirement of a trans double bond at carbon 2 and a hydroxyl group at carbon 4 (Natarajan et al., 1993). These data provide evidence that 4-HNE, a metabolite of membrane lipid peroxidation, is involved in endothelial cell (EC) signal transduction, through the activation of PLD and the generation of second messengers like PA and DAG (Cummings et al., 2002; Natarajan et al., 1993; Natarajan et al., 1997).
3.2.5. 4-HYDROXYHEXENAL MODULATES NF-kB SIGNAL TRANSDUCTION
Although 4-HHE is the most abundant hydroxyalkenal in tissues, very little is known about its signaling functions under normal or pathological situations. 4-HHE stimulated NF-kB via NIK/IKK and p38 MAPK (Je et al., 2004) and enhanced iNOS gene expression in vascular ECs (Lee et al., 2004). The 4-HHE mediated activation of NF-kB and induction of iNOS in YPEN-1 cells contrasts with the lack of NF-kB activation and iNOS induction by 4-HNE in RAW 264.7 macrophage cell line (Kumagai et al., 2004). The ability of 4-HHE to modulate endothelial barrier function is unknown; however, the biological activity of 4-HHE is distinct from 4-HNE suggesting the importance of 4-HHE in tissues rich in n-3 PUFAs.
3.2.6. OXIDIZED PHOSPHOLIPIDS MODULATE SIGNAL TRANSDUCTION PATHWAYS AND GENE EXPRESSION
Based on cell types, Ox-PLs exhibit both pro- and anti-inflammatory properties and modulate a variety of cell responses such as cytoskeletal remodeling, barrier function, inflammation, pro-coagulant activity, stress responses, angiogenesis and gene expression. Numerous studies have shown that Ox-PLs activate monocytes and its binding to the vascular ECs via monocyte-specific chemo-attractants such as monocyte chemotactic protein 1 (MCP1), monocyte/EC adhesion proteins, PI3K and α5β1 integrin (Cole et al., 2003; Lee et al., 2000). Ox-PL stimulated monocyte adhesion to endothelium seems to depend on binding to a G-protein coupled receptor. The mechanism of Ox-PL-induced monocyte binding to ECs is different from that mediated by other inflammatory agents, such as LPS or TNFa (Bochkov et al., 2002a; Ma et al., 2004). Ox-PLs do not enhance expression of intercellular adhesion molecule-1 (ICAM-1), VCAM-1, and E-selectin expression; however, it up-regulates MCP1, CS1, and P-selectin resulting in monocyte, but not neutrophils, adhesion to the endothelium (Bochkov et al., 2010; Catala, 2009). There is evidence for increased expression of IL-8, IL-6, MCP-1 and other inflammatory chemokines by Ox-PLs (Papadopoulou et al., 2008). Unlike LPS and TNFα, the induction of inflammatory mediators by Ox-PLs is NF–κB independent and mediated by Src/JAK2/STAT3 signal transduction pathways (Yeh et al., 2004). Recent findings suggest a role for the unfolded protein response (UPR), an adaptive signaling pathway triggered by endoplasmic reticulum stress (Ron and Walter, 2007), signaling cascade in Ox-PLs-induced induction of inflammatory cytokines in human atherosclerotic lesions. In ECs, signaling mechanisms activated by Ox-PLs include changes in intracellular Ca2+, elevation of cyclic AMP, activation of protein kinases such as PKA, PKC, and MAPKs, and also induction of MAP-kinase phosphatase 1, resulting in the activation of transcription mediated by several transcription factors such as Egr-1 and NFAT, CREB, PPARα, and PPARγ (Bochkov et al., 2010; Negre-Salvayre et al., 2010). Further, Ox-PLs induce generation of ROS in ECs by activation of NADPH oxides that leads to depletion of GSH and loss of redox balance (Honjo et al., 2008; Lee et al., 2009; Rouhanizadeh et al., 2005; Takeshita et al., 2000). Ox-PLs also induce expression of HO-1 in HUVECs that involve ROS/MAPK-dependent phosphorylation of CREB (Kronke et al., 2003). Ox-PLs also induce expression of OKL 38 and ATF4 through transcription factor nuclear factor E2-related factor (Nrf2) mediated signal transduction (He et al., 2001). 4-HNE treatment of cells alters the expression of several transcription factors including c-Myc, c-Myb and c-Jun (Barrera et al., 1994; Barrera et al., 2005; Barrera et al., 1996), suggesting that it have more global effects on protein expression and cell function.
4. HYDROXYALKENALS AND OXIDIZED PHOSPHOLIPIDS MODULATE ENDOTHELIAL BARRIER FUNCTION
Endothelial cells, lining the blood vessels, serve as a semi-permeable barrier to circulating cells, plasma albumin, macromolecules, and bioactive agents. Maintenance of EC barrier integrity is critical for vessel wall homeostasis and normal organ function. Among various circulating edemic agents, ROS, generated at sites of inflammation and injury by activated polymorphonuclear leukocytes or ECs, play an important role in the disruption of barrier function (Mehta and Malik, 2006).
