INTRODUCTION
Exposure to early life adversity has been identified in both animal and human models as a risk factor for developing anxiety later in life (Coplan et al., 1996; Huot et al., 2001; Heim and Nemeroff, 2001). In nonhuman primates, these effects have been studied in infant monkeys exposed to different early rearing environments (see review by Stevens et al., 2009). Infants reared with other naïve infants for the first 8 months of life (peer rearing) develop most species-typical patterns of behavior but show more intense reactions to social separation (Higley et al., 1991) and also display heightened anxiety, particularly in response to novel events, compared to mother-peer-reared (MPR) monkeys (Harlow, 1969; Suomi, 1991). As juveniles, peer-reared (PR) monkeys show lower levels of affiliative behavior and are less likely to have their stress levels reduced by the presence of a companion than normally reared monkeys (Winslow et al., 2003). Juvenile PR monkeys also show a heightened acoustic startle response (Parr et al., 2002), enhanced fear potentiated startle (Nelson et al., 2009), and disrupted sleep patterns (Barrett et al., 2009) compared to mother reared controls. Very little information is available on PR adults. However, in one study, adult PR animals showed heightened vulnerability to alcohol consumption compared with normally reared adults (Fahlke et al., 2000), a finding consistent with the view that increased anxiety may persist into adulthood following peer rearing.
Despite the link between peer rearing and anxiety, no consistent pattern has emerged from an examination of hypothalamic-pituitary-adrenocortical (HPA) axis function in these infants. PR monkeys have been reported to show higher basal levels of cortisol (Barrett et al., 2009; Higley et al,. 1992), lower basal levels but no difference in stress responsivity (Shannon et al., 1998), no difference in basal levels but lower stress responsivity (Clarke, 1993), higher stress responsivity (Fahlke et al., 2000), a lower cortisol set point (Capitanio et al., 2005), and lastly no effects in either baseline or stress responsivity (Winslow et al., 2003). Variation in rearing practices or other differences between primate facilities might account for some of these inconsistencies.
HPA function has also been examined in a second kind of early nursery rearing environment: surrogate-peer rearing (SPR). In this condition, infants are reared with inanimate surrogate mothers and given up to 2 hours of daily play experience with other infants. The purpose of this rearing condition is to reduce the profound attachment that occurs between infants, thereby presumably reducing anxiety and facilitating play behavior. Although less is known about the effects of this rearing procedure, infant monkeys appear to acquire all the typical forms of species-normative behavior without showing excessive clinging (Hansen, 1966). As adults, SPR monkeys are indistinguishable from mother reared monkeys in their reproductive and parental behavior (Novak et al., 1992; Sackett et al., 2002). Converging lines of evidence suggest that infant SPR monkeys have significantly lower basal concentrations of circulating cortisol (Capitanio et al., 2005) than MPR and PR counterparts, although in one study (Davenport et al., 2003), this difference was present only in the first month of life. Other studies have revealed that SPR monkeys respond significantly less to the stress of brief social separation (Meyer et al., 1975; Shannon et al., 1998) than both MPR monkeys and PR monkeys, which is perhaps not surprising given the inanimate nature of their mother and their restricted interaction with peers.
One of the limitations of previous research on HPA activity in differently reared monkeys is a reliance on blood plasma as the primary sample matrix, given the well-known lability of circulating cortisol to circadian variation and environmental disturbances. Moreover, studies of HPA function over the course of development necessitate repeated sample collections, each requiring capture and sedation of the animals which not only further stresses the subjects but may also confound the results. A long-term measure of circulating glucocorticoids would greatly reduce the frequency of sampling required and would eliminate the confounds imposed by capture and restraint.
As a potential alternative to measuring cortisol “point samples” in plasma or saliva, hair cortisol has recently gained attention as an index of chronic HPA activity (Davenport et al., 2006; Sauvé et al., 2007; Kirschbaum et al., 2009) and as a possible biomarker for stress-related health disorders in both human and nonhuman primate populations. Hair cortisol has proven to be a reliable indicator of the effects of various stressors. In adult rhesus monkeys, relocation to a different environment resulted in elevated hair cortisol along with marked behavioral changes and sleep disturbances (Davenport et al., 2008). These hair cortisol samples were also strongly correlated with salivary cortisol samples obtained in the same time frame (Davenport et al., 2006). In human studies, increased hair cortisol concentrations have been associated with perceived stress in healthy pregnant women (Kalra et al., 2007), hospitalization of at-term infants (Yamada et al., 2007), periods of elevated cortisol secretion in patients with Cushing’s syndrome (Thomson et al., 2010), and long-term unemployment (Dettenborn et al., 2010).
