Abstract
A significant subset of gliomas arises after activation of the pro-proliferative platelet-derived growth factor (PDGF) pathway. The progression of low-grade gliomas to more malignant tumors may be due to oncogenic cellular programs combining with those suppressing apoptosis. Anti-apoptotic genes are overexpressed in a variety of cancers and the anti-apoptotic gene, BCL2, is associated with treatment resistance and tumor recurrence in gliomas. However, the impact of anti-apoptotic gene expression to tumor formation and progression is unclear. We overexpressed Bcl-2 in a PDGFB-dependent mouse model of oligodendroglioma, a common glioma subtype, to assess its effect in vivo. We hypothesized that the anti-apoptotic effect would complement the pro-proliferative effect of PDGFB to promote tumor formation and progression to anaplastic oligodendroglioma (AO). Here, we show that co-expression of PDGFB and Bcl-2 results in a higher overall tumor formation rate compared to PDGFB alone. Co-expression of PDGFB and Bcl-2 promotes progression to AO with prominent foci of necrosis, a feature of high-grade gliomas. Median tumor latency was shorter in mice injected with PDGFB and Bcl-2 compared to those injected with PDGFB alone. Although independent expression of Bcl-2 was insufficient to induce tumors, suppression of apoptosis (detected by cleaved caspase-3 expression) was more pronounced in AOs induced by PDGFB and Bcl-2 compared to those induced by PDGFB alone. Tumor cell proliferation (detected by phosphohistone H3 activity) was also more robust in high-grade tumors induced by PDGFB and Bcl-2. Our results indicate that suppressed apoptosis enhances oligodendroglioma formation and engenders a more malignant phenotype.
Keywords: Glioma, Oligodendroglioma, Bcl-2, Apoptosis, Necrosis
Introduction
Gliomas are the most common and deadliest primary brain tumors in humans. These diffuse neoplasms are classified as astrocytomas or oligodendrogliomas but can be considered mixed when displaying features of both lineages. A widely used classification scheme based on histopathology recognizes two grades of oligodendrogliomas, oligodendroglioma (grade II) and anaplastic oligodendroglioma (grade III)1. The median survival time for patients who have oligodendroglioma ranges from seven to ten years, but for patients with anaplastic oligodendrogliomas (AO), it is significantly shorter, ranging from three to five years 2, 3. Despite the use of aggressive measures including surgery, radiation therapy, and chemotherapy, these tumors become resistant to treatment and inevitably progress. Upregulation of genes that permit evasion of apoptotic cell death has been observed in recurrent high-grade tumors and is a mechanism by which they develop treatment resistance. These genes are critical for neoplastic transformation because they permit oncogene-induced tumor cell proliferation by disabling cell programs that mediate apoptosis4. Anti-apoptotic genes are upregulated in glioma5, prompting our hypothesis that apoptotic suppression is necessary for glioma formation and progression. To analyze the consequences of anti-apoptotic signaling on tumorigenesis, we studied overexpression of Bcl-2, which confers a strong anti-apoptotic effect, in a platelet-derived growth factor beta (PDGFB)-dependent mouse model system of oligodendroglioma.
Both PDGF and its receptor (PDGFR) are highly expressed in many cancers including sarcomas, germ cell tumors, and brain tumors 6, 7. Genetic alterations in PDGF and PDGFR characterize a significant subset of high-grade gliomas 8. Homodimerization of the B isoform of the ligand (PDGFB) is oncogenic and is observed in low- and high-grade tumors7, 9. Successful binding of the receptor by its cognate ligand on tumor cells results in activation of oncogenicsignaling pathways which promote tumor cell proliferation (reviewed in 6). The relevance of PDGFB in glioma development has been validated in animal model systems in which its overexpression results in both grade II and grade III tumors, although grade II tumors predominate 10, 11. This variability in tumor grade has been described as “dose dependent” in that mice exposed to higher levels of PDGFB in the brain develop more malignant tumors 9.
In mouse model systems of oligodendroglioma, it has been shown that whereas ectopic expression of PDGFB is sufficient to initiate tumor formation, PDGFB cooperates with other signaling molecules that drive cell cycle progression to enhance tumor formation and progression. Insulin-like growth factor binding protein 2 (IGFBP2), drives cell cycle progression, and when combined with PDGFB increases tumor formation and promotes the anaplastic phenotype 12, 13. Combined expression of PDGFB with tumor-promoting microRNA (miR-26a) also enhances tumor formation 14. Expression of PDGFB in an Ink4a-Arf−/−background increases tumor incidence and grade 15.