4.1. 4-HYDROXYNONENAL MODULATES ENDOTHELIAL BARRIER FUNCTION THROUGH FOCAL ADHESION KINASE
Previous studies from various laboratories have demonstrated that exposure of ECs to ROS or edemic agents induce actin stress fibers, enhance protein tyrosine phosphorylation of Src, focal adhesion, and adherens junction proteins, and alter EC permeability through paracellular pathway (Mehta and Malik, 2006; Mehta et al., 2002). Whereas the role of intracellular Ca2+, Src family kinases, myosin light chain kinase, and PKC in mediating EC barrier dysfunction has been described (Hu and Minshall, 2009; Tiruppathi et al., 2006), the involvement of focal adhesions and adherens junctions in barrier regulation is not well understood. Focal adhesions, sites of close contact between cell-cell and cell-extracellular matrix, are essential for normal cell growth, differentiation, inter- and intracellular communication, and tissue integrity (Mehta and Malik, 2006; Mehta et al., 2002). Among the protein complexes associated with focal adhesions, focal adhesion kinase (FAK) and paxillin play an important role in the transmission of integrin-induced cytoplasmic signals and in the reorganization of actin cytoskeleton (Katsumi et al., 2004; Parsons, 2003; Su et al., 2007). FAK, an ~125-kDa tyrosine kinase, is activated primarily through integrin-mediated cell adhesion to extracellular matrix and to a lesser extent by growth factors, bioactive lipids, neuropeptides, and ROS (Mehta and Malik, 2006). Autophosphorylation of FAK at Y397 induced by its localization to focal adhesions is central to agonist-dependent phosphorylation of other tyrosine residues of FAK and FAK-dependent cellular responses (Parsons, 2003). Although several studies suggest a role of FAK and other cytoskeletal proteins in agonist- or ROS-mediated endothelial permeability, specific mechanisms of FAK- and cytoskeletal regulation of EC barrier function remain poorly defined. It was hypothesized that 4-HNE-induced changes in cellular thiol redox status may contribute to modulation of cell signaling pathways and endothelial barrier dysfunction involving focal adhesion, adherens and tight junction proteins as well as integrin signal transduction (Usatyuk et al., 2006). Treatment of ECs with 4-HNE, in dose- and time-dependent fashion, decreased intracellular GSH suggesting that 4-HNE caused depletion of cellular GSH and causes cellular thiol redox status in ECs (Usatyuk et al., 2006). Furthermore, 4-HNE decreased FAK tyrosine phosphorylation in a time-dependent fashion without altering the levels of non-phosphorylated FAK (Usatyuk et al., 2006). NAC and MPG attenuated reduction in FAK phosphorylation by 4-HNE (Usatyuk et al., 2006). These results suggest that 4-HNE suppresses FAK phosphorylation, which in turn affect focal adhesion, adherens and tight junction proteins, cell-cell contacts and gap formation and ultimately ECs barrier function. There are several possible mechanisms underlying the role of FAK in EC barrier regulation: 1) agonist-dependent activation of FAK leads to disassembly and redistribution of focal adhesions leading to EC rounding and gap formation; 2) activation of FAK tightens the intercellular gaps resulting in assembly rather than disassembly; 3) activation of FAK regulates myosin light chain phosphorylation, development of contractile force and gap formation; 4) inactivation of FAK by FRNK or dn FAK reduces tyrosine phosphorylation of adherens junction proteins and tightening of the intercellular junctions and 5) activation of FAK modulates intracellular signaling molecules such as Src-family of non-receptor kinases, paxillin and Rho that alter barrier function.
4.2. 4-HYDROXYNONENAL MODULATES ENDOTHELIAL BARRIER FUNCTION THROUGH CYTOSKELETAL REORGANIZATION
There is considerable evidence for the role of cytoskeletal proteins including actin and actin binding proteins, focal adhesion and adherens junction proteins in regulation of EC barrier function. Recent studies have demonstrated that ROS potently and rapidly polymerized G-actin to F-actin and decreased TER in lung ECs, suggesting participation of actin reorganization in barrier function (Dudek and Garcia, 2001). We and others observed that 4-HNE induced actin stress fibers as well as intercellular gap formation suggesting a link between F-actin cytoskeletal rearrangement and permeability changes (Aldini et al., 2005; Gadoni et al., 1993; Gayarre et al., 2006; Huot et al., 1997; Ozeki et al., 2005; Usatyuk and Natarajan, 2004; VanWinkle et al., 1994). The effect of 4-HNE on actin rearrangement was partially prevented by MAPK inhibitors and thiol protectants suggesting an important role for ERK, p38 MAPK and JNK and redox-dependent mechanisms in cytoskeletal regulation (Usatyuk and Natarajan, 2004). It has been recently reported that actin can be modified by the Michael addition of 4-HNE to Cys374 (Aldini et al., 2005; Ozeki et al., 2005). Exposure of purified actin at increasing reactive aldehyde (4-HNE and acrolein) concentrations have identified the sites of these aldehyde additions using LC-ESI-MS/MS (Aldini et al., 2005). As Cys374 is a preferential target for various oxidative/nitrosative modifications, and actin is one of the main carbonylated proteins in vivo, it was suggested that the highly reactive Cys374 could serve as a carbonyl scavenger of reactive α, β-unsaturated aldehydes and other electrophilic lipids (Ozeki et al., 2005). Glutathionylation of Cys374 regulates actin polymerization (Dalle-Donne et al., 2003). Further LC-ESI-MS/MS analysis indicated that the reactivity of Cys374 residue is due to a significant accessible surface and substantial thiol acidity due to the particular microenvironment surrounding Cys374 (Aldini et al., 2005). Native actin forms long regular double-helical filaments. Pre-treatment of actin with 4-HNE markedly altered filament structure leading to the formation of shorter and more disperse filaments (Gayarre et al., 2006). Immunoaffinity separation and sequencing revealed that actin is a major target protein for HNE modification in vivo (Dalle-Donne et al., 2007; Ozeki et al., 2005). We observed that 4-HNE induced actin stress fibers, actin aggregation as well as intercellular gap formation in lung ECs (Usatyuk and Natarajan, 2004). These data point out that 4-HNE-dependent destabilization or stabilization of actin microfilaments with other cytoskeletal proteins regulate EC barrier function. Modification of cytoskeletal proteins, including actin and microtubules, by 4-HNE and other α, β-unsaturated aldehydes plays an important role in the regulation of cell-cell contacts and endothelial barrier function (Usatyuk et al., 2006). Cross-talk between FAK, and cytoskeleton seems to be involved in FAK autophosphorylation and activation by integrins (Mehta et al., 2002; Parsons, 2003; Romer et al., 2006; Sun et al., 2009).
