Abstract
Psychological, physical, and/or immune stressors during pregnancy are associated with negative birth outcomes, such as preterm birth and developmental abnormalities. In rodents, prenatal stressors can alter the expression of 5α-reductase enzymes in the brain and may influence cognitive function and anxiety-type behaviour in the offspring. Progesterone plays a critical role in maintaining gestation. Here it was hypothesised that 5α-reduced progesterone metabolites influence birth outcomes and/or the cognitive and neuroendocrine function of the offspring. 5α-reduced steroids were manipulated in pregnant Long-Evans rats via administration of vehicle, the 5α-reduced, neuroactive metabolite of progesterone, 5α-pregnan-3α-ol-20-one (3α,5α-THP, allopregnanolone; 10 mg/kg/ml, SC), or the 5α-reductase inhibitor, finasteride (50 mg/kg/ml, SC), daily from gestational days 17–21. Compared to vehicle or 3α,5α-THP treatment, finasteride, significantly reduced the length of gestation and the number of pups per litter found in the dams’ nests after parturition. The behaviour of the offspring in hippocampus-dependent tasks (object recognition, open field) was examined on post-natal days 28–30. Compared to vehicle-exposed controls, prenatal 3α,5α-THP treatment significantly increased motor behaviour in females compared to males, decreased progesterone content in the medial prefrontal cortex (mPFC) and diencephalon, increased 3α,5α-THP and 17β-estradiol content in the hippocampus, mPFC, and diencephalon, and significantly increased serum corticosterone concentrations in males and females. Prenatal finasteride treatment significantly reduced object recognition, decreased hippocampal 3α,5α-THP content, increased progesterone concentration in the mPFC and diencephalon, and increased serum corticosterone concentration in female (but not male) juvenile offspring, compared with vehicle-exposed controls. Thus, inhibiting formation of 5α-reduced steroids during late gestation in rats reduces gestational length, the number of viable pups/litter, and impairs cognitive and neuroendocrine function in the juvenile offspring.
Keywords: allopregnanolone, anxiety, finasteride, prenatal stress, progesterone
Introduction
Prenatal stress can have negative effects on birth outcomes and has been implicated in preterm birth (parturition prior to 37 weeks of gestation in women) [1]. Environmental stressors associated with low socio-economic status (such as unemployment, crowded urban living conditions) are associated with low birth-weight and/or preterm birth in women [2]. More than a two-fold increase in the incidence of preterm birth has been reported among pregnant women residing in an area severely hit by hurricane Katrina, compared to pregnant survivors in an area that was less severely damaged [3]. The consequences of preterm birth include greater fetal mortality and, among surviving offspring, neurological and developmental disabilities [4,5]. Moreover, impairment of cognitive, social, and emotional development has been observed in school-age children that were born preterm [6,7], and the severity of these phenotypes is increased when gestation is shorter [6]. Maternal stress has been reported to result in similar neurodevelopmental impairments in full-term infants [8,9] to those observed following preterm birth, suggesting that they may have common underlying causes. Despite the potential contributions of stress, the etiopathology of preterm birth and/or associated effects on negative birth outcomes are not well understood. Further, the mechanisms that mediate stress-related influences on pregnancy, parturition onset, and birth outcomes need to be better understood.
Progestogens are critical for maintaining pregnancy [10] and also importantly influence function of the major neuroendocrine stress response system, the hypothalamo-pituitary-adrenal (HPA) axis. Progesterone is metabolised by 5α-reductase to form dihydroprogesterone (DHP), which is subsequently converted to the neuroactive metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP; also known as allopregnanolone) by the actions of 3α-hydroxysteroid dehydrogenase. In women, circulating concentrations of progesterone and its metabolites increase as gestation progresses and their decline occurs concurrent with parturition [11,12]. Studies using rodent models have revealed that central concentrations of progesterone decline earlier in pregnancy than central concentrations of 3α,5α-THP, and it is the 3α,5α-THP decline in the brain that is most proximately associated with parturition [13]. Hence, 3α,5α-THP may serve an important regulatory role, not only in the maintenance of pregnancy, but also in the onset of parturition via its withdrawal.
In addition to effects on pregnancy, progestogens have activational effects to facilitate social interaction, reduce stress axis responding, and exert anti-anxiety, cognitive enhancing and neuroprotective effects [14]. Despite pregnancy and early exposure to maternal progestogens being the sine qua non, the organizing role of progestogens is not well understood. While there is little direct evidence that stress exposure during pregnancy alters 3α,5α-THP formation, prenatal stressors alter the expression of 5α-reductase in the brain of sheep offspring [15] and can have detrimental effects on cognitive function and anxiety-type behaviour [16,17]. Moreover, rats that are bred for high anxiety responses to maternal separation show differences in anxiety, reproductive behavior, and 3α,5α-THP levels in midbrain compared to their low-anxiety conspecifics [18]. Moreover, perinatal administration of supra-physiological levels of 3α,5α-THP ameliorates neonatal anxiety and adult depressive-type behavior in this model [19]. Thus, in addition to activating effects in adult, these findings may indicate a pervasive, organizational role for 3α,5α-THP on offspring neurodevelopment.
The present study investigated the role of a 5α-reduced, progesterone metabolite, 3α,5α-THP, on pregnancy maintenance, birth outcomes and offspring neurodevelopment. Given that progestins are presently utilized as tocolytic agents [20], it is important to understand not only the immediate consequences of effects of 3α,5α-THP on pregnancy outcomes, such as length of pregnancy and fecundity, but also the long-term neuroendocrine and behavioural consequences for the gestationally-exposed offspring. We exposed pregnant rat dams to either vehicle (oil), 3α,5α-THP (10 mg/kg), or the 5α-reductase inhibitor, finasteride (50 mg/kg), on gestational days (GD) 17–21. Pregnancy outcomes (gestational length and the number of viable offspring) were assessed, as well as cognitive, affective, and motor function in the juvenile offspring. Endogenous progestogen (progesterone, DHP, 3α,5α-THP), and 17β-estradiol contents were measured in blood and in brain regions important in affective and cognitive function, and/or stress processing). We hypothesised that administration of 3α,5α-THP would prolong gestation, whereas inhibition of 3α,5α-THP formation via finasteride would reduce the length of gestation. Moreover, we anticipated that 3α,5α-THP would not alter pup viability, but would enhance anti-anxiety-type/cognitive behaviour of offspring, while finasteride would reduce pup viability, enhance anxiety-type behaviour and impair cognitive function of surviving offspring, concomitant with altered progestogen formation in the brains of the offspring.
Materials and Methods
Ethical Approval
These methods were pre-approved by the Institutional Care and Use Committee at The University at Albany-SUNY and were conducted in accordance with ethical guidelines defined by The National Institutes of Health (NIH Publication No. 85-23).
Animals and housing
Subjects were primigravid, timed-pregnant, adult female Long-Evans rats (N = 24) purchased from Taconic Farms (Germantown, NY). Rats were packed on gestational day (GD) 14, shipped on GD 15, and were housed in a temperature- (21 ± 1 °C) and humidity-controlled room in the Life Sciences Research Building Laboratory Animal Care Facility at The University at Albany-SUNY. Rats were group-housed (3–4/cage) until GD 18, after which they were singly-housed. The housing room was maintained on a reverse 12:12 h light cycle (lights off at 08:00 h) and rats were given ad libitum access to Purina Rat Chow and water.
Evaluation of Pregnancy Status and Fecundity
Pregnancy status and duration of gestation were assessed daily. All rats were handled, examined, and weighed from GD 16 until the day of parturition. Criteria for confirmation of pregnancy were weight gain of > 5 g per day, with visible teats, and palpable pups. Rats not meeting these criteria were excluded from the study (n = 3). The cages of all rats were checked for pups hourly from 06:00 to 22:00 h daily from GD 18–23. The number of hours after 00:00 h on GD 18 that pups were first observed was recorded as the latency to deliver. GD 18 was chosen as this is the earliest time that we have observed prenatal manipulations to promote parturition [21]. The number of pups in the nest following the completion of parturition was counted and recorded, as a measure of pup viability. No dead pups were found in the nest immediately following parturition or at any other time-points during the study.