The present study was designed to determine how differences in early experience affect the response of infant rhesus monkeys to the major stress event of relocation by measuring both anxious behavior and chronic concentrations of cortisol derived from hair. Repeated assessment of both variables over 2 years enabled us to test the hypothesis that heightened HPA activity (as measured by hair cortisol concentrations) early in development predicts later anxious behavior in response to stress. Another novel and important aspect of this study was examining anxious behavior and HPA axis activity in two different nursery-rearing environments (PR vs. SPR) in comparison to MPR controls. In this regard, the rhesus monkey infants used this study were part of a larger project aimed at understanding biobehavioral development at the Laboratory of Comparative Ethology at the National Institute of Child Health and Human Development. Infants were reared in all three rearing conditions described above for the first 8 months of life and then, as is standard practice at this laboratory, were relocated and placed together in one large social group. That this relocation can be considered initially stressful is based not only on previous findings of substantial relocation effects in adult rhesus monkeys (Davenport et al., 2008) but also on the facts that the new environment was substantially different from the monkeys’ previous housing environments, both in size and complexity, and in sheer number of animals with which to interact.
Thus, the aims of the present study were 1) to characterize hair cortisol concentrations in differently-reared infant rhesus monkeys across the first 2 years of life both before and after the stressor of relocation and 2) determine whether hair cortisol was a predictor of anxious behavior after the major social stressor of weaning and relocation in these young monkeys.
METHODS
Subjects and Rearing
The subjects were 61 infant rhesus monkeys born and raised at the Laboratory for Comparative Ethology (LCE) at the National Institutes of Health in two birth cohorts (2006, n=25; 2007, n=36; 33 males, 28 females). Animals from three different rearing conditions were studied: mother-peer-reared (MPR, n=21), peer-reared (PR, n=20), or surrogate-peer-reared (SPR, n=20; Table 1). Rearing conditions in this laboratory have previously been described in detail (Ruppenthal, 1979; Shannon et al., 1998). Briefly, MPR infants were born and reared in harem groups consisting of 6–8 adult females, 1–2 adult males, and several same-aged infants. PR and SPR infants were separated from their mothers within the first 3 days of life and reared in a nursery where they were housed in incubators and hand-fed for the first 14 days of life. From days 15–37, these infants were placed alone in a nursery cage with an inanimate cloth-covered surrogate, and on day 37 PR infants were permanently placed in a large cage with three other agemates while SPR infants began daily 2-h play sessions with three other peers. These monkeys were part of a larger, long-term longitudinal research program at the LCE to examine the effects of early rearing environments on socio-emotional development.
Table 1.
Subject characteristics for this study.
| Rearing | Male | Female | Total |
|---|---|---|---|
| MPR | 11 | 10 | 21 |
| PR | 12 | 8 | 20 |
| SPR | 10 | 10 | 20 |
| Total | 33 | 28 | 61 |
Major Life Stress Event
Infants remained in the MPR, PR, or SPR condition for the first 8 months of life, at which time all infants in a cohort (birth year 2006 or 2007) representing each of the three rearing conditions were removed from their housing situations and placed together into a single social group comprised of approximately 60 animals. MPR infants were weaned from their mothers while PR and SPR infants were weaned from their surrogates. All subjects were housed together in one large social group and were moved back and forth between two different housing environments for husbandry purposes (i.e., two connected indoor enclosures vs. a combined indoor/outdoor enclosure). The indoor enclosures each measured 7.3 × 3.4 × 3.7m and were equipped with perches, barrels, swings, and wood shavings. The outdoor enclosure was a circular corn-crib enclosure measuring 5.03m in diameter by 5.49m high. All subjects had free access between the indoor and outdoor housing areas, except when they were partitioned to either side for routine cleaning or for social behavior observations, or to the inside during inclement weather (e.g., 4°C or below). Water was provided ad libitum and monkeys were fed Purina Monkey Chow (#5038) and provided enrichment daily. Relocation occurred for all infants in a cohort on the same day and represented a major social challenge in the lives of the young monkeys, as they had to adapt to a new environment and new peers, including the establishment of a new social hierarchy. This major life stress served as a valuable opportunity to study anxious behaviors and physiological responses following such an event.