4.3. 4-HYDROXYNONENAL MODULATES ENDOTHELIAL BARRIER FUNCTION THROUGH INTEGRINS
Cross-talk between FAK, integrin and cytoskeleton has been proposed to be responsible for FAK activation and autophosphorylation by integrin in cell adhesion (Mehta et al., 2002; Parsons, 2003; Romer et al., 2006; Sun et al., 2009). Integrins are a family of transmembrane α and β heterodimers consisting of matrix binding sites for collagen, laminin, vitronectin and fibronectin (Wang et al., 2006). The cytoplasmic domain of integrin interacts with a number of signaling and adaptor proteins that link to focal adhesion and cytoskeleton (Katsumi et al., 2004). It is well established that integrins are critical mediators of lung vascular permeability and acute lung injury. As cell-matrix adhesion and cell shape are mediated by integrins, loss of this binding could result in intercellular gaps and disruption of EC barrier function. In addition, it is shown that integrin-mediated activation of focal adhesion proteins could be induced in cells derived from FAK-deficient (FAK−/−) animals (Ueki et al., 1998). Integrin-mediated adhesion to the extracellular matrix regulate RhoA signaling (Vigil et al.) which play pivotal roles in the regulation of endothelial integrity and permeability (Carbajal and Schaeffer, 1999). Suppression of Rho kinase activity in mice with Y-27632, a specific Rho kinase inhibitor, decreased infarct volume substantiated the relevance of Rho kinase overactivity to ischemic cerebral damage (Rikitake et al., 2005). The ability of FAK to down-regulate RhoA activity is well documented (Holinstat et al., 2006), and it has been shown that FAK may interact with the Rho GTPase-activating protein (GAP) GRAF (Vigil et al.). It was recently demonstrated for the first time that 4-HNE caused a rapid (15 min) decline in surface integrins and tyrosine phosphorylation of FAK (Usatyuk et al., 2006), which explain the dramatic alteration EC barrier dysfunction leading to tissue damage and edema during heart infarction, brain stroke and lung diseases.
The mechanism(s) by which 4-HNE affects integrins signaling is not completely understood. One of the possible pathways may involve 4-HNE-induced activation of Ephrin kinases which in turn can inhibit integrin-mediated cell adhesion (Miao et al., 2000). Further, integrin-mediated ROS production required inhibition of a low molecular weight protein tyrosine phosphatase that prevented dephosphorylation and activation of FAK (Chiarugi et al., 2003; Hernandez-Hernandez et al., 2005; Hernandez-Hernandez et al., 1999); however, it is unclear if such a mechanism is involved in attenuation of tyrosine phosphorylation of FAK by 4-HNE in lung ECs. One of the main mechanism(s) explaining 4-HNE-induced integrin signaling in EC barrier dysfunction may involve 4-HNE-mediated Michael adducts formation with focal adhesion and adherens junction proteins as well as with α5 and β3 integrins, modification of their structure and function which in turn alter EC barrier function (Usatyuk et al., 2006). How 4-HNE alters interaction Rho GAPs or Rho GEFs (Pirone et al., 2006) and, thus, modulate RhoA activity and EC fuction is completely understood.
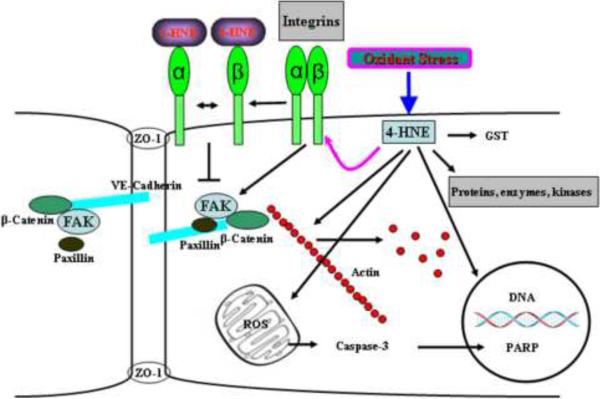
Thus, several studies support a role for integrins-mediated tyrosine phosphorylation of FAK and other focal adhesion and adherens junction proteins in endothelial barrier regulation under oxidative stress. In recent years, considerable advance has been made in a better understanding of molecular mechanisms and potential signaling pathways involved in 4-HNE-mediated endothelial barrier regulation; however, there is a gap in integrating several aspects of focal adhesion kinases, adherens junction proteins, integrins, and the cytoskeletal proteins in endothelial barrier dysfunction by 4-HNE (Fig. 5).
Fig. 5. Mechanism(s) of 4-HNE mediated modulation of signal transduction in ECs.
4-HNE generated by oxidative stress results in: DNA and protein modification by formation of Michael adducts and Schiff bases, depletion of intracellular thiols including GSH and ROS production in mitochondria. Dramatic increase of mitochondrial ROS causes activation of caspases, which in turn modulates PARP cleavage and apoptosis. Depletion of intracellular thiols generated by exposure to 4-HNE, results in cytoskeleton reorganization, the activation of phospholipase D, PKC, and mitogen-activated protein kinases. Furthermore, Michael adducts generated in the presence of 4-HNE, modulate focal adhesion and integrin signaling. Thus, 4-HNE dependent formation of Michael adducts and depletion of intracellular thiols modulates integrin and FAK resulting in reorganization of cytoskeletal and focal adhesion proteins, decrease in cell-cell contacts resulting in barrier dysfunction in ECs.