Procedure
Assessment of Enhanced or Inhibited 3α,5α-THP Milieu on Gestational Outcome
In order to assess the role of 3α,5α-THP on gestational outcome, 3α,5α-THP availability was enhanced or inhibited on GD 17–21. Control dams (n = 7) were administered vehicle (10% EtOH in vegetable oil, 0.1 ml/100 g, SC), daily on GD 17–21. Some dams (n = 7) were administered a subcutaneous (SC) injection of 3α,5α-THP (10 mg/kg/ml; Sigma-Aldrich Corp., St. Louis, MO) in vegetable oil (10% EtOH), daily on GD 17–21. Some dams (n = 7) were administered the 5α-reductase inhibitor, finasteride (50 mg/kg/ml, SC) in vegetable oil (10% EtOH), daily on GD 17–21. In rodents, finasteride inhibits the type I 5α-reductase isozyme acutely and competitively binds irreversibly to the type II isozyme [22]. We have observed that the present regimen reduces the production of 3α,5α-THP from progesterone by ~50–75% [23]. Others have utilised this regimen in rats to demonstrate that it allows for maintenance of pregnancy [24,25]. Manipulations were conducted on GD 17–21 given that this period represents a critical time for the development of offspring cortico-limbic structures [17,26,27]. Moreover, late pregnancy represents a time when endogenous protective factors (such as maternal progestogens [13] and placental 11β-hydroxysteroid dehydrogenase [28]) are declining, which may promote vulnerability to the harmful effects of elevated glucocorticoid levels. We have previously observed that maternal stress during this time can reduce the length of offspring gestation in rats, reduce synaptic density in dorsal hippocampus of juvenile offspring, and yields poorer cognitive outcomes for juvenile offspring [16,17].
Phenotype of Offspring
Following parturition, all pups were cross-fostered to non-manipulated dams that were matched for postpartum stage in order to minimise potential confounds produced by any aberrant maternal behaviour (reviewed in [29]). To achieve this, litters were culled following birth, such that 15 males and 15 females per condition could be cross-fostered to non-manipulated dams. The female-to-male ratios prior to culling did not differ significantly across prenatal treatments (assessed via χ2 analysis), and were 1.1: 1.0 for vehicle-exposed control offspring, 0.8: 1.0 for 3α,5α-THP-exposed offspring, and 1.4: 1.0 for finasteride-exposed offspring. Litter birth weights were not assessed. For cross-fostering, 2–4 pups were taken from each litter (i.e. either 1 male and 1 female, 1 male and 2 females, 2 males and 1 female, or 2 males and 2 females, in a counter-balanced manner) and cross-fostered to non-manipulated dams. In total, 12 non-manipulated dams (n = 4 dams/condition) were used as surrogate mothers to experimental offspring. Litter sizes were maintained across the surrogate dams, who had their own pups to care for in addition to experimentally-manipulated pups, such that all dams had 16–20 pups to care for following cross-fostering. Experimental pups were identified by a tail mark (using a Sharpie™ marker) until post-natal day 21, when pups were weaned from surrogate dams, identified via ear notch, and housed 4–5 per cage until testing. Behavioural testing was carried out between post-natal days 28 and 30. All cross-fostered pups (n = 30 male, n = 30 female pups; yielding n = 15 pups/condition) survived to weaning and were subjected to behavioural testing. At the time of testing, all juveniles were weighed and assessed in the object recognition [hippocampus- and prefrontal cortex (PFC)-dependent] and open field (hippocampus-dependent) tasks. There were no differences in the body weight of the pups across groups at this time.
Behavioural Testing
All behavioural testing was conducted after 09:00 h, during the dark phase of the 24 h light cycle. Rats were assessed in the open field task prior to the object recognition task. This allows for assessment of anxiety-like behaviour and habituation to the novel testing chamber prior to assessment of object recognition. All dependent measures were hand recorded by an observer who was blind to the experimental condition of the animals and by ANY-maze animal tracking software (Stoelting, Chicago, IL). There was greater than 95 % concordance between hand-collected and software-calculated data. As such, the computer-generated data were used for analyses.
Open Field
Behaviour in the open field, such as exploration, and behaviour that is indicative of anxiety, is dependent upon the hippocampus [30] and was examined as previously-described prior to assessment in the cognitive task [17,31]. Briefly, the open field consisted of an arena (76 × 57 × 35 cm) divided into a grid of 48 squares. The arena was located in a brightly-lit testing room. Rats were placed in the lower right-hand corner and allowed to explore the arena for 5 min. The number of entries made into all 48 squares (total entries), and into the inner 8 squares (central entries), were recorded using animal tracking software (as above). The tracking software uses the centre of an animal’s body in order to objectively quantify grid entries in the maze. The number of total entries made in the task was considered an index of exploratory/motor behaviour. The percentage of entries made into the inner 8 squares as a function of all squares entered [(frequency of entries into the inner 8 squares/frequency of entries into all squares) × 100] was utilised as an index of reduced anxiety in order to minimise the contribution of any individual disparities in overall locomotor behaviour.
Object Recognition
Object recognition, a working memory task that primarily relies on an intact hippocampus and PFC [32], was used per previously published methods [16,17]. Briefly, rats were placed in an open field (76 × 57 × 35 cm) in a brightly-lit testing room with two identical round objects (plastic toys in the shape of oranges; 6 cm diameter) for the training phase of this task. Rats were allowed 3 min to explore the open field with the objects. Following training, rats were placed in their home-cages in a dark, sound-dampened room for 4 h. Rats were returned to the open field for the testing phase of this task, wherein, one of the spherical objects was replaced with a cone-shaped object (a plastic toy in the shape of a triangular buoy; 6.25 cm tall, 6 cm wide at base, 1 cm at apex). Rats were allowed 3 min to explore the open field with the familiar and novel objects. Exploration of the objects was recorded via the animal tracking software. Rats did not show a side-preference in their exploration of objects; however, placement of the novel object was counterbalanced across treatment groups and testing sessions to minimise potential confounds. In this task, a greater percentage of time spent exploring the object in the novel location as a function of the total amount of time spent exploring both objects during testing [duration spent with novel object/(duration spent with novel object + duration spent with familiar object) × 100] was considered an index of enhanced cognitive performance.
Tissue Collection
Immediately following behavioural testing, offspring were euthanised via rapid decapitation without anaesthetic. Trunk blood was collected in a chilled glass culture test tube following decapitation, allowed to clot, centrifuged at 3,000 × g, and serum was decanted. Whole brains were rapidly removed from the skull and frozen on dry ice. All tissue were stored at −80 °C until radioimmunoassay for concentrations of progesterone, DHP, 3α,5α-THP, 17β-oestradiol and corticosterone (serum only).
Tissue Preparation
The brains of offspring were thawed on ice. The hippocampus, medial prefrontal cortex (mPFC), and diencephalon were grossly dissected as previously described [33].
Radioimmunoassays for Steroid Hormones
Progesterone, DHP, 3α,5α-THP, 17β-oestradiol, and corticosterone concentrations were measured as described below, using previously reported methods [16,17].
Radioactive Probes
[3H] progesterone (NET-208: specific activity = 47.5 Ci/mmol), and [3H]3α,5α-THP (used for DHP and 3α,5α-THP, NET-1047: specific activity = 65.0 Ci/mmol), [3H]oestradiol (NET-317: specific activity = 51.3 Ci/mmol), and [3H]corticosterone were purchased from Perkin Elmer (Boston, MA).
Extraction of Steroids from Serum
Serum (300 μl) was incubated with water and 800 cpm of [3H] steroid, and 17β-oestradiol, progesterone, DHP, and 3α,5α-THP were extracted with ether. Test tubes containing steroid and ether were snap frozen twice and evaporated to dryness. Tubes containing lyophilised steroid were reconstituted with phosphate assay buffer to the pre-lyophilised serum volume (300 μl). Corticosterone was extracted by heating at 60 °C for 30 min.