Hair Cortisol Measurements
Hair was shaved from each infant at the back of the neck during a routine neonatal assessment on day 14. Shaving at this time allowed for adequate re-growth of hair for cortisol analysis over the next 5.5 months as well as a precise window of time to which the accumulated cortisol would correspond. Additionally, removing the hair at this age insured that prenatal sources of cortisol (both fetal and maternal) would be removed from the sample (Dettmer et al., 2009). Hair was allowed to re-grow and shaved again during routine health procedures at months 6, 12, 18, and 24. For these very young monkeys, a 6-month delay between sample collections was necessary to ensure that an adequate amount of hair was present for cortisol analysis (~250mg; see Davenport et al., 2006). The samples at 6, 12, 18, and 24 months were analyzed by means of a sensitive and specific enzyme immunoassay kit (#1–3002; Salimetrics, State College, PA) for cortisol content according to procedures developed and validated by our laboratory (Davenport et al, 2006; 2008). Briefly, hair was washed twice with isopropanol and allowed to dry for 5 to 7 days before being ground to a fine powder. Approximately 50mg of the powder was incubated overnight in 1mL of methanol to extract the cortisol, then centrifuged, and the supernatant transferred to a new tube to be dried down under nitrogen gas. The cortisol extract was reconstituted with 400 µL assay buffer and analyzed according to the manufacturer’s recommendations. Hair cortisol samples were assayed after each 6-month period, and samples from all three rearing conditions were approximately balanced across each assay plate. Inter- and intra-assay coefficients of variation were <10%.
Anxiety Scores
From day 37, when the PR and SPR infants were placed into social groups, through two years of age, each infant was observed for 5 min twice per week (once in the morning and once in the afternoon) using focal animal sampling. The order of infants to be observed was randomized across morning and afternoon sessions, and from months 2–8 all infants were observed in their respective social situations (i.e., harem group housing for MPR infants, nursery continuous group housing for PR infants, and nursery 2-h daily play sessions for SPR infants). From months 8–24 all infants were observed in the large mixed-sex housing condition described above. Durations and frequencies of exploratory, social, and solitary behaviors were recorded using JWatcher software (Blumstein at al., 2006). Based on previous research describing anxious behavior in rhesus monkeys (Maestripieri et al., 1991; Suomi, 1997; Erickson et al., 2005; Karere et al., 2009), we focused particularly on clinging, fear vocalizations, huddling, self-rocking, self-clasping, self-biting, and scratching. For each observational session, a composite anxiety score was calculated by summing the total duration of these behaviors as many of these behaviors commonly occur with low frequency (Maestripieri et al., 1991; Erickson et al., 2005; Karere et al., 2009). To relate the hair cortisol measurements to behavior, mean durations of anxiety were first calculated for the following blocks: months 2–6, 6–8, 8–12, 12–18 and 18–24. The 6–8 and 8–12 month blocks were calculated separately to reflect the time periods before and after housing relocation occurred at 8 months of age. The hair cortisol concentrations at each 6-month interval were correlated with the corresponding anxiety blocks (i.e., month 6 hair with anxiety from months 2–6; month 12 hair for anxiety from months 6–8 and 8–12; etc.). Due to the low frequency of the individual behaviors, they were not separately correlated with hair cortisol.
Statistical Analysis
Hair cortisol and composite anxiety scores at each 6-month interval were examined for normal distribution using the Shapiro-Wilk normality test. Hair cortisol at months 6, 18, and 24, as well as anxiety at months 6, 12, 18, and 24, were all right-skewed and Tukey’s ladder of transformations was employed to identify the best transformation for the data prior to using the variable in any analyses (Tukey, 1977).
Mixed-design ANOVAs were used to analyze the change in both hair cortisol and composite anxiety scores across the first 2 years of life, with age (6, 12, 18, and 24 months) as the within-subjects factor and sex and rearing condition (MPR, PR, and SPR) as the between-subjects factors. If the assumptions of sphericity were violated in any of these analyses, the Greenhouse-Geisser correction was employed.
Spearman rank correlations were used to determine whether individuals with higher hair cortisol values at month 6 also exhibited more anxious behavior at 6, 12, 18, and 24 months. These correlations were used to further examine this relationship from 6–8 months of age (immediately pre-relocation) and from 8–12 months of age (immediately post-relocation). Correlations were performed first for all subjects together and then for each rearing group separately.
Between months 18–24, 9 MPR and 8 PR infants were unexpectedly removed from the social group for reassignment to another protocol. Thus, hair cortisol data were available for all 61 infants up through month 18, but for only 44 of the infants at month 24. As this transfer of animals occurred at approximately 20 months of age, some behavioral data were available for all subjects during the 18–24 month period. Thus the repeated measures ANOVA for hair cortisol was performed on the 44 subjects for which a hair cortisol sample was available at each time point.