4.4. OXIDIZED PHOSPHOLIPIDS AND ENDOTHELIAL BARRIER REGULATION INVOLVING SMALL GTPases, FOCAL ADHESION AND ADHERENS JUNCTION PROTEINS
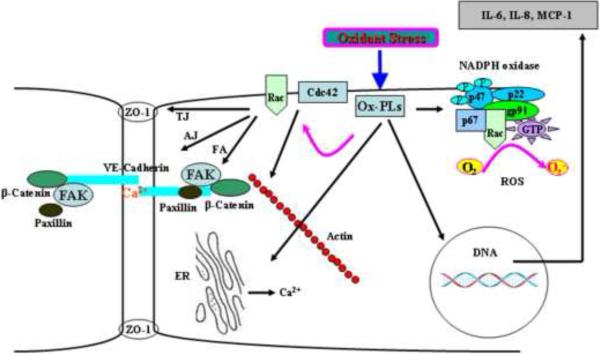
In contrast to barrier disruptive property of hydroxyalkenals (Mertsch et al., 2001; Usatyuk and Natarajan, 2004; Usatyuk et al., 2006), Ox-PLs exhibit anti-inflammatory and protective effects (Berliner and Gharavi, 2008; Bochkov et al., 2010). In certain instances, Ox-PLs also show pro-inflammatory property, which is dependent on concentration and route of delivery. The anti-inflammatory effects are mediated by modulation of signal transduction pathways that enhance barrier function, and expression of transcription factors regulating anti- and pro-inflammatory genes (Berliner and Gharavi, 2008; Bochkov et al., 2010). Intratracheal instillation of Ox-PLs protected LPS- and VILI-induced lung injury in animal models (Bochkov et al., 2002a) and endothelial cells exposed to thrombin and cyclic stretch (Birukova et al., 2007b). However, higher doses of instilled Ox-PLs induced lung injury by causing barrier disruptive effects on the epithelial cells (Birukova et al., 2007b). In vitro, exposure of pulmonary ECs to Ox-PLs protected LPS- or thrombin-induced barrier disruption (Birukova et al., 2007b). Mechanisms that regulate EC barrier protective effects of Ox-PLs include balance between Rac and Rho small GTPases, reorganization and interaction of cytoskeletal, focal adhesions, and adhesion junction proteins (Birukov et al., 2004a; Birukov et al., 2004b; Birukova et al., 2007a; Birukova et al., 2007c; Bochkov et al., 2002a). Ox-PLs-induced barrier protection of pulmonary ECs results from activation of Rac and Cdc42 leading to the enhancement of coffilin phosphorylation, actin polymerization at cell periphery (Birukov et al., 2004a; Birukova et al., 2007c), and redistribution of adherens junction and focal adhesion proteins via paxillin binding to beta-catenin (Birukova et al., 2007c). Furthermore, upstream activation of PKA, Src and PKC was involved in Ox-PL mediated Rac/Cdc42 pathway in the endothelium (Bochkov et al., 2010). The barrier-protective effects of PKA due to its ability to attenuate the endothelial myosin light chain (MLC) kinase activity that leads to a decreased basal level of MLC phosphorylation, PAK-mediated inhibition of Rho activity, and phosphorylation of the actin-binding proteins filamin, adductin, dematin, as well as paxillin and FAK (Bochkov et al., 2010). In a recent study it was demonstrated that Src kinase inhibition abolished Ox-PAPC-induced phosphorylation of FAK and its substrate paxillin, a focal adhesion protein, which functions as structural and adaptor protein (Birukova et al., 2007a). Further, this study showed that inhibitors of PKA and PKC abolished Ox-PAPC-induced Rac activation, whereas MEK/ERK-1,2 and p38 MAP kinase inhibitors were without effect. Also, down-regulation of small GTPases by pretreatment with toxin B attenuated phosphorylation of Src, FAK and paxillin (Birukova et al., 2007a). Since Ox-PAPC does not activate Rho, these results suggest involvement of Rac-dependent tyrosine phosphorylation of Src, FAK, and paxillin, which may be an important part of coordinated focal adhesion and cytoskeletal remodeling associated with barrier-protective effects of Ox-PAPC. Thus, Ox-PLs exhibit a broad range of biologic activities, including pro-inflammatory and anti-inflammatory effects as well as the regulation of vascular permeability (Fig. 6). Spatial distribution, concentration and type of Ox-PLs generated under different pathological situations may dictate activation or inhibition of signaling pathways that regulate pro- or anti-inflammatory effects of these bioactive mediators in vivo.
Fig. 6. Oxidized phospholipids and signal transduction in ECs.
Oxidized phospholipids (Ox-PLs) generated by lipid peroxidation of phospholipids containing polyunsaturated fatty acids at sn-2 position of the glycerol backbone modulate wide range of signaling pathways. Ox-PLs stimulate ROS production by activation of NADPH oxidase, enhance intracellular calcium release from endoplasmic reticulum, regulate gene expression and cytokine production. Ox-PL-mediated activation of Rac/Cdc42 in the endothelium induces reorganization of cytoskeleton, activation of FAK and enhancement of tight junction (TJ), adherent junction (AJ) and focal adhesion (FA) proteins, increase in cell-cell contacts resulting in protection of EC barrier function.
5. CONCLUSSION
The early studies of Dr. Esterbauer and co-workers (Benedetti et al., 1980; Benedetti et al., 1982; Esterbauer et al., 1986) on generation of cytotoxic lipid peroxidation products led to the identification and characterization of several α, β-unsaturated aldehydes including the most abundant family member, 4-HNE. Later work in the 1990s identified Ox-PLs, the major components in minimally modified LDL, for their ability to initiate inflammation in atherosclerotic lesions. Accumulating evidence now supports a role for hydroxyalkenals and Ox-PLs in different human pathologies including atherosclerosis, inflammation, acute lung injury and diabetes. In addition to cytotoxic effects, hydroxyalkenals and Ox-PLs are reorganized as bioactive lipid mediators that form adducts with proteins, lipids and other macromolecules resulting in modified membrane architecture, protein function and cellular metabolism. Hydroxyalkenals- and Ox-PLs-induced changes in cellular thiol redox status may contribute to modulation of cell signaling pathways and endothelial barrier function involving focal adhesion, adherens and tight junction proteins as well as integrin signal transduction. Mechanisms by which hydroxyalkenals and Ox-PLs alter EC permeability include: (i) modulation of activity of proteins/enzymes by Michael adducts formation; (ii) enhancing the level of protein tyrosine phosphorylation of the target proteins by the inhibition of phosphatases; (iii) redox-dependent mechanisms due to the depletion of cellular thiols; (iv) reorganization of focal adhesion and adherens junction proteins; and (v) integrin-mediated regulation of cell-cell adhesion. While this short review summarizes the potential role of 4-HNE and Ox-PLs in modulating endothelial signal transduction and barrier regulation, identification of new cellular targets and characterization of cellular compartmentalization would lead to a better understanding on the biological functions of these bioactive lipid peroxidation products to the pathophysiology of cardio-pulmonary diseases.
RESEARCH HIGHLIGHTS.
-
▶
4-HNE and Ox-PLs exhibit pro- and anti-inflammatory effects in the lung.
-
▶
4-HNE and Ox-PLs modulate endothelial cell signaling and gene expression.
-
▶
4-HNE and Ox-PLs induce reorganization of cytoskeletal and adherens junction proteins.
-
▶
4-HNE and Ox-PLs alter endothelial barrier function through focal adhesion kinases.
6. ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants P01 HL 58064 and R01 HL 085553 to V.N. The authors thank Dr. Prasad Kanteti for his invaluable assistance in reading and editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aldini G, Dalle-Donne I, Vistoli G, Maffei Facino, R., Carini M. Covalent modification of actin by 4-hydroxy-trans-2-nonenal (HNE): LC-ESI-MS/MS evidence for Cys374 Michael adduction. J Mass Spectrom. 2005;40:946–954. doi: 10.1002/jms.872. [DOI] [PubMed] [Google Scholar]
- Auge N, Garcia V, Maupas-Schwalm F, Levade T, Salvayre R, Negre-Salvayre A. Oxidized LDL-induced smooth muscle cell proliferation involves the EGF receptor/PI-3 kinase/Akt and the sphingolipid signaling pathways. Arterioscler Thromb Vasc Biol. 2002;22:1990–1995. doi: 10.1161/01.atv.0000043453.21629.3b. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Sharma R, Cheng JZ, Yang Y, Sharma A, Singhal SS, Awasthi S. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med. 2003;24:219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Bacot S, Bernoud-Hubac N, Baddas N, Chantegrel B, Deshayes C, Doutheau A, Lagarde M, Guichardant M. Covalent binding of hydroxy-alkenals 4-HDDE, 4-HHE, and 4-HNE to ethanolamine phospholipid subclasses. J Lipid Res. 2003;44:917–926. doi: 10.1194/jlr.M200450-JLR200. [DOI] [PubMed] [Google Scholar]
- Barrera G, Muraca R, Pizzimenti S, Serra A, Rosso C, Saglio G, Farace MG, Fazio VM, Dianzani MU. Inhibition of c-myc expression induced by 4-hydroxynonenal, a product of lipid peroxidation, in the HL-60 human leukemic cell line. Biochem Biophys Res Commun. 1994;203:553–561. doi: 10.1006/bbrc.1994.2218. [DOI] [PubMed] [Google Scholar]
- Barrera G, Pizzimenti S, Laurora S, Briatore F, Toaldo C, Dianzani MU. 4-hydroxynonenal and cell cycle. Biofactors. 2005;24:151–157. doi: 10.1002/biof.5520240118. [DOI] [PubMed] [Google Scholar]
- Barrera G, Pizzimenti S, Serra A, Ferretti C, Fazio VM, Saglio G, Dianzani MU. 4-hydroxynonenal specifically inhibits c-myb but does not affect c-fos expressions in HL-60 cells. Biochem Biophys Res Commun. 1996;227:589–593. doi: 10.1006/bbrc.1996.1550. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Esterbauer H, Ferrali M, Fulceri R, Comporti M. Evidence for aldehydes bound to liver microsomal protein following CCl4 or BrCCl3 poisoning. Biochim Biophys Acta. 1982;711:345–356. doi: 10.1016/0005-2760(82)90044-3. [DOI] [PubMed] [Google Scholar]
- Berliner JA, Gharavi NM. Endothelial cell regulation by phospholipid oxidation products. Free Radic Biol Med. 2008;45:119–123. doi: 10.1016/j.freeradbiomed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004a;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Leitinger N, Bochkov VN, Garcia JG. Signal transduction pathways activated in human pulmonary endothelial cells by OxPAPC, a bioactive component of oxidized lipoproteins. Microvasc Res. 2004b;67:18–28. doi: 10.1016/j.mvr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Chatchavalvanich S, Oskolkova O, Bochkov VN, Birukov KG. Signaling pathways involved in OxPAPC-induced pulmonary endothelial barrier protection. Microvasc Res. 2007a;73:173–181. doi: 10.1016/j.mvr.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007b;292:L924–935. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-beta-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2007c;293:L199–211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002a;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 2002b;99:199–206. doi: 10.1182/blood.v99.1.199. [DOI] [PubMed] [Google Scholar]
- Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbiev T, Birukova A, Liu F, Nurmukhambetova S, Gerthoffer WT, Garcia JG, Verin AD. p38 MAP kinase-dependent regulation of endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L911–918. doi: 10.1152/ajplung.00372.2003. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, Schaeffer RC., Jr. RhoA inactivation enhances endothelial barrier function. Am J Physiol. 1999;277:C955–964. doi: 10.1152/ajpcell.1999.277.5.C955. [DOI] [PubMed] [Google Scholar]
- Catala A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, Singhal SS, Rauniyar N, Prokai L, Awasthi S, Awasthi YC. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry. 49:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001a;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Singhal SS, Sharma A, Saini M, Yang Y, Awasthi S, Zimniak P, Awasthi YC. Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Arch Biochem Biophys. 2001b;392:197–207. doi: 10.1006/abbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- Chiarpotto E, Domenicotti C, Paola D, Vitali A, Nitti M, Pronzato MA, Biasi F, Cottalasso D, Marinari UM, Dragonetti A, et al. Regulation of rat hepatocyte protein kinase C beta isoenzymes by the lipid peroxidation product 4-hydroxy-2,3-nonenal: A signaling pathway to modulate vesicular transport of glycoproteins. Hepatology. 1999;29:1565–1572. doi: 10.1002/hep.510290510. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol. 2003;161:933–944. doi: 10.1083/jcb.200211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23:1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- Compton CN, Franko AP, Murray MT, Diebel LN, Dulchavsky SA. Signaling of apoptotic lung injury by lipid hydroperoxides. J Trauma. 1998;44:783–788. doi: 10.1097/00005373-199805000-00007. [DOI] [PubMed] [Google Scholar]
- Cummings R, Parinandi N, Wang L, Usatyuk P, Natarajan V. Phospholipase D/phosphatidic acid signal transduction: role and physiological significance in lung. Mol Cell Biochem. 2002;234–235:99–109. [PubMed] [Google Scholar]