Extraction of Steroids from Brain Tissues
Progesterone, DHP, 3α,5α-THP, and 17β-oestradiol were extracted from brain tissues following homogenization with a glass/glass homogeniser in 50% methanol (MeOH), 1% acetic acid. Tissues were centrifuged at 3,000 x g and the supernatant was subjected to chromatography using Sepak-cartridges equilibrated with 50% MeOH:1% acetic acid. Steroids were eluted with increasing concentrations of MeOH (50% MeOH followed by 100% MeOH). Solvents were removed using a speed drier and samples were reconstituted in 350 μl assay buffer.
Antibodies
The progesterone antibody (P#337 from Dr. G.D. Niswender, Colorado State University) was used at a 1:30,000 dilution and has very low (< 4%) cross-reactivity with DHP and 3α,5α-THP [34]. This antibody typically binds between 30% and 50% of [3H] progesterone and bound 48% in the present study. The DHP (X-947) and 3α,5α-THP (#921412-5) antibodies were purchased from Dr. Robert Purdy (Veterans Medical Affairs, La Jolla, CA) and used at a 1:5000 dilution. The DHP antibody cross-reacts with 3α,5α-THP (100%), 5α-pregnan-3,20-dione (50%), 4-pregnen-3α-ol-20-one (50%), and progesterone (17%) [35]. The 3α,5α-THP antibody cross-reacts with 3α-hydroxypregn-4en-20-one (84%) and DHP (11%), and its β-isomer (7%), progesterone (6%), and pregnenolone (< 2%) [35,36]. These antibodies typically bind between 40 and 60% of [3H] 3α,5α-THP and bound 47% in the present study. The corticosterone antibody (#B3-163, Endocrine Sciences) was used at a 1:20,000 dilution and has little cross-reactivity with deoxycorticosterone (4%), negligible (< 1%) cross-reactivity with cortisol, aldosterone, and progesterone [37]. This antibody typically binds between 40 and 60% of [3H]corticosterone and bound 45% in the present study. The 17β-oestradiol antibody (E#244, Dr. G.D. Niswender, Colorado State University, Fort Collins, CO) was used at a 1:40,000 dilution and has negligible (< 1%) cross-reactivity with oestrone, 17β-oestradiol, progesterone, and 17-hydroxyprogesterone [38]. This antibody typically binds between 40% and 60% of [3H]17β-oestradiol and bound 54% in the present study.
Set-up and Incubation of Radioimmunoassays
Standard curves ranged from 0–8000 pg for progesterone, DHP, and 3α,5α-THP, 0–1000 pg for 17β-oestradiol, and 0–4 ng for corticosterone. Standards were added to assay buffer followed by addition of the appropriate antibody and [3H]steroid. Total assay volumes were 800 μl for progesterone, 950 μl for DHP and 3α,5α-THP, 800 μl for 17β-oestradiol, and 950 μl for corticosterone. All assays were incubated overnight at 4 °C with the exception of corticosterone which was incubated for 60 min at room temperature.
Termination of Binding
Dextran-coated charcoal was rapidly added to assay tubes in order to separate bound and free steroid. Following incubation with charcoal, samples were centrifuged at 3000 x g and the supernatant was decanted into a glass scintillation vial with 5 ml scintillation cocktail. Steroid concentrations were calculated using the logit-log method of Rodbard and Hutt [39], interpolation of standards, and correction for recovery with Assay Zap (Biosoft, Cambridge, UK). The inter- and intra-assay reliability co-efficients were: progesterone - 0.11 and 0.10, DHP - 0.11 and 0.09, 3α,5α-THP - 0.09 and 0.09, 17β-oestradiol - 0.07 and 0.06, and corticosterone - 0.04 and 0.06.
Statistical Analyses
Weight gain in the dams [expressed as a percentage of body weight; i.e. (body weight at GD 16/body weight at term) × 100], length of gestation (expressed in hours after 00:00h on GD 18 to parturition) and fecundity (mean number of pups per litter) were assessed via one-way analyses of variance (ANOVA) with gestational manipulation as a factor (vehicle, 3α,5α-THP, or finasteride administration). Behavioural and neuroendocrine status of offspring was assessed via two-way ANOVAs with gestational manipulation (vehicle, 3α,5α-THP, or finasteride administration) and sex (male or female) as factors. A ratio of progestogen metabolites reduced from the parent hormone, progesterone (DHP+3α,5α-THP:Progesterone), was calculated per previous methods [16], as an index of progesterone utilization. Fisher’s PLSD post hoc tests were utilised to determine group differences. When interactions were present, one-way ANOVAs with p value corrected for multiple comparisons (given considerations of inflated error in post-hoc analyses [40]) were utilised to determine simple main effects. Alpha level for statistical significance was p ≤ 0.05. Simple regressions were performed to assess whether variances in behavior could be explained by concentrations of steroid hormones, but significant relationships were not observed for any measure.
Results
Inhibition of 5α-reductase during pregnancy decreased the length of gestation and the number of pups found in the nest following parturition
Dam weights (mean ± SEM; n = 7/group) did not significantly differ on GD 16, prior to manipulations (vehicle-administered control dam = 264 ± 10 g, 3α,5α-THP-administered dams = 247 ± 2 g, finasteride-administered dams = 267 ± 12 g), nor did they significantly differ at term (control dam = 302 ± 10 g, 3α,5α-THP-administered dams = 292 ± 3 g, finasteride-administered dams = 292 ± 19 g). However, weight gained during pregnancy as a percentage of body weight significantly differed among groups [F(2,18) = 5.08, p < 0.05]. While experimental groups were not different from vehicle-administered control dams (115 ± 3 %), 3α,5α-THP-administered dams gained significantly more weight (118 ± 1 %) than did the finasteride-administered dams (109 ± 3 %; p = 0.005).
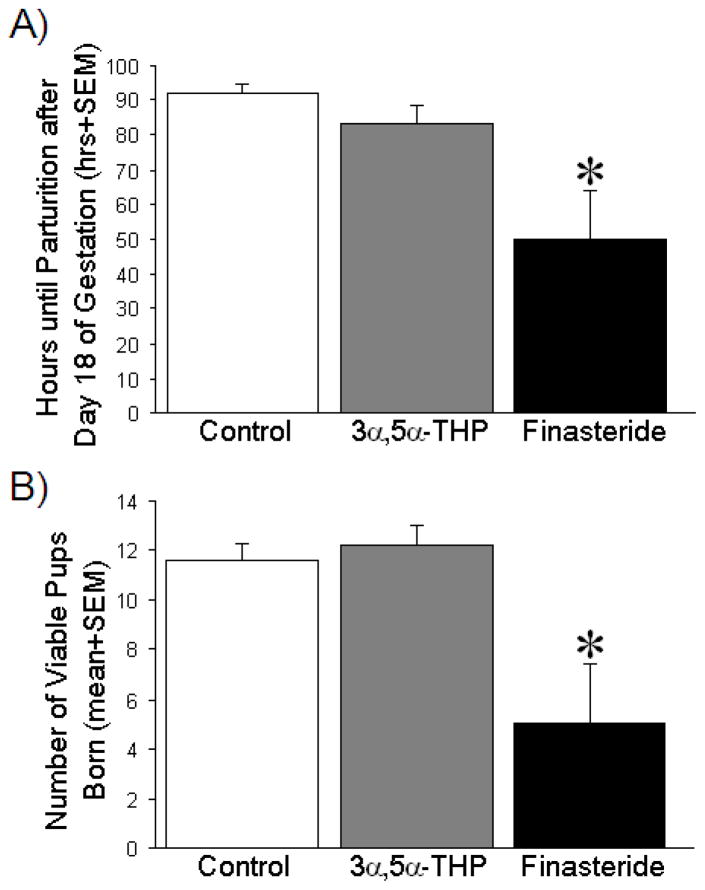
Compared to treatment with vehicle (p = 0.004) or 3α,5α-THP (p = 0.02), blockade of 3α,5α-THP formation with finasteride administration during gestational days 17–21, significantly reduced the length of gestation [F(2,18) = 6.30, p < 0.05] (Fig. 1A). Administration of 3α,5α-THP, during gestational days 17–21, did not significantly alter gestational length compared to administration of vehicle (Fig. 1A).