SPSS software was used for all analyses. An α<0.05 was considered statistically significant for the ANOVAs, whereas we adjusted the alpha level for rejection to 0.005 to adjust for the number of comparisons for the correlational analyses.
RESULTS
Hair Cortisol Concentrations Across Development
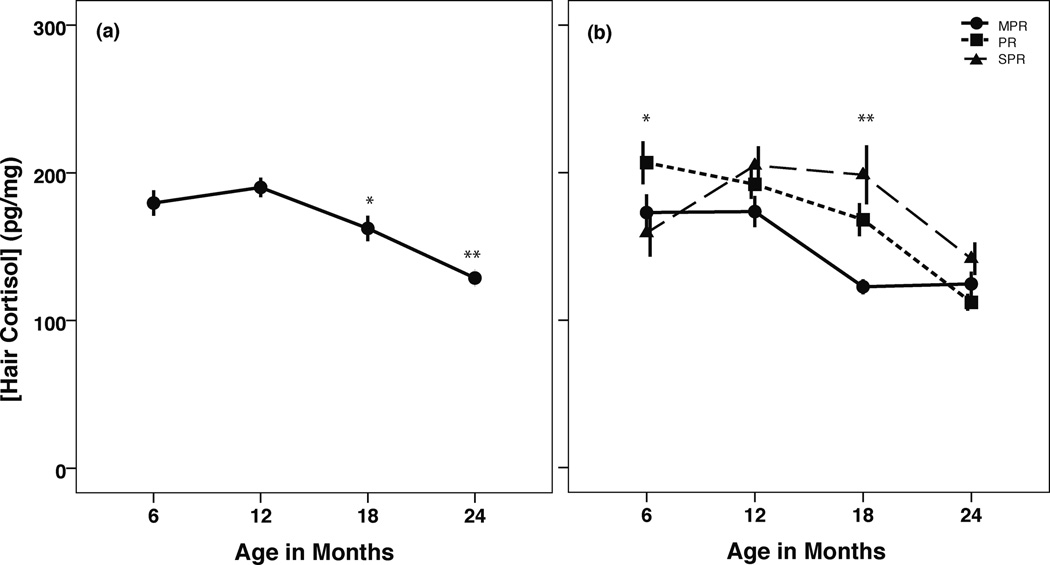
Repeated measures ANOVA revealed a significant within-subjects effect of age on hair cortisol concentrations across the first 2 years of life (F(3,123)=24.25, p<0.001), reflecting an overall decrease with age (Figure 1a). When specific time points were analyzed using paired sample t-tests, overall hair cortisol concentrations (i.e., collapsed across rearing condition) significantly decreased between months 12 and 18 (t(58)=−4.52, p<0.001) and again between months 18 and 24 (t(44)=−4.43, p<0.001). A significant age×rearing interaction was also observed (F(6,123)=4.95, p<0.001; Figure 1b), indicating different patterns of hair cortisol levels across time in the various rearing groups. When we compared the groups with each other at each time point using simple effects ANOVAs, the PR infants exhibited the highest hair cortisol concentrations at month 6 (F(2,59)=4.78, p=0.01), and both PR and SPR infants exhibited higher concentrations than MPR infants at month 18 (F(2,60)=12.56, p<0.001). Interestingly, by month 24 the rearing groups were indistinguishable from one another. When changes over time were analyzed according to rearing condition, we found a significant rise in hair cortisol values for SPR infants from months 6–12 (during the period involving the relocation; t(19)=−3.30, p=0.004). Significant decreases in hair cortisol were observed in the MPR infants between months 12 and 18 (t(19)=−5.84, p<0.001), and in the PR (t(11)=3.93, p=0.002) and SPR (t(19)=4.04, p=0.001) infants between months 18 and 24. No main effects of rearing or sex were observed, nor was there any interaction between these two variables.
Figure 1.
(a) Hair cortisol concentrations declined across the first two years of life. *month 18 < month 12 (p<0.05); **month 24 < months 6, 12, and 18 (p<0.01). (b) Rearing differences in hair cortisol across the first 2 years of life. *PR > MPR=SPR (p<0.05); ** PR=SPR > MPR (p<0.01). Data shown as mean ± SEM.