- Dalle-Donne I, Carini M, Vistoli G, Gamberoni L, Giustarini D, Colombo R, Maffei Facino, R., Rossi R, Milzani A, Aldini G. Actin Cys374 as a nucleophilic target of alpha,beta-unsaturated aldehydes. Free Radic Biol Med. 2007;42:583–598. doi: 10.1016/j.freeradbiomed.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I, Giustarini D, Rossi R, Colombo R, Milzani A. Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic Biol Med. 2003;34:23–32. doi: 10.1016/s0891-5849(02)01182-6. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Donath B, Fischer C, Page S, Prebeck S, Jilg N, Weber M, da Costa C, Neumeier D, Miethke T, Brand K. Chlamydia pneumoniae activates IKK/I kappa B-mediated signaling, which is inhibited by 4-HNE and following primary exposure. Atherosclerosis. 2002;165:79–88. doi: 10.1016/s0021-9150(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Dubinina EE, Dadali VA. Role of 4-hydroxy-trans-2-nonenal in cell functions. Biochemistry (Mosc) 75:1069–1087. doi: 10.1134/s0006297910090014. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol. 1999;276:H935–943. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- Escargueil-Blanc I, Salvayre R, Vacaresse N, Jurgens G, Darblade B, Arnal JF, Parthasarathy S, Negre-Salvayre A. Mildly oxidized LDL induces activation of platelet-derived growth factor beta-receptor pathway. Circulation. 2001;104:1814–1821. doi: 10.1161/hc4001.097179. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Benedetti A, Lang J, Fulceri R, Fauler G, Comporti M. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochim Biophys Acta. 1986;876:154–166. doi: 10.1016/0005-2760(86)90329-2. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Forman HJ. Reactive oxygen species and alpha,beta-unsaturated aldehydes as second messengers in signal transduction. Ann N Y Acad Sci. 1203:35–44. doi: 10.1111/j.1749-6632.2010.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Birukov KG. Oxidized phospholipids in control of inflammation and endothelial barrier. Transl Res. 2009;153:166–176. doi: 10.1016/j.trsl.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadoni E, Olivero A, Miglietta A, Bocca C, Gabriel L. Cytoskeletal modifications induced by 4-hydroxynonenal. Cytotechnology. 1993;11(Suppl 1):S62–64. doi: 10.1007/BF00746057. [DOI] [PubMed] [Google Scholar]
- Gayarre J, Sanchez D, Sanchez-Gomez FJ, Terron MC, Llorca O, Perez-Sala D. Addition of electrophilic lipids to actin alters filament structure. Biochem Biophys Res Commun. 2006;349:1387–1393. doi: 10.1016/j.bbrc.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Goyal J, Wang K, Liu M, Subbaiah PV. Novel function of lecithin-cholesterol acyltransferase. Hydrolysis of oxidized polar phospholipids generated during lipoprotein oxidation. J Biol Chem. 1997;272:16231–16239. doi: 10.1074/jbc.272.26.16231. [DOI] [PubMed] [Google Scholar]
- Groeneweg M, Vergouwe MN, Scheffer PG, Vermue HP, Sollewijn Gelpke, M. D., Sijbers AM, Leitinger N, Hofker MH, de Winther MP. Modification of LDL with oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC) results in a novel form of minimally modified LDL that modulates gene expression in macrophages. Biochim Biophys Acta. 2008;1781:336–343. doi: 10.1016/j.bbalip.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci. 1997;110(Pt 3):357–368. doi: 10.1242/jcs.110.3.357. [DOI] [PubMed] [Google Scholar]
- Guichardant M, Bacot S, Moliere P, Lagarde M. Hydroxy-alkenals from the peroxidation of n-3 and n-6 fatty acids and urinary metabolites. Prostaglandins Leukot Essent Fatty Acids. 2006;75:179–182. doi: 10.1016/j.plefa.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guichardant M, Bernoud-Hubac N, Chantegrel B, Deshayes C, Lagarde M. Aldehydes from n-6 fatty acid peroxidation. Effects on aminophospholipids. Prostaglandins Leukot Essent Fatty Acids. 2002;67:147–149. doi: 10.1054/plef.2002.0412. [DOI] [PubMed] [Google Scholar]
- Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Jr., Li L, Eschenbacher WL, Szweda L, Holian A. Potential involvement of 4-hydroxynonenal in the response of human lung cells to ozone. Am J Physiol. 1998;274:L8–16. doi: 10.1152/ajplung.1998.274.1.L8. [DOI] [PubMed] [Google Scholar]
- He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- He NG, Singhal SS, Chaubey M, Awasthi S, Zimniak P, Partridge CA, Awasthi YC. Purification and characterization of a 4-hydroxynonenal metabolizing glutathione S-transferase isozyme from bovine pulmonary microvessel endothelial cells. Biochim Biophys Acta. 1996;1291:182–188. doi: 10.1016/s0304-4165(96)00064-5. [DOI] [PubMed] [Google Scholar]
- Herbst U, Toborek M, Kaiser S, Mattson MP, Hennig B. 4-Hydroxynonenal induces dysfunction and apoptosis of cultured endothelial cells. J Cell Physiol. 1999;181:295–303. doi: 10.1002/(SICI)1097-4652(199911)181:2<295::AID-JCP11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hernandez A, Garabatos MN, Rodriguez MC, Vidal ML, Lopez-Revuelta A, Sanchez-Gallego JI, Llanillo M, Sanchez-Yague J. Structural characteristics of a lipid peroxidation product, trans-2-nonenal, that favour inhibition of membrane-associated phosphotyrosine phosphatase activity. Biochim Biophys Acta. 2005;1726:317–325. doi: 10.1016/j.bbagen.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hernandez A, Sanchez-Yague J, Martin-Valmaseda EM, Llanillo M. Oxidative inactivation of human and sheep platelet membrane-associated phosphotyrosine phosphatase activity. Free Radic Biol Med. 1999;26:1218–1230. doi: 10.1016/s0891-5849(98)00306-2. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006;281:2296–2305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- Honjo T, Otsui K, Shiraki R, Kawashima S, Sawamura T, Yokoyama M, Inoue N. Essential role of NOXA1 in generation of reactive oxygen species induced by oxidized low-density lipoprotein in human vascular endothelial cells. Endothelium. 2008;15:137–141. doi: 10.1080/10623320802125433. [DOI] [PubMed] [Google Scholar]