Figure 1. Hours until parturition after day 18 of gestation and number of viable pups per litter.
(A) The mean length of gestation is expressed as the number of hours after 00:00 h on gestational day 18 until parturition (mean + SEM) among dams administered vehicle (oil, control; n = 7), 3α,5α-THP (10 mg/kg daily, n = 7), or finasteride (50 mg/kg daily, n = 5) on gestational days 17–21 (mean + SEM). (B) The mean number of viable pups per litter that were found at parturition (mean + SEM), born to dams administered either vehicle, 3α,5α-THP, or finasteride (as described above). * indicates significant difference from control rats via one-way ANOVA, p < 0.05.
In comparison to vehicle (p = 0.007) or 3α,5α-THP (p = 0.004) administration, finasteride significantly reduced the number of pups/litter that were present following parturition [F(2,18) = 6.69, p < 0.05] (Fig. 1B). Administration of 3α,5α-THP, compared to vehicle-control manipulation, did not significantly alter fecundity (Fig. 1B).
Altering prenatal 3α,5α-THP availability influenced object recognition and locomotor behaviour, but not anxiety-type behaviour
Prenatal blockade of 3α,5α-THP formation on GD 17–21 significantly impaired object recognition in the juvenile offspring [F(2,84) = 4.31, p < 0.05]. Compared to prenatal vehicle (p = 0.01) or 3α,5α-THP (p = 0.01) exposure, male and female offspring of mothers exposed to finasteride in late gestation spent significantly less time with the novel object during testing, irrespective of sex (Fig. 2). Offspring born to dams that had been administered 3α,5α-THP during GD 17–21 did not significantly differ from vehicle-exposed control offspring in the object recognition task (Fig. 2). No sex differences were observed for object recognition.
Figure 2. Cognitive performance in the male and female offspring in the object recognition task.
Data are the percentage time (in 3 min; mean + SEM) spent investigating a novel object in the object recognition task among male and female offspring (n = 15/group) that were prenatally-exposed to vehicle (oil, control), 3α,5α-THP (10 mg/kg daily), or finasteride (50 mg/kg daily) on days 17–21 of gestation. ** indicates a significant main effect for finasteride-exposed offspring to differ from control, or 3α,5α-THP exposed, offspring (irrespective of sex) via two-way ANOVA, p ≤ 0.05.
There was no significant main effect for locomotor behaviour in the open field, with respect to prenatal manipulations. However, there was a significant interaction between treatment and sex [F(2,84) = 3.23, p < 0.05], wherein only prenatal 3α,5α-THP administration significantly increased the total number of entries made in the open field by females compared to males (p = 0.0005; Table 1).
Table 1. Locomotor activity of male and female offspring in the open field test.
Locomotor behaviour (expressed as the mean total number of grid entries ± SEM in 5 min) in male and female offspring born to dams that were administered vehicle, 3α,5α-THP (10 mg/kg), or finasteride (50 mg/kg) on gestational days 17–21.
| Locomotor activity in Open Field (mean ± SEM) | ||||||
|---|---|---|---|---|---|---|
| Control | 3α,5α-THP | Finasteride | ||||
| Male (n=15) | Female (n=15) | Male (n=15) | Female (n=15) | Male (n=15) | Female (n=15) | |
| Total Entries | 170±14 | 196±17 | 133±19^ | 223±12^ | 174±17 | 185±20 |
indicates an interaction wherein the denoted groups significantly differ from each other via two-way ANOVA, p ≤ 0.05.
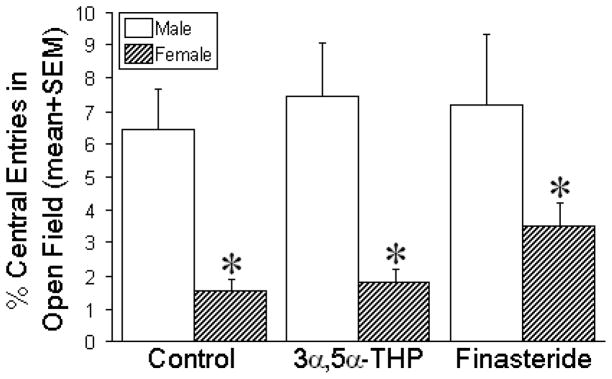
Gestational treatment did not significantly alter the anxiety-type behaviour among male or female offspring (Fig. 3). However, irrespective of gestational manipulation, a significant sex difference was observed, with males making a greater proportion of entries into the center of the open field compared to females [F(1,84) = 21.69, p < 0.05], (Fig. 3).
Figure 3. Anxiety-type behavior of the male and female offspring in the open field test.
Data are the percentage of entries (in 5 min; mean + SEM) into the center of an open field among male and female offspring (n = 15/group) that were prenatally-exposed to vehicle (oil, control), 3α,5α-THP (10 mg/kg daily), or finasteride (50 mg/kg daily) on days 17–21 of gestation. * indicates a significant main effect wherein females differ from males via two-way ANOVA, p < 0.05.
Steroid concentrations in the hippocampus, medial prefrontal cortex, and diencephalon of the juvenile offspring were altered by gestational manipulation of 3α,5α-THP availability
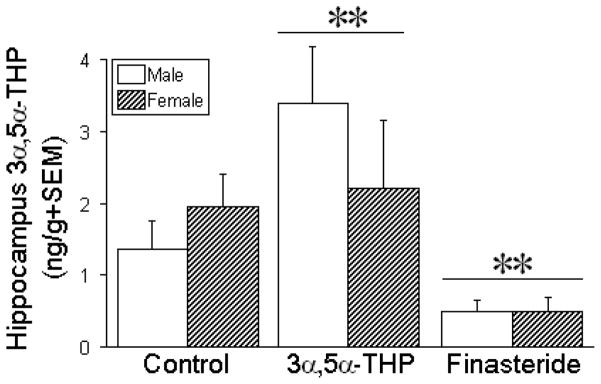
Administration of 3α,5α-THP, or inhibition of its formation from progesterone with finasteride, influenced 3α,5α-THP concentrations in the hippocampus [F(2,84) = 7.97, p < 0.05], mPFC [F(2,84) = 4.35, p < 0.05], and/or diencephalon [F(2,84) = 4.63, p < 0.05] of the juvenile offspring. Compared to vehicle, prenatal treatment with 3α,5α-THP significantly increased 3α,5α-THP content in juvenile hippocampus (p = 0.05; Fig. 4), mPFC (p = 0.007; Table 2), and diencephalon (p = 0.007; Table 2), regardless of sex. Irrespective of sex, the hippocampus was the only region wherein prenatal finasteride exposure significantly reduced 3α,5α-THP content of male and female offspring compared to vehicle-exposed controls (p = 0.047) or 3α,5α-THP-exposed offspring (p = 0.0004; Fig. 4).
Figure 4. Hippocampal 3α,5α-THP concentrations in juvenile male and female offspring.
Hippocampal 3α,5α-THP concentrations (mean + SEM) in male and female offspring (n = 15/group) that were prenatally-exposed to vehicle (oil, control), 3α,5α-THP (10 mg/kg daily), or finasteride (50 mg/kg daily) on days 17–21 of gestation. ** indicates a significant main effect for offspring exposed to 3α,5α-THP or finasteride to differ from control offspring (irrespective of sex) via two-way ANOVA, p < 0.05.
Table 2. Central steroid concentrations in male and female offspring.