Anxious Behavior Across Development
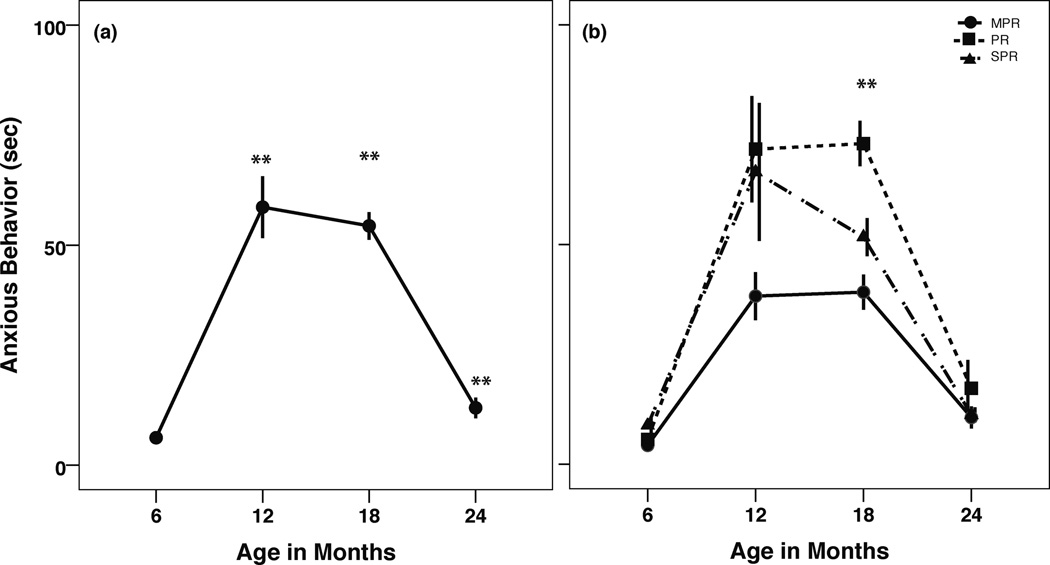
Repeated measures ANOVA (Greenhouse-Geisser estimate) revealed a significant within-subjects effect of age on anxiety-like behavior across the first 2 years of life (F(2.62,146.42)=116.52, p<0.001). Composite anxiety scores sharply increased between months 6 and 12, soon after the major life stressor of housing relocation (t(58)=−11.53, p<0.001), stayed elevated between months 12 and18, and declined to below pre-stress levels by 24 months of age (t(60)=−4.20, p<0.001; Figure 2a). A main effect of rearing was observed (F(2,56)=5.44, p=0.007) such that overall, PR infants exhibited the most anxious behavior, followed by SPR then MPR infants (data not shown). Simple effects ANOVA at each time point revealed that anxious behavior remained elevated between months 12 and 18 in the PR group (F(2,60)=13.77, p<0.001; Figure 2b), whereas MPR and SPR infants did not differ at this age. By 24 months of age, the rearing groups were indistinguishable from one another. No other interactions were revealed.
Figure 2.
(a) Anxious behavior rose and remained elevated after the major life stressor at month 8, then returned to pre-move levels. **differs from every other time point (p<0.001). (b) Rearing differences in anxious behavior across the first 2 years of life. **PR > MPR=SPR (p≤0.01). Data shown as mean ± SEM.
Hair Cortisol and Anxiety Score Relationships
Spearman correlations revealed a significant positive correlation between month 6 hair cortisol and anxious behavior from months 6 to 12 for all subjects combined (rs=0.53, p<0.001). When each rearing group was examined separately, only individuals from the PR group exhibited significant positive correlations between month 6 hair cortisol and anxious behavior between months 6 to 12 (rs=0.71, p=0.001). To further determine whether this association held for the time period immediately before relocation (i.e., 6–8 months of age) or immediately after relocation (i.e., 8–12 months of age), the data were analyzed separately at each of these time points. Month 6 hair cortisol was not significantly correlated with anxious behavior between months 6–8 for any rearing group, but was strongly correlated with anxious behavior from months 8–12 for all infants combined (rs=0.41, p<0.001; Table 2). Once again, when each rearing group was examined separately, only the PR infants exhibited this relationship (rs=0.75, p<0.001; Table 2). PR infants tended to maintain a positive correlation between month 6 hair cortisol and anxiety-like behavior for the subsequent 6 months (i.e., from month 12–18; rs=0.47, p=0.037). Post hoc regression analysis revealed that month 6 hair cortisol was indeed a significant predictor of anxious behavior from months 12 to 18 for PR infants (R2=0.24; p=0.03), but not for MPR (R2=0.02; p=0.58) or SPR (R2=0.08; p=0.23) infants.
Table 2.
