Abstract
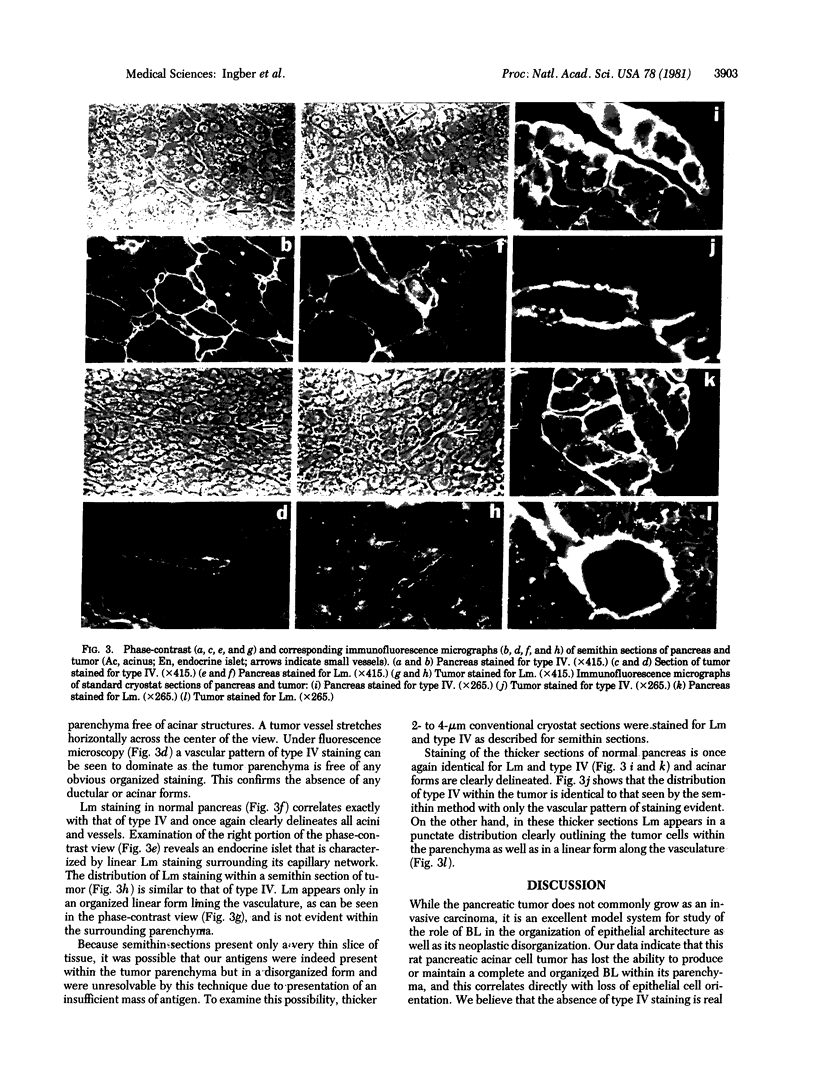
We have studied a transplantable carcinoma of the rat pancreas [Reddy, J. K. & Rao, M. S. (1977( Science 198, 78-80] that is composed of cytologically differentiated acinar cells that have lost their epithelial orientation and do not form acini. Light microscopy shows, however, consistent palisading, reorientation, and polarization of these cells in areas of contact with the vasculature. Electron microscopy reveals a normal basal lamina (BL) along the basal portions of repolarized tumor cells that is physically separate from the endothelial BL. We used indirect immunofluorescence to examine the distribution of BL constituents, laminin (Lm) and type IV collagen (type IV), within the different microenvironments of this tumor. In normal pancreas, Lm and type IV are distributed linearly, outlining acini and blood vessels. In the tumor parenchyma, type IV is not detected, whereas Lm appears in a punctate distribution outlining cells. Reorientation of tumor cells is observed only along linearly deposited Lm and type IV bordering vessels. These data indicate that this nonmetastatic tumor has lost the ability to produce or maintain a complete BL within its disorganized parenchyma, while its cells retain the capacity to produce and reorganize along liner BL when in contact with vascular adventitia. We suggest that failure to maintain a complete BL may be involved in the neoplastic disorganization of normal tissue architecture as well as in the breakdown of boundaries during the development of invasive carcinomata.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S. D., Cohn R. H., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977 May;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Tokuyasu K. T., Barondes S. H. Localization of an endogenous lectin in chicken liver, intestine, and pancreas. J Cell Biol. 1979 Aug;82(2):565–571. doi: 10.1083/jcb.82.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. A., Chan P. C., Wynder E. L. The role of a high-fat diet in enhancing the development of mammary tumors in ovariectomized rats. Cancer. 1981 Jan 1;47(1):66–71. doi: 10.1002/1097-0142(19810101)47:1<66::aid-cncr2820470113>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson J. W., Hay E. D. Secretion of collagen by corneal epithelium. II. Effect of the underlying substratum on secretion and polymerization of epithelial products. J Exp Zool. 1974 Jul;189(1):51–72. doi: 10.1002/jez.1401890106. [DOI] [PubMed] [Google Scholar]
- Dodson J. W., Hay E. D. Secretion of collagenous stroma by isolated epithelium grown in vitro. Exp Cell Res. 1971 Mar;65(1):215–220. doi: 10.1016/s0014-4827(71)80069-1. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Alitalo K., Vaheri A., Timpl R., Saxén L. Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):485–489. doi: 10.1073/pnas.77.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- LUIBEL F. J., SANDERS E., ASHWORTH C. T. An electron microscopic study of carcinoma in situ and invasive carcinoma of the cervix uteri. Cancer Res. 1960 Apr;20:357–361. [PubMed] [Google Scholar]