The concentrations of 17β-oestradiol (pg/g), progesterone (ng/g), dihydroprogesterone (DHP, ng/g), 5α-pregnan-3α-ol-20-one (3α,5α-THP; ng/g), and progesterone (P) utilization (DHP+3α,5α-THP:P ratio) in the hippocampus, medial prefrontal cortex, and diencephalon of male and female offspring born to dams that were exposed to vehicle, 3α,5α-THP (10 mg/kg), or finasteride (50 mg/kg) on gestational days 17–21.
| Brain Steroid Concentrations (mean ± SEM) | ||||||
|---|---|---|---|---|---|---|
| Control | 3α,5α-THP | Finasteride | ||||
| Male (n=15) | Female (n=15) | Male (n=15) | Female (n=15) | Male (n=15) | Female (n=15) | |
| Hippocampus | ||||||
| 17β-Oestradiol | 0.33±0.11 | 0.22±0.04† | 0.60±0.10‡ | 0.32±0.03†‡ | 0.16±0.03 | 0.16±0.04† |
| Progesterone (P) | 0.5±0.1 | 0.5±0.1 | 0.2±0.1 | 0.3±0.1 | 0.6±0.2 | 0.5±0.1 |
| Dihydroprogesterone | 0.3±0.2 | 0.4±0.1 | 0.5±0.2 | 0.8±0.4 | 0.8±0.2 | 0.5±0.1 |
| P Utilization | 3.3±0.8 | 4.7±2.1 | 64.1±32.8‡ | 39.7±21.1‡ | 7.3±3.7 | 1.5±0.4 |
| Medial Prefrontal Cortex | ||||||
| 17β-Oestradiol | 0.32±0.12 | 0.11±0.03† | 0.79±0.13‡ | 0.44±0.13†‡ | 0.28±0.08 | 0.14±0.02† |
| Progesterone | 0.4±0.1 | 0.4±0.1 | 0.1±>0.0‡ | 0.4±0.1‡ | 0.6±0.1‡ | 0.5±0.1‡ |
| Dihydroprogesterone | 0.2±0.1 | 0.5±0.1 | 0.8±0.3^ | 0.5±0.2 | 0.9±0.1^ | 0.4±0.1 |
| 3α,5α-THP | 0.5±0.1 | 1.4±0.7 | 2.2±0.7‡ | 4.4±1.7‡ | 1.1±0.3 | 1.8±0.6 |
| P Utilization | 2.8±0.9 | 4.8±1.4 | 26.3±12.6‡ | 19.6±8.6‡ | 7.1±3.3 | 4.2±1.2 |
| Diencephalon | ||||||
| 17β-Oestradiol | 0.15±0.06 | 0.11±0.02 | 0.35±0.08‡ | 0.31±0.12‡ | 0.09±0.02 | 0.09±0.03 |
| Progesterone | 0.15±0.04 | 0.23±0.03† | 0.05±0.03‡ | 0.15±0.04†‡ | 0.23±0.04‡ | 0.30±0.03†‡ |
| Dihydroprogesterone | 0.2±0.1 | 0.6±0.1† | 0.2±0.1 | 1.2±0.6† | 0.3±0.1 | 0.7±0.1† |
| 3α,5α-THP | 0.5±0.1 | 0.2±0.1 | 1.1±0.4‡ | 1.4±0.7‡ | 0.3±0.1 | 0.6±0.2 |
| P Utilization | 10.2±4.0 | 3.2±0.5 | 53.9±20.0‡ | 54.3±33.3‡ | 3.9±1.2 | 4.0±0.7 |
indicates a main effect (collapsing on the factor of prenatal manipulation) wherein females differ significantly from males via two-way ANOVA.
indicates a main effect (collapsing on the factor of sex) wherein 3α,5α-THP-, and/or finasteride-, exposed offspring significantly differ from control offspring via two-way ANOVA.
indicates an interaction wherein the denoted group significantly differs from same-sex controls via two-way ANOVA, p ≤ 0.05.
Progesterone content was also influenced by prenatal 3α,5α-THP and finasteride manipulations in the juvenile hippocampus [F(2,84) = 5.89, p < 0.05], mPFC [F(2,84) = 11.54, p < 0.05], and/or diencephalon [F(2,84) = 12.63, p < 0.05]. Compared to vehicle-treated offspring, prenatal 3α,5α-THP significantly reduced progesterone content in the mPFC (p = 0.009) and diencephalon (p = 0.007) of male and female offspring, but not hippocampus (Table 2). Prenatal finasteride treatment resulted in significantly greater progesterone content in the mPFC (p = 0.04) and diencephalon (p = 0.03), but not hippocampus (Table 2). Notably, progesterone utilization (ratio of metabolites to progesterone) was altered in the hippocampus [F(2,84) = 5.93, p < 0.05], mPFC [F(2,84) = 5.36, p < 0.05] and diencephalon [F(2,84) = 6.23, p < 0.05]. Prenatal 3α,5α-THP treatment significantly increased progesterone utilization in the juvenile hippocampus (p = 0.004), mPFC (p = 0.004), and diencephalon (p = 0.004), of male and female offspring compared to vehicle-exposed controls (Table 2). Prenatal finasteride exposure did not significantly influence the ratio of progesterone to its metabolites.
Prenatal manipulations altered 17β-oestradiol content in the offspring hippocampus [F(2,84) = 10.21, p < 0.05], mPFC [F(2,84) = 11.48, p < 0.05], and diencephalon [F(2,84) = 7.71, p < 0.05]. Irrespective of sex, prenatal 3α,5α-THP treatment significantly increased 17β-oestradiol content in the hippocampus (p = 0.007), mPFC (p < 0.0001), and diencephalon compared to vehicle-exposed controls (p = 0.003; Table 2). No differences were observed in 17β-oestradiol content in any of the brain regions studied following gestational finasteride exposure.
Some sex differences were observed in central progestogen formation. There was a significant interaction [F(2,84) = 3.00, p = 0.05], whereby DHP content in the mPFC was influenced by perinatal manipulations in a sex-dependent manner. In the male offspring, prenatal 3α,5α-THP (p = 0.01) or finasteride treatment (p = 0.02) significantly increased DHP content in the mPFC compared to that seen in the vehicle-exposed males; however, no differences were observed across the female groups (Table 2). Additionally, sex differences were observed wherein females had greater progesterone [F(1,84) = 10.31, p < 0.05] and DHP [F(1,84) = 8.28, p < 0.05] concentrations in the diencephalon, and lower 17β-oestradiol levels in the hippocampus [F(1,84) = 8.28, p < 0.05] and mPFC [F(1,84) = 5.46, p < 0.05] compared to the male offspring (Table 2).
Circulating steroid concentrations in the juvenile offspring were influenced by gestational manipulation of 3α,5α-THP availability
Serum corticosterone concentrations showed a significant interaction with gestational manipulation and sex [F(2,84) = 9.51, p < 0.05]. Prenatal 3α,5α-THP administration resulted in increased corticosterone concentration in both male (p = 0.02) and female (p = 0.03) offspring compared to their respective same-sex controls (Table 3). Prenatal finasteride treatment significantly increased circulating corticosterone concentrations compared with same-sex controls, in the female (p < 0.0001) but not the male offspring.
Table 3. Circulating steroid concentrations in the male and female offspring.