Month 6 hair cortisol is correlated with anxious behavior immediately following relocation (8–12 months) for PR infants. This relationship tends to persist for the subsequent 6 months (12–18 months).
| Rearing Condition |
Anxiety 1–6mo |
Anxiety 6–8mo |
Anxiety 8–12mo |
Anxiety 12–18mo |
Anxiety 18–24mo |
|
|---|---|---|---|---|---|---|
| ALL | Hair Cortisol 6mo | NS | NS | rs=0.41** | NS | NS |
| MPR | Hair Cortisol 6mo | NS | NS | NS | NS | NS |
| PR | Hair Cortisol 6mo | NS | NS | rs=0.75** | rs=0.47* | NS |
| SPR | Hair Cortisol 6mo | NS | NS | NS | NS | NS |
p<0.05;
p<0.001.
DISCUSSION
The present study is the first to observe differently-reared monkeys from birth continuously through the first 2 years of life and the first to use a chronic measure of HPA axis activity to characterize changes in this system in response to the stress of relocation. Our study demonstrated that hair cortisol is a potential biomarker for the development of anxiety-like behavior in response to a major life stressor in young monkeys, particularly in animals exposed to adverse early rearing. It also revealed significant divergence in the responses of the two nursery rearing groups (PR and SPR) to relocation in comparison to MPR controls.
A major finding of the present study is that PR infants with higher hair cortisol levels measured early in life before relocation exhibited more anxious behaviors in the months immediately following relocation, a relationship that tended to persist for the subsequent 6 months. The PR condition is a well-established model of anxiety in humans (Suomi et al., 1981; Higley and Suomi, 1989; Higley et al., 1990; Fahlke et al., 2000), and our findings are consistent with both the human and animal literature in which individuals exposed to adverse conditions early in life are more prone to exhibiting anxiety after a subsequent major stressor (Suomi, 1997; Francis et al., 1999; Ladd et al., 2000; Heim and Nemeroff, 2001). However, the present results are the first to demonstrate a prolonged reaction in PR infants over many months and are also unique in showing that a biological measure obtained early in life significantly predicts later stress-related anxious behavior in these same subjects.
Another major finding is the diversity of response to relocation in the two nursery rearing groups both in terms of anxious behavior and HPA axis activity. Consistent with previous studies, we found that PR infants exhibited more anxious behavior than MPR infants following exposure to a stressor (Barrett et al., 2009; Higley et al., 1991); however, this is the first study to show that SPR infants exhibited less anxious behavior than their PR counterparts in reaction to the same stressor. Our findings lend support to previous results indicating that SPR infants are behaviorally more similar to MPR than are PR infants (Sackett, 1982; Ruppenthal et al., 1991; Strand and Novak, 2005). Indeed, MPR and SPR infants showed more rapid behavioral adaptation to the relocation stressor as evidenced by their reduced anxiety scores in the 12- to 18-month period, whereas PR infants showed elevated anxiety scores across two consecutive 6-month periods (months 6–12 and 12–18). One possible explanation for this finding is that like MPR infants, SPR infants develop an attachment bond with their inanimate mother (i.e., surrogate) and develop playful relationships with their peers, whereas PR infants lack this distinction. PR infants must serve as both attachment figures and playmates, making it difficult for the monkeys to dissociate the two roles.
A somewhat different pattern of results was obtained for HPA axis activity. Our finding that PR infants exhibited the highest hair cortisol at 6 months of age prior to relocation is in agreement with previous studies describing elevated “point” measures of cortisol (e.g., in CSF or blood plasma) in these infants (Barrett et al., 2009; Higley et al., 1992). However, only one group, SPR infants, showed a significant increase in hair cortisol concentrations from 6–12 months during the period in which the housing relocation occurred. Because all groups exhibited increases in anxious behavior during the same time period, we cannot attribute this rearing condition effect to an overall lack of responsiveness of the PR and MPR groups. For the PR group, it is possible that the absence of a change was due, in part, to the already high cortisol levels expressed in these monkeys prior to relocation. The lack of HPA axis reactivity in the MPR group may be related to the relatively lower anxiety levels shown by this group in response to relocation. Naturally-occurring age-related declines in basal cortisol values also might have influenced this pattern (Lewis and Ramsay, 1995; Guazzo et al., 1996). However, this hypothesis would best be tested by examining hair cortisol from infants reared with their mothers who did not undergo the relocation stress; indeed, we are currently collecting and examining such data on a subset of infants at the LCE. Finally, rearing condition differences in hair cortisol were also observed in the present study at month 18, a time at which the MPR infants exhibited lower hair cortisol concentrations than either PR or SPR infants. Taken together with the behavioral data, these findings suggest that although the level of HPA activity prior to stress onset predicted subsequent anxiety responses to the stressor, changes in HPA activity after stress onset did not parallel the changes in anxiety when all of the rearing conditions are considered.