- Hu G, Minshall RD. Regulation of transendothelial permeability by Src kinase. Microvasc Res. 2009;77:21–25. doi: 10.1016/j.mvr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- Je JH, Lee JY, Jung KJ, Sung B, Go EK, Yu BP, Chung HY. NF-kappaB activation mechanism of 4-hydroxyhexenal via NIK/IKK and p38 MAPK pathway. FEBS Lett. 2004;566:183–189. doi: 10.1016/j.febslet.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Ji C, Kozak KR, Marnett LJ. IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J Biol Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- Kaminski K, Bonda T, Wojtkowska I, Dobrzycki S, Kralisz P, Nowak K, Prokopczuk P, Skrzydlewska E, Kozuch M, Musial WJ. Oxidative stress and antioxidative defense parameters early after reperfusion therapy for acute myocardial infarction. Acute Card Care. 2008;10:121–126. doi: 10.1080/17482940701744334. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003;278:51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- Landar A, Zmijewski JW, Dickinson DA, Le Goffe C, Johnson MS, Milne GL, Zanoni G, Vidari G, Morrow JD, Darley-Usmar VM. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- Lee H, Shi W, Tontonoz P, Wang S, Subbanagounder G, Hedrick CC, Hama S, Borromeo C, Evans RM, Berliner JA, Nagy L. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res. 2000;87:516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- Lee JY, Je JH, Jung KJ, Yu BP, Chung HY. Induction of endothelial iNOS by 4-hydroxyhexenal through NF-kappaB activation. Free Radic Biol Med. 2004;37:539–548. doi: 10.1016/j.freeradbiomed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Lee S, Gharavi NM, Honda H, Chang I, Kim B, Jen N, Li R, Zimman A, Berliner JA. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radic Biol Med. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonarduzzi G, Robbesyn F, Poli G. Signaling kinases modulated by 4-hydroxynonenal. Free Radic Biol Med. 2004;37:1694–1702. doi: 10.1016/j.freeradbiomed.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Lu C, Chan SL, Fu W, Mattson MP. The lipid peroxidation product 4-hydroxynonenal facilitates opening of voltage-dependent Ca2+ channels in neurons by increasing protein tyrosine phosphorylation. J Biol Chem. 2002;277:24368–24375. doi: 10.1074/jbc.M201924200. [DOI] [PubMed] [Google Scholar]
- Ma Z, Li J, Yang L, Mu Y, Xie W, Pitt B, Li S. Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2004;286:L808–816. doi: 10.1152/ajplung.00220.2003. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Mehta D, Tiruppathi C, Sandoval R, Minshall RD, Holinstat M, Malik AB. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol. 2002;539:779–789. doi: 10.1113/jphysiol.2001.013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertsch K, Blasig I, Grune T. 4-Hydroxynonenal impairs the permeability of an in vitro rat blood-brain barrier. Neurosci Lett. 2001;314:135–138. doi: 10.1016/s0304-3940(01)02299-6. [DOI] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Miura D, Kusano KF, Fujimoto Y, Sumita-Yoshikawa W, Fuke S, Nishii N, Nagase S, Hata Y, Morita H, et al. 4-Hydroxy-2-nonenal induces calcium overload via the generation of reactive oxygen species in isolated rat cardiac myocytes. J Card Fail. 2009;15:709–716. doi: 10.1016/j.cardfail.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Scribner WM, Taher MM. 4-Hydroxynonenal, a metabolite of lipid peroxidation, activates phospholipase D in vascular endothelial cells. Free Radic Biol Med. 1993;15:365–375. doi: 10.1016/0891-5849(93)90036-t. [DOI] [PubMed] [Google Scholar]
- Natarajan V, Scribner WM, Vepa S. Phosphatase inhibitors potentiate 4-hydroxynonenal-induced phospholipase D activation in vascular endothelial cells. Am J Respir Cell Mol Biol. 1997;17:251–259. doi: 10.1165/ajrcmb.17.2.2623. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, et al. Pathological aspects of lipid peroxidation. Free Radic Res. 44:1125–1171. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negre-Salvayre A, Vieira O, Escargueil-Blanc I, Salvayre R. Oxidized LDL and 4-hydroxynonenal modulate tyrosine kinase receptor activity. Mol Aspects Med. 2003;24:251–261. doi: 10.1016/s0098-2997(03)00020-7. [DOI] [PubMed] [Google Scholar]
- Ozeki M, Miyagawa-Hayashino A, Akatsuka S, Shirase T, Lee WH, Uchida K, Toyokuni S. Susceptibility of actin to modification by 4-hydroxy-2-nonenal. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:119–126. doi: 10.1016/j.jchromb.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Page S, Fischer C, Baumgartner B, Haas M, Kreusel U, Loidl G, Hayn M, Ziegler-Heitbrock HW, Neumeier D, Brand K. 4-Hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- Paola D, Domenicotti C, Nitti M, Vitali A, Borghi R, Cottalasso D, Zaccheo D, Odetti P, Strocchi P, Marinari UM, et al. Oxidative stress induces increase in intracellular amyloid beta-protein production and selective activation of betaI and betaII PKCs in NT2 cells. Biochem Biophys Res Commun. 2000;268:642–646. doi: 10.1006/bbrc.2000.2164. [DOI] [PubMed] [Google Scholar]
- Papadopoulou C, Corrigall V, Taylor PR, Poston RN. The role of the chemokines MCP-1, GRO-alpha, IL-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine. 2008;43:181–186. doi: 10.1016/j.cyto.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, Romer LH, Chen CS. An inhibitory role for FAK in regulating proliferation: a link between limited adhesion and RhoA-ROCK signaling. J Cell Biol. 2006;174:277–288. doi: 10.1083/jcb.200510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med Res Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- Raza H, John A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–318. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Renner A, Sagstetter MR, Harms H, Lange V, Gotz ME, Elert O. Formation of 4-hydroxy-2-nonenal protein adducts in the ischemic rat heart after transplantation. J Heart Lung Transplant. 2005;24:730–736. doi: 10.1016/j.healun.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robino G, Parola M, Marra F, Caligiuri A, De Franco RM, Zamara E, Bellomo G, Gentilini P, Pinzani M, Dianzani MU. Interaction between 4-hydroxy-2,3-alkenals and the platelet-derived growth factor-beta receptor. Reduced tyrosine phosphorylation and downstream signaling in hepatic stellate cells. J Biol Chem. 2000;275:40561–40567. doi: 10.1074/jbc.M007694200. [DOI] [PubMed] [Google Scholar]