Serum concentrations of corticosterone (μg/dl), 17β-oestradiol (pg/g), progesterone (P, ng/g), dihydroprogesterone (ng/g), 5α-pregnan-3α-ol-20-one (3α,5α-THP; ng/g), and progesterone utilization (DHP+3α,5α-THP:P ratio) of male and female offspring born to dams that were exposed to vehicle, 3α,5α-THP (10 mg/kg), or finasteride (50 mg/kg) on gestational days 17–21.
| Serum Steroid Concentrations (mean±SEM) | ||||||
|---|---|---|---|---|---|---|
| Control | 3α,5α-THP | Finasteride | ||||
| Male (n=15) | Female (n=15) | Male (n=15) | Female (n=15) | Male (n=15) | Female (n=15) | |
| Corticosterone | 0.2±0.1 | 0.4±0.1 | 1.8±0.8^ | 1.7±0.4^ | 0.3±0.1 | 3.6±0.6^ |
| 17β-Oestradiol | 4.7±0.7 | 3.8±1.1 | 3.0±0.6 | 2.9±0.8 | 2.9±1.1 | 2.1±0.4 |
| Progesterone (P) | 6.6±1.1 | 8.4±1.4 | 9.5±1.1 | 4.8±1.0^ | 5.0±1.1 | 6.6±1.3 |
| Dihydroprogesterone | 16.6±1.5 | 14.2±2.0 | 14.7±2.8 | 17.0±2.6 | 9.7±1.0 | 14.6±1.3 |
| 3α,5α-THP | 5.4±1.0 | 6.3±1.1 | 4.1±0.8 | 6.5±1.2 | 6.8±1.4 | 5.5±1.5 |
| P Utilization | 7.3±2.3 | 2.5±0.6 | 2.6±0.6 | 8.0±2.4^ | 6.6±2.4 | 5.8±1.4 |
indicates a main effect (collapsing on the factor of prenatal manipulation) wherein females differ significantly from males via two-way ANOVA.
indicates a main effect (collapsing on the factor of sex) wherein 3α,5α-THP-, and/or finasteride-, exposed offspring significantly differ from control offspring via two-way ANOVA.
indicates an interaction wherein the denoted group significantly differs from same-sex controls via two-way ANOVA, p ≤ 0.05.
Serum progesterone concentrations were significantly reduced [F(2,84) = 5.05, p < 0.05] in 3α,5α-THP-exposed females compared to vehicle-exposed females (p = 0.045), but no such differences were observed for males (Table 3). There was also an interaction for serum measurements reflecting progesterone utilization to be enhanced [F(2,84) = 4.01, p < 0.05] among 3α,5α-THP-exposed females compared to vehicle-exposed females (p = 0.02); this was not observed among males (Table 3). No differences were observed in serum concentrations of 17β-oestradiol, DHP, or 3α,5α-THP concentrations in any of the groups.
Discussion
The hypothesis that inhibition of 3α,5α-THP formation via finasteride would reduce the length of gestation and pup viability is supported by the results of the present study. While, 3α,5α-THP administration to pregnant dams between GD 17–21 did not extend pregnancy or alter fecundity, finasteride administration significantly decreased gestational length and the number of pups found after birth, compared to dams administered vehicle. These findings are in accord with the known teratogenic effects of finasteride when administered to pregnant women [41] and support the notion that 5α-reduced metabolites play an important role in the mechanisms that maintain pregnancy and facilitate parturition [42]. Given that systemic finasteride (50 mg/kg/ml) has also been demonstrated to enhance circulatory progesterone among late-pregnant Sprague-Dawley rats [25], 5α-reduction of progesterone may underlie a critical mechanism in regulating rodent parturition. In support, female mice with a disruption in the gene encoding for 5α-reductase type I have poor pregnancy outcomes, including embryonic resorption and reduced litter size [43].
An important question to consider is: what happened to the finasteride-exposed pups that were gestated but not observed following birth? Pup resorption is unlikely to explain the reduced litter sizes observed among finasteride-administered dams given the proximity of the manipulations to the end of pregnancy and the similarities in weights of dams late in gestation. As such, reductions in offspring number may be attributable to either death in utero or at birth (as a consequence of being born prematurely) and/or cannibalism/infanticide at birth (perhaps as a consequence of being born under-developed or as a consequence of finasteride-induced disruption of maternal behaviour). Indeed, finasteride administration delays the onset of maternal behaviour among primigravid Sprague-Dawley rats [25]. While deceased pups were not found in any dam cages, it cannot be ruled out that dams cannibalised pups between observation periods. Despite average dam weights not being significantly different at term, dams administered finasteride did gain a significantly lower percentage of weight by term than did 3α,5α-THP-administered dams, which may be indicative of fetal loss or under-development at birth. As such, actions of 5α-reduced metabolites may be critical for the maintenance of rodent pregnancy and/or offspring survival.
Another important question to consider is: what are the mechanisms through which 3α,5α-THP exerts its effects to maintain pregnancy? One possible mechanism may be via inhibitory actions to dampen HPA axis or neurohypophysial oxytocin responses to stress. In lambs, prenatal glucocorticoids promote preterm birth [44] and studies utilizing sheep models have demonstrated low fetal birth weight following prenatal exposure to synthetic glucocorticoids [45]. Inhibitory influences of 3α,5α-THP extend to magnocellular oxytocin neurones in late pregnancy [46]. At this time, 3α,5α-THP prevents oxytocin neurones from responding to stimuli, such as stressors [47,48]. Since oxytocin is an important and potent stimulator of uterine contractions at term, this action of 3α,5α-THP may minimize the risk of preterm birth through maintaining quiescence of oxytocin neurones. As such, inhibitory actions of 3α,5α-THP on oxytocin-producing neurones may be important for maintaining gestation [48] and may play an important role in preventing premature delivery.
The hypotheses that exposure to 3α,5α-THP, or inhibition of its formation by finasteride, would enhance or perturb cognitive behaviour of surviving offspring, respectively, concomitant with altered progestogen availability in the brains of offspring, were supported, in part. While exposure to 3α,5α-THP did not alter offspring cognitive performance, offspring born to dams administered finasteride displayed significantly poorer performance in the object recognition task compared to those administered vehicle. Of the brain regions investigated, prenatal finasteride had lasting effects to reduce 3α,5α-THP levels only in the hippocampus of the juvenile male and female offspring. These data are in accord with previous findings in adults rats, which show that restraint stress during late pregnancy (GD 17–20) reduces maternal hippocampal 3α,5α-THP levels [49]. Moreover, acutely-administered 3α,5α-THP improves memory in hippocampus- and frontal cortex-mediated tasks of adult rats [50]. We have recently observed prenatal exposure to stressors (thrice daily restraint or chronic unpredictable stressors) to reduce cognitive performance in juveniles concomitant with dysregulation in central progestogen formation [16,17]. The present data indicate that inhibition of 5α-reduction during late pregnancy can have lasting effects to alter cognitive performance into adolescence.
Some of the effects of finasteride to reduce cognitive performance may be due, in part, to a role of 3α,5α-THP as a neurotrophic modulator. The hippocampus is particularly sensitive to glucocorticoid exposure during fetal development [27] and prenatal stress can promote reduced hippocampal volume and decreased dendritic spine density [17,51]. Others have demonstrated that rats administered 3α,5α-THP prior to a prefrontal cortex-targeted traumatic brain injury have less central cell loss and less cognitive impairment on hippocampus-dependent tasks following recovery [52]. 3α,5α-THP has similar neuroprotective effects following ischemic brain injury in adult rats [53]. Thus, inhibition of 5α-reduction during pregnancy perturbs later cognitive performance of juvenile offspring, which may be due in part to reduced neurotrophic actions of 3α,5α-THP.
Our hypotheses that enhanced exposure to 3α,5α-THP, or inhibition of its formation by finasteride, would alter anxiety-type behaviour of the surviving offspring was not supported. Neither prenatal exposure to 3α,5α-THP, nor finasteride, significantly altered anxiety-like behaviour in the open field test. We did, however, observe a significant sex difference, wherein juvenile males demonstrated significantly lower anxiety-type behaviour than females. This finding is in contrast to studies with adult, group-housed rats and mice, in which females typically demonstrate lower anxiety-type behavior than do males [54–56]. However, when the influence of gonadal hormones is taken into account, we and others have observed that reductions in anxiety-type behaviour of females coincide with cyclical enhancement of gonadal progestogen levels [57,58]. Given that the present studies were performed in juvenile rats (when the activational role of gonadal hormones can be assumed to be minimal), it is of interest that a sex difference was observed, although the explanation is not clear. Prenatal stress differentially affects anxiety-type behaviour in male and female offspring [59,60] or commensurately enhances anxiety-type behavior in both sexes [61]. Some of these apparent discrepancies may be due to variations in the prenatal stress paradigm used and/or may result from differences in the strain of rat used (given that opposing prenatal stress effects have been observed for morphological and affective abberations observed among Sprague-Dawley vs. Long-Evans rats) [62–65].