One of the intriguing outcomes of this study was the finding that at 24 months of age (16 months after the relocation), all rearing groups showed similar levels of anxious behavior and HPA axis activity. The extent to which this outcome may have resulted from the mixing together of the different rearing groups (as opposed to maintaining them with animals only of the same rearing condition) is an important question that cannot be answered with the available data. It is interesting to note that a number of different primate facilities routinely house young monkeys in mixed rearing groups for variable periods of time after the first 6–8 months of life. However, despite the present finding of similar levels of anxiety-like behavior across rearing groups at 24 months of age, other studies have demonstrated that the mixing of differently reared monkeys does not eliminate all behavioral differences later in life. For example, juvenile PR monkeys with this mixed rearing exposure show sleep disturbances (Barrett et al., 2009) and enhanced fear-potentiated startle (Nelson et al., 2009) compared to mother-reared controls. Furthermore, studies at the LCE where animals typically receive extensive mixed rearing exposure show heightened vulnerability of PR monkeys to alcohol consumption in adulthood compared to their MPR counterparts (Fahlke et al., 2000). Thus, the effects observed in our study are best viewed from the perspective of adaptation to the mixed housing condition following establishment of a new set of relatively stable social relationships. When additional stressors or novel situations are encountered, rearing condition differences may once again be discerned, thus explaining some of the disparate findings mentioned above. In any case, further research is needed to determine the role that physical and social contact with same- vs. differently-reared age mates might play in modulating anxious behavior in young monkeys. Additionally, further research is warranted to examine normative age-related changes in hair cortisol concentrations in mother-reared monkeys who do not undergo the stress of relocation, as the current study did not include such infants. Indeed, we are currently collecting and beginning to analyze hair samples from mother-reared infants who remain with their mothers for at least the first year of life at the LCE.
In summary, we have demonstrated that PR, SPR, and MPR infant rhesus monkeys showed rearing condition-dependent patterns of behavioral and physiological reactivity to a major social stressor. All infants exhibited increased anxiety-like behavior following imposition of the stressor, but subsequent recovery from the anxiety response as well as the HPA response measured using hair cortisol differed across the three groups. Interestingly, early hair cortisol levels were shown to predict later anxious responses to a stressor in the at-risk PR monkeys. Finally, the present data also address a gap that has existed in nonhuman primate research, as the majority of previous studies have focused on infants in the first 6 months of life and/or on animals in early adulthood and beyond. While further studies are needed to resolve some of the questions raised by the present findings, it seems likely that measurement of hair cortisol concentrations will prove increasingly valuable in the search for valid biomarkers of stress-related and psychiatric disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barrett CE, Noble P, Hanson E, Pine DS, Winslow JT, Nelson EE. Early adverse rearing experiences alter sleep-wake patterns and plasma cortisol levels in juvenile rhesus monkeys. Psychoneuroendocrinology. 2009;34:1029–1040. doi: 10.1016/j.psyneuen.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC, Evans CS. JWatcher (Version 1.0) [Software] 2006 Available from http://www.jwatcher.ucla.edu. [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulations in young rhesus monkeys (Macaca mulatta) Dev. Psychobiol. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Clarke AS. Social rearing effects on HPA axis activity over early development and in response to stress in rhesus monkeys. Dev. Psychobiol. 1993;26:433–446. doi: 10.1002/dev.420260802. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotrophin releasing hormone in adult nonhuman primates exposed to early life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc. Natl. Acad. Sci. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biol. Psychiatry. 2008;63:990–996. doi: 10.1016/j.biopsych.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport MD, Novak MA, Meyer JS, Tiefenbacher S, Higley JD, Lindell SG, Champoux M, Shannon C, Suomi SJ. Continuity and change in emotional reactivity in rhesus monkeys throughout the prepubertal period. Motiv. Emot. 2003;27:57–76. [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.04.006. In press. PMID: 20471757. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MFSX, Novak MA, Meyer JS, Suomi SJ. Hair cortisol predicts object permanence performance in infant rhesus macaques (Macaca mulatta) Dev. Psychobiol. 2009;51:706–713. doi: 10.1002/dev.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K, Gabry KE, Lindell S, Champoux M, Schulkin J, Gold P, Suomi SJ, Higley JD. Social withdrawal behaviors in nonhuman primates and changes in neuroendocrine and monoamine concentrations during a separation paradigm. Dev. Psychobiol. 2005;46:331–339. doi: 10.1002/dev.20061. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi S, Higley JD. Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alc. Clin. Exp. Res. 2000;24:644–650. [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotrophin-releasing factor – norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol. Psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. J. Clin. Endocrinol. Metab. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- Hansen EW. The development of maternal and infant behavior in the rhesus monkey. Behaviour. 1996;27:107–149. doi: 10.1163/156853966x00128. [DOI] [PubMed] [Google Scholar]