- Roede JR, Carbone DL, Doorn JA, Kirichenko OV, Reigan P, Petersen DR. In vitro and in silico characterization of peroxiredoxin 6 modified by 4-hydroxynonenal and 4-oxononenal. Chem Res Toxicol. 2008;21:2289–2299. doi: 10.1021/tx800244u. [DOI] [PubMed] [Google Scholar]
- Roede JR, Jones DP. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environ Mol Mutagen. 51:380–390. doi: 10.1002/em.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer LH, Birukov KG, Garcia JG. Focal adhesions: paradigm for a signaling nexus. Circ Res. 2006;98:606–616. doi: 10.1161/01.RES.0000207408.31270.db. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rouhanizadeh M, Hwang J, Clempus RE, Marcu L, Lassegue B, Sevanian A, Hsiai TK. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radic Biol Med. 2005;39:1512–1522. doi: 10.1016/j.freeradbiomed.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J Biol Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- Schwarzer E, Muller O, Arese P, Siems WG, Grune T. Increased levels of 4-hydroxynonenal in human monocytes fed with malarial pigment hemozoin. A possible clue for hemozoin toxicity. FEBS Lett. 1996;388:119–122. doi: 10.1016/0014-5793(96)00523-6. [DOI] [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Dwivedi S, Popov VL, Boor PJ, Singhal SS, Awasthi S, Awasthi YC. Mechanisms and physiological significance of the transport of the glutathione conjugate of 4-hydroxynonenal in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:3438–3449. doi: 10.1167/iovs.03-0051. [DOI] [PubMed] [Google Scholar]
- Sochman J. N-acetylcysteine in acute cardiology: 10 years later: what do we know and what would we like to know?! J Am Coll Cardiol. 2002;39:1422–1428. doi: 10.1016/s0735-1097(02)01797-7. [DOI] [PubMed] [Google Scholar]
- Song BJ, Soh Y, Bae M, Pie J, Wan J, Jeong K. Apoptosis of PC12 cells by 4-hydroxy-2-nonenal is mediated through selective activation of the c-Jun N-terminal protein kinase pathway. Chem Biol Interact. 2001;130–132:943–954. doi: 10.1016/s0009-2797(00)00247-7. [DOI] [PubMed] [Google Scholar]
- Spite M, Baba SP, Ahmed Y, Barski OA, Nijhawan K, Petrash JM, Bhatnagar A, Srivastava S. Substrate specificity and catalytic efficiency of aldoketo reductases with phospholipid aldehydes. Biochem J. 2007;405:95–105. doi: 10.1042/BJ20061743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Spite M, Trent JO, West MB, Ahmed Y, Bhatnagar A. Aldose reductase-catalyzed reduction of aldehyde phospholipids. J Biol Chem. 2004;279:53395–53406. doi: 10.1074/jbc.M403416200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D, Campbell DG, Nakielny S, Hidaka H, Leevers SJ, Marshall C, Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. Embo J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Hodnett M, Wu N, Atakilit A, Kosinski C, Godzich M, Huang XZ, Kim JK, Frank JA, Matthay MA, et al. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol. 2007;36:377–386. doi: 10.1165/rcmb.2006-0238OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Shikata Y, Wang L, Ohmori K, Watanabe N, Wada J, Shikata K, Birukov KG, Makino H, Jacobson JR, et al. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res. 2009;77:304–313. doi: 10.1016/j.mvr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S, Inoue N, Gao D, Rikitake Y, Kawashima S, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J Atheroscler Thromb. 2000;7:238–246. doi: 10.5551/jat1994.7.238. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- Tjalkens RB, Luckey SW, Kroll DJ, Petersen DR. Alpha, beta-unsaturated aldehydes increase glutathione S-transferase mRNA and protein: correlation with activation of the antioxidant response element. Arch Biochem Biophys. 1998;359:42–50. doi: 10.1006/abbi.1998.0895. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- Ueki K, Mimura T, Nakamoto T, Sasaki T, Aizawa S, Hirai H, Yano S, Naruse T, Nojima Y. Integrin-mediated signal transduction in cells lacking focal adhesion kinase p125FAK. FEBS Lett. 1998;432:197–201. doi: 10.1016/s0014-5793(98)00862-x. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem. 2004;279:11789–11797. doi: 10.1074/jbc.M311184200. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem. 2006;281:35554–35566. doi: 10.1074/jbc.M607305200. [DOI] [PubMed] [Google Scholar]
- Usatyuk PV, Vepa S, Watkins T, He D, Parinandi NL, Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- VanWinkle WB, Snuggs M, Miller JC, Buja LM. Cytoskeletal alterations in cultured cardiomyocytes following exposure to the lipid peroxidation product, 4-hydroxynonenal. Cell Motil Cytoskeleton. 1994;28:119–134. doi: 10.1002/cm.970280204. [DOI] [PubMed] [Google Scholar]
- Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Doerschuk CM. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J Immunol. 2001;166:6877–6884. doi: 10.4049/jimmunol.166.11.6877. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin G, Miao H, Li JY, Usami S, Chien S. Integrins regulate VE-cadherin and catenins: dependence of this regulation on Src, but not on Ras. Proc Natl Acad Sci U S A. 2006;103:1774–1779. doi: 10.1073/pnas.0510774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sharma A, Sharma R, Patrick B, Singhal SS, Zimniak P, Awasthi S, Awasthi YC. Cells preconditioned with mild, transient UVA irradiation acquire resistance to oxidative stress and UVA-induced apoptosis: role of 4-hydroxynonenal in UVA-mediated signaling for apoptosis. J Biol Chem. 2003a;278:41380–41388. doi: 10.1074/jbc.M305766200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003b;50:319–336. [PubMed] [Google Scholar]
- Yang Y, Trent MB, He N, Lick SD, Zimniak P, Awasthi YC, Boor PJ. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis. Atherosclerosis. 2004;173:211–221. doi: 10.1016/j.atherosclerosis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Yeh M, Gharavi NM, Choi J, Hsieh X, Reed E, Mouillesseaux KP, Cole AL, Reddy ST, Berliner JA. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J Biol Chem. 2004;279:30175–30181. doi: 10.1074/jbc.M312198200. [DOI] [PubMed] [Google Scholar]