- Leivo I., Vaheri A., Timpl R., Wartiovaara J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol. 1980 Apr;76(1):100–114. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- Levine S., Pictet R., Rutter W. J. Control of cell proliferation and cytodifferentiation by a factor reacting with the cell surface. Nat New Biol. 1973 Nov 14;246(150):49–52. doi: 10.1038/newbio246049a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Vembu D., Kleinman H. K., Martin G. R., Boone C. Collagen required for proliferation of cultured connective tissue cells but not their transformed counterparts. Nature. 1978 Apr 13;272(5654):622–624. doi: 10.1038/272622a0. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZZELLO L. The behavior of basement membranes in intraductal carcinoma of the breast. Am J Pathol. 1959 Jul-Aug;35(4):887–899. [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S. Transplantable pancreatic carcinoma of the rat. Science. 1977 Oct 7;198(4312):78–80. doi: 10.1126/science.897688. [DOI] [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Albert J., Furthmayr H. Codistribution of collagen types IV and AB2 in basement membranes and mesangium of the kidney. an immunoferritin study of ultrathin frozen sections. J Cell Biol. 1980 Jun;85(3):597–616. doi: 10.1083/jcb.85.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Rutter W. J. Effects of a partially purified factor from chick embryos on macromolecular synthesis of embryonic pancreatic epithelia. Dev Biol. 1973 Feb;30(2):307–320. doi: 10.1016/0012-1606(73)90091-2. [DOI] [PubMed] [Google Scholar]
- Rubio C. A., Biberfeld P. The basement membrane in experimentally induced atypias and carcinoma of the uterine cervix in mice. An immunofluorescence study. Virchows Arch A Pathol Anat Histol. 1979 Feb 9;381(2):205–209. doi: 10.1007/BF01257885. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Cohen H. I., Faubion J. Development of the embryonic mammalian pancreas: the relationship between morphogenesis and cytodifferentiation. Dev Biol. 1977 Dec;61(2):119–130. doi: 10.1016/0012-1606(77)90285-8. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Wick G., Martin G. R. Immunochemical study on basement membrane (type IV) collagens. Immunology. 1979 Sep;38(1):109–116. [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Martin G. R., Bruckner P., Wick G., Wiedemann H. Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem. 1978 Mar;84(1):43–52. doi: 10.1111/j.1432-1033.1978.tb12139.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembu D., Liotta L. A., Paranjpe M., Boone C. W. Correlation of tumorigenicity with resistance to growth inhibition by cis-hydroxypyroline. Exp Cell Res. 1979 Dec;124(2):247–252. doi: 10.1016/0014-4827(79)90200-3. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Walker F. Basement-membrane turnover in the rat. J Pathol. 1972 Jun;107(2):119–121. doi: 10.1002/path.1711070206. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Garbisa S., Kidwell W. R. Basement membrane collagen requirements for attachment and growth of mammary epithelium. Exp Cell Res. 1979 Nov;124(1):181–190. doi: 10.1016/0014-4827(79)90268-4. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Vonderhaar B. K., Kidwell W. R. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol. 1980 Dec;80(2):253–256. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]