In addition to enhanced anxiety-type behavior among female offspring, prenatal finasteride treatment elevated corticosterone levels among females only, which may contribute to the male bias observed for anxiety-type behaviour in the open field task. Among all juveniles, corticosterone levels were observed to be relatively low. Long-Evans rats [66] have reportedly lower concentrations of circulatory corticosterone in adolescence and adulthood compared to other strains, such as Sprague-Dawley rats [67]. Albeit, corticosterone levels are expected to be lower among juvenile rats compared to adults. In support, even after a significant stressor (confinement to an open elevated arm for 15 mins), corticosterone concentrations are lower among pubescent Long-Evans rats (~1–3 μg/dl at 45 days of age) compared to adult Long-Evans rats (~3–12 μg/dl at 70 days of age [66]). Prior investigations in rats indicate that males and females can have a divergent 5α-reductase type II profile under stress (with males displaying increased, and females decreased, mRNA expression in the prefrontal cortex) [68], which may be involved in these effects. The extent to which effects of prenatal finasteride exposure persists, are ameliorated, or are exacerbated, in adulthood is not known and will be the target of future investigation. In addition, the characterization of genetic differences that influence affective and HPA axis profiles should be systematically addressed in future studies using different rat strains.
Enhancement of prenatal 3α,5α-THP exposure influenced neuroendocrine programming. While prenatal 3α,5α-THP administration did not significantly alter cognitive performance or affective behaviour compared to vehicle-control manipulations, salient changes in the neuroendocrine milieu were observed among 3α,5α-THP-exposed, juvenile rats. Hence, prenatal 3α,5α-THP administration significantly enhanced 17β-oestradiol and 3α,5α-THP concentrations in all brain regions examined. The enhancement of 17β-oestradiol level may be due, in part, to actions of 3α,5α-THP that dampen corticotropin releasing hormone (CRH) transcription [69]. Typically, CRH inhibits gonadotropin releasing hormone secretion via enhancement of β-endorphin [70]; hence, actions of 3α,5α-THP that reduce CRH secretion may promote oestradiol accumulation in the brain that would otherwise be regulated via negative feedback. In addition, prenatal 3α,5α-THP exposure promoted modestly elevated corticosterone levels in response to behavioural testing. At the time of puberty, patterns for distinct GABAergic receptor subunit composition are observed in rodents which can attenuate neurosteroid efficacy in HPA axis modulation [71]. This transient GABA receptor subunit expression may underlie the observed effects for prenatal 3α,5α-THP to increase corticosterone at the time of puberty. Future investigations will be needed to assess these effects in adulthood. As such, the gestational 3α,5α-THP milieu may have lasting effects to influence offspring neuroendocrine development.
In conclusion, these data demonstrate that inhibition of 5α-reductase activity during late pregnancy (GD 17–21) can induce preterm birth and reduce fecundity, as well as having long-term consequences for cognitive function and hippocampal 3α,5α-THP formation in the surviving offspring. These findings may present a paradigm to investigate the involvement of 3α,5α-THP in a rodent model of preterm birth. Future investigations will aim to utilize 3α,5α-THP administration to prevent the negative effects of finasteride on birth outcomes. A limitation of this study is that litter birth weights were not assessed. While, there were no differences in the weight of the juvenile offspring across groups at the time of behavioural testing, assessment of differences at birth may be indicative of fetal effects that are transient or pervasive throughout life. Future investigations should aim to assess effects from birth throughout the lifespan. In addition, further investigation of the reduction in number of viable offspring is warranted given that it remains to be delineated how blockade of 5α-reduction in pregnancy reduces fecundity.
These data have important clinical implications. Among women that are at-risk for preterm birth, intramuscular injections of 17α-hydroxyprogesterone (17α-OHP) reduce the incidence of parturition before 34 or 37 weeks of gestation, respectively [20]; albeit, effectiveness of 17α-OHP is not always observed among women with a history of prior preterm birth and/or pregnancy complications [72]. Analyses of 11 randomised, controlled trials (2,425 pregnant women) revealed that treatment with progesterone, compared to placebo, significantly reduced the incidence of birth before 34 weeks of gestation among those with a history of preterm birth [73]. Metabolism to 3α,5α-THP may partly underlie the mechanisms mediating efficacy of progesterone as a tocolytic agent. It should be noted that the decrease in peripheral progestogen concentrations that precedes parturition in rodents is not characteristic of human pregnancy. In women, peripheral progestogen levels are maintained via the placenta, which acts as a progestogen-secreting gland [74]. However, concentrations of 3α,5α-THP have been reported to be markedly reduced in the umbilical artery, umbilical vein, maternal cubital vein, and amniotic fluid in the last three weeks of birth compared to other times in the last trimester of pregnancy in women [12]. Moreover, changes in central 3α,5α-THP levels are observed in rats prior to parturition and cannot be directly assessed in women [13]. Preterm birth, which occurs relatively frequently in human pregnancy, may deprive the brain of key steroid exposure. Further investigation is required to elucidate the roles of 3α,5α-THP in preterm birth and offspring neurodevelopment.
Acknowledgments
CAF, JJP and AAW appreciate technical help by Kassandra Edinger and are supported by funding from the National Institute of Mental Health (MH06769801). PJB and JAR are supported by the Biotechnology and Biological Sciences Research Council (BBSRC). CAF and AAW were pregnant during the implementation and reporting of this study, respectively.
References
- 1.Giurgescu C. Are maternal cortisol levels related to preterm birth? J Obstet Gynecol Neon Nurs. 2009;38:377–390. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues T, Barros H. Maternal unemployment: an indicator of spontaneous preterm delivery risk. Eur J Epidemiol. 2008;23:689–693. doi: 10.1007/s10654-008-9283-x. [DOI] [PubMed] [Google Scholar]
- 3.Xiong X, Harville EW, Mattison DR, Elkind-Hirsch K, Pridjian G, Buekens P. Exposure to Hurricane Katrina, post-traumatic stress disorder and birth outcomes. Am J Med Sci. 2008;336:111–115. doi: 10.1097/MAJ.0b013e318180f21c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakulinen A, Heinonen K, Jokela V, Launiala K. Prematurity-associated morbidity during the 1st 2 yrs of life. A population-based study. Acta Paediatr Scand. 1988;77:340–348. doi: 10.1111/j.1651-2227.1988.tb10658.x. [DOI] [PubMed] [Google Scholar]
- 5.Petrini JR, Dias T, McCormick MC, Massolo ML, Green NS, Escobar GJ. Increased risk of adverse neurological development for late preterm infants. J Pediatr. 2009;154:169–176. doi: 10.1016/j.jpeds.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Kerstjens JM, de Winter AF, Bocca-Tjeertes IF, Ten Vergert EM, Reijneveld SA, Bos AF. Developmental delay in moderately preterm-born children at school entry. J Pediatr. 2011;159:92–98. doi: 10.1016/j.jpeds.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 7.Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, Breslau N. Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. 2010;126:1124–1131. doi: 10.1542/peds.2010-1536. [DOI] [PubMed] [Google Scholar]
- 8.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the HPA axis response to stress among full-term infants. Dev Psychobiol. 2011;53:175–183. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–152. doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3α-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21:268–279. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- 12.Hill M, Pařízek A, Kancheva R, Jirásek JE. Reduced progesterone metabolites in human late pregnancy. Physiol Res. 2011;60:225–241. doi: 10.33549/physiolres.932077. [DOI] [PubMed] [Google Scholar]
- 13.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci U S A. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye CA. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology. 2009;34:S143–S161. doi: 10.1016/j.psyneuen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirst JJ, Yawno T, Nguyen P, Walker DW. Stress in pregnancy activates neurosteroid production in the fetal brain. Neuroendocrinology. 2006;84:264–274. doi: 10.1159/000097990. [DOI] [PubMed] [Google Scholar]