- Harlow HF. Age-mate or peer affectional system. Adv. Study Behav. 1969;2:333–353. [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ. Temperamental reactivity in non-human primates. In: Kohnstamm G, Bates JE, Rothbart MK, editors. Temperament in childhood. New York: Wiley & Sons; 1989. pp. 153–167. [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. Serotonin in nonhuman primates: Gender, rearing, and developmental correlates with behavioral timidity and affective psychopathology. In: Coccaro EF, Murphy DL, editors. Serotonin in major psychiatric disorders. Washington, DC: American Psychiatric Press, Inc.; 1990. pp. 27–46. [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. CSF monamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology. 1991;103:551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol. Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology. 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clin. Invest. Med. 2007;30:E103–E107. doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol. Psychiatry. 2009;65:770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production – Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experiences. Prog. Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay DS. Developmental change in infants’ responses to stress. Child Dev. 1995;66:50–59. doi: 10.1111/j.1467-8624.1995.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Martel FL, Nevison CM, Simpson MJA, Keverne EB. Anxiety in rhesus monkey infants in relation to interactions with their mother and other social companions. Dev. Psychobiol. 1991;24:571–581. doi: 10.1002/dev.420240805. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Novak MA, Bowman RE, Harlow HF. Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Dev. Psychobiol. 1975;8:425–435. doi: 10.1002/dev.420080507. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Herman KN, Barrett CE, Noble PL, Wojteczko K, Chisholm K, Delaney D, Ernst M, Fox NA, Suomi SJ, Winslow JT, Pine DS. Adverse rearing experiences enhance responding to both aversive and rewarding stimuli in juvenile rhesus monkeys. Biol. Psychiatry. 2009;66:702–704. doi: 10.1016/j.biopsych.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, O'Neill P, Suomi SJ. Adjustments and adaptations to indoor and outdoor environments: continuity and change in young adult rhesus monkeys. Am. J. Primatol. 1992;28:125–138. doi: 10.1002/ajp.1350280205. [DOI] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Davis M. Rearing experience differentially affects somatic and cardiac startle responses in rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2002;116:378–386. doi: 10.1037//0735-7044.116.3.378. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC. Survey of protocols for nursery rearing infant macaques. In: Ruppenthal GC, Reese DJ, editors. Nursery care of nonhuman primates. New York: Plenum Press; 1979. pp. 165–185. [Google Scholar]
- Ruppenthal GC, Walker CG, Sackett GP. Rearing infant monkeys (Macaca nemestrina) in pairs produces deficient social development compared with rearing in single cages. Am. J. Primatol. 1991;25:103–113. doi: 10.1002/ajp.1350250204. [DOI] [PubMed] [Google Scholar]
- Sackett GP. Can single processes explain effects of postnatal influences on primate development? In: Emde RN, Harmon RJ, editors. The development of attachment and affiliative systems. New York: Plenum Press; 1982. pp. 3–12. [Google Scholar]
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Invest. Med. 2007;30:E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am. J. Primatol. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Stevens H, Leckman JF, Coplan JD, Suomi SJ. Risk and resilience: Early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:114–127. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- Strand SC, Novak MA. Examination of behavior in differently reared monkeys housed together. Am. J. Primatol. 2005;66 S1:120–121. [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. Brit. Med. Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Genetic, maternal, and environmental influences on social development in rhesus monkeys. In: Chiarelli AB, Corruccini RS, editors. Primate Behavior and Sociobiology. New York: Springer-Verlag; 1981. pp. 81–87. [Google Scholar]
- Suomi SJ. Early stress and adult emotional reactivity in rhesus monkeys. Ciba Found. Symp. 1991;156:171–183. doi: 10.1002/9780470514047.ch11. [DOI] [PubMed] [Google Scholar]
- Thomson S, Koren G, Fraser L-A, Rieder M, Friedman TC, Van Uum SHM. Hair analysis provides a historical record of cortisol levels in Cushing’s syndrome. Exper. Clin. Endocr. Diab. 2010;118:133–138. doi: 10.1055/s-0029-1220771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Behavioral Science-Quantitative Methods. Reading, MA: Addison-Wesley; 1977. Exploratory Data Analysis. [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007;92:42–49. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]