- 16.Paris JJ, Frye CA. Juvenile offspring of rats exposed to restraint stress in late gestation have impaired cognitive performance and dysregulated progestogen formation. Stress. 2011;14:23–32. doi: 10.3109/10253890.2010.512375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paris JJ, Frye CA. Gestational exposure to variable stressors produces decrements in cognitive and neural development of juvenile male and female rats. Curr Top Med Chem. 2011;11:1706–1713. doi: 10.2174/156802611796117649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frye CA, Sumida K, Zimmerberg B, Brunelli SA. Rats bred for high versus low anxiety responses neonatally demonstrate increases in lordosis, pacing behavior, and midbrain 3α,5α-THP levels as adults. Behav Neurosci. 2006;120:281–289. doi: 10.1037/0735-7044.120.2.281. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerberg B, Martinez AR, Skudder CM, Killien EY, Robinson SA, Brunelli SA. Effects of gestational allopregnanolone administration in rats bred for high affective behavior. Physiol Behav. 2010;99:212–217. doi: 10.1016/j.physbeh.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O’Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17α-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 21.Paris JJ, Brunton PJ, Russell JA, Frye CA. Immune stress in late pregnant rats decreases length of gestation, fecundity, and alters later cognitive and affective behaviour of surviving offspring. Stress. doi: 10.3109/10253890.2011.628719. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM. A new look at the 5α-reductase inhibitor finasteride. CNS Drug Rev. 2006;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci. 2001;1:287–296. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- 24.Bowman CJ, Barlow NJ, Turner KJ, Wallace DG, Foster PM. Effects of in utero exposure to finasteride on androgen-dependent reproductive development in the male rat. Toxicol Sci. 2003;74:393–406. doi: 10.1093/toxsci/kfg128. [DOI] [PubMed] [Google Scholar]
- 25.Mann PE. Finasteride delays the onset of maternal behavior in primigravid rats. Physiol Behav. 2006;88:333–338. doi: 10.1016/j.physbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 27.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 28.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–357. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- 29.Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–141. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86:449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 31.Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 1975;14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- 32.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 33.Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5α-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niswender GD. Influence of the site of conjugation on the specificity of antibodies to progesterone. Steroids. 1973;22:413–424. doi: 10.1016/0039-128x(73)90104-9. [DOI] [PubMed] [Google Scholar]
- 35.Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. Radioimmunoassay of 3α-hydroxy-5α-pregnan-20- one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- 36.Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther. 1994;271:164–170. [PubMed] [Google Scholar]
- 37.McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 38.England BG, Niswender GD, Midgley AR., Jr Radioimmunoassay of estradiol- 17β without chromatography. J Clin Endocrinol Metab. 1974;38:42–50. doi: 10.1210/jcem-38-1-42. [DOI] [PubMed] [Google Scholar]
- 39.Rodbard D, Hutt DM. Int Atomic Energy Agency: Symposium on RIA and Related Procedures in Medicine. New York: Uni-pub; 1974. Statistical analysis of radioimmunoassay and immunoradiometric (labeled antibody) assays: a generalized, weighted, iterative, least-squares method for logistic curve fitting; p. 165. [Google Scholar]
- 40.Keppel G, Wickens TD. Design and analysis: a researcher’s handbook. 4. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. p. 125. [Google Scholar]
- 41.Stout SM, Stumpf JL. Finasteride treatment of hair loss in women. Ann Pharmacother. 2010;44:1090–1097. doi: 10.1345/aph.1M591. [DOI] [PubMed] [Google Scholar]
- 42.Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M, Reis FM, Luisi M, Genazzani AR. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85:2429–2433. doi: 10.1210/jcem.85.7.6675. [DOI] [PubMed] [Google Scholar]
- 43.Mahendroo MS, Cala KM, Russell DW. 5α-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 44.Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45:515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 45.Kerzner LS, Stonestreet BS, Wu KY, Sadowska G, Malee MP. Antenatal dexamethasone: effect on ovine placental 11β-hydroxysteroid dehydrogenase type 2 expression and fetal growth. Pediatr Res. 2002;52:706–712. doi: 10.1203/00006450-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends Neurosci. 2000;23:190–195. doi: 10.1016/s0166-2236(99)01540-4. [DOI] [PubMed] [Google Scholar]
- 47.Brunton PJ, McKay AJ, Ochedalski T, Piastowska A, Rebas E, Lachowicz A, Russell JA. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J Neurosci. 2009;29:6449–6460. doi: 10.1523/JNEUROSCI.0708-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell JA, Leng G, Douglas AJ. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol. 2003;24:27–61. doi: 10.1016/s0091-3022(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 49.Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brummelte S, Pawluski JL, Galea LA. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: A model of post-partum stress and possible depression. Horm Behav. 2006;50:370–382. doi: 10.1016/j.yhbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Sayeed I, Stein DG. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog Brain Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- 54.Alstott J, Timberlake W. Effects of rat sex differences and lighting on locomotor exploration of a circular open field with free-standing central corners and without peripheral walls. Behav Brain Res. 2009;196:214–219. doi: 10.1016/j.bbr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Masur J, Schutz MT, Boerngen R. Gender differences in open-field behavior as a function of age. Dev Psychobiol. 1980;13:107–110. doi: 10.1002/dev.420130202. [DOI] [PubMed] [Google Scholar]
- 56.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 57.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 58.Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 59.Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 60.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- 61.Fride E, Weinstock M. Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci. 1988;42:1059–1065. doi: 10.1016/0024-3205(88)90561-9. [DOI] [PubMed] [Google Scholar]
- 62.Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 63.Fleming DE, Anderson RH, Rhees RW, Kinghorn E, Bakaitis J. Effects of prenatal stress on sexually dimorphic asymmetries in the cerebral cortex of the male rat. Brain Res Bull. 1986;16:395–398. doi: 10.1016/0361-9230(86)90062-6. [DOI] [PubMed] [Google Scholar]
- 64.Steiner MA, Lecourt H, Rakotoariniaina A, Jenck F. Favoured genetic background for testing anxiolytics in the fear-potentiated and light-enhanced startle paradigms in the rat. Behav Brain Res. 2011;221:34–42. doi: 10.1016/j.bbr.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 65.Zuena AR, Mairesse J, Casolini P, Cinque C, Alemà GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One. 2008;3:e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Cao J, Belluzzi JD, Loughlin SE, Dao JM, Chen Y, Leslie FM. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:82–90. doi: 10.1016/j.pbb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sànchez P, Torres JM, Olmo A, O’Valle F, Ortega E. Effects of environmental stress on mRNA and protein expression levels of steroid 5α-Reductase isozymes in adult rat brain. Horm Behav. 2009;56:348–353. doi: 10.1016/j.yhbeh.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- 70.Petraglia F, De Leo V, Nappi C, Facchinetti F, Montemagno U, Brambilla F, Genazzani AR. Differences in the opioid control of luteinizing hormone secretion between pathological and iatrogenic hyperprolactinemic states. J Clin Endocrinol Metab. 1987;64:508–512. doi: 10.1210/jcem-64-3-508. [DOI] [PubMed] [Google Scholar]
- 71.Smith SS, Aoki C, Shen H. Puberty, steroids and GABAA receptor plasticity. Psychoneuroendocrinology. 2009;34:S91–S103. doi: 10.1016/j.psyneuen.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rafael TJ, Mackeen AD, Berghella V. The effect of 17α-hydroxyprogesterone caproate on preterm birth in women with an ultrasound-indicated cerclage. Am J Perinatol. 2011;28:389–394. doi: 10.1055/s-0031-1272967. [DOI] [PubMed] [Google Scholar]
- 73.Dodd JM, Flenady VJ, Cincotta R, Crowther CA. Progesterone for the prevention of preterm birth: a systematic review. Obstet Gynecol. 2008;112:127–134. doi: 10.1097/AOG.0b013e31817d0262. [DOI] [PubMed] [Google Scholar]
- 74.Milewich L, Gant NF, Schwarz BE, Chen GT, MacDonald PC. 5α-reductase activity in human placenta. Am J Obstet Gynecol. 1979;133:611–617. doi: 10.1016/0002-9378(79)90006-1. [DOI] [PubMed] [Google Scholar]