Although a single oncogene (e.g. PDGFB) can initiate tumor formation, the ability of a cancerous cell to evade apoptosis is necessary for tumor growth 16. Members of the B-cell lymphoma-2 (Bcl-2) family of genes are potent suppressors of apoptosis, and alterations in their expression contribute to tumorigenesis 5. Overexpression of the Bcl-2 gene has been described in a variety of human malignancies 17–19. In high-grade gliomas, pro-survival and anti-apoptotic members of the Bcl-2 gene family show upregulated expression in tumors exhibiting recurrence and progression 20, 21. The anti-apoptotic effect of these genes may shift the tumor to the anaplastic phenotype by inducing a different mechanism of cell death – necrosis, a cardinal feature of high-grade gliomas 22. Still, the functional consequence of apoptotic suppression on glioma development and progression remains unclear, with some studies correlating increased Bcl-2 expression with a protective effect on patient survival 23.
We modeled apoptotic suppression on glioma formation in mice using a transgenic mouse system (RCAS/Ntv-a). In this system, a gene of interest is cloned into a modified avian retrovirus (RCAS) that is replication defective in mammalian cells. This vector is introduced into a transgenic mouse line (Ntv-a) that expresses TVA (the avian leukosis virus subtype A receptor, the receptor for RCAS) under control of the Nestin promoter. Nestin expressing cells include glioneuronal precursors, which are the presumed cells of origin for glial tumors. The gene is randomly incorporated into the host cell’s genome and is expressed by the constitutive retroviral promoter long terminal repeat. This method of somatic cell gene transfer can be used to assess the functional consequence of ectopic gene expression in vivo. Further, multiple RCAS vectors can be expressed together, permitting the study of cooperative effects of different genes on tumorigenesis. This system has been used to analyze the effect of cellular programs via the expression of specific genes (e.g. PDGFB to model proliferation) on the formation and progression of various brain tumors including glioma and medulloblastoma 24–26. We studied the anti-apoptotic effect conferred by Bcl-2 expression using the RCAS/Ntv-a system to examine its tumorigenic effect in high-grade gliomas20, 27. We studied the coexpression of PDGFB and Bcl-2, hypothesizing that the pro-proliferative effect of PDGFB and the anti-apoptotic effect of Bcl-2 would combine to enhance tumor formation and grade. Here, we show that Bcl-2 enhances the tumor formation rate, promotes anaplastic histologic features, and decreases symptom-free survival time in a PDGFB-dependent mouse model system of oligodendroglioma.
Materials and methods
Vector constructs
The details of the creation of RCAS-Bcl-2 are described in 28. Briefly, this vector was constructed by ligating a PCR-generated cDNA corresponding to the entire coding sequence of human BCL-2 into the retroviral vector RCASBP(A). RCAS- PDGFB was constructed with a hemagglutinin epitope tag and is described in 10. The details of the creation of RCAS-IGFBP2 are described in 12. Briefly, the RCAS-IGFBP2 vector was constructed by subcloning the 1.4kb cDNA fragment of IGFPB2 into the RCAS-X vector using NotI and ClaI restriction enzymes.
Transfection of DF-1 cells
DF-1 immortalized chicken fibroblasts were grown in DMEM containing 10% FBS (GIBCO, Carlsbad, CA) in an humidified atmosphere of 95% air/5% CO2 at 37°C. To produce live virus, we transfected plasmid versions of RCAS vectors into immortalized chicken fibroblasts (DF-1 cells) using FuGene6 (Roche, Nutley, NJ) and allowed them to replicate in culture.
Verification of transgene expression
To verify Bcl-2 expression after infection with RCAS-Bcl-2, we grew untransfected DF-1 cells in culture. At 50% confluency, the cells were exposed for 48 hours to filtered medium conditioned by DF-1/RCAS-Bcl-2-transfected cells. Cells were fixed with 4% paraformaldehyde in phospate-buffered saline (PBS) followed by treatment with cold methanol. Immunocytochemical labeling was performed using standard methods. A mouse monoclonal antibody against human Bcl-2 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and goat anti-mouse Alexa Fluor® 594 fluorescent conjugate (1:500; Molecular Probes, Carlsbad, CA) were used for detection. Prolong Gold antifade reagent with DAPI (Molecular Probes, Carlsbad, CA) was used for labeling cell nuclei and for mounting. Staining was observed using a Zeiss Axioskop 40 microscope. Verification of PDGFB and IGFBP2 expression from DF-1 cells after infection was performed by Western blot methodology. We prepared whole-cell lysates from DF-1 cell cultures 48 hours after infection with virus-containing medium conditioned by DF-1 cells expressing either RCAS-PDGFB or RCAS-IGFBP2. Protein samples (10 μg) were fractionated by SDS-PAGE using gels containing 10% polyacrylamide, transferred to PVDF membrane, and probed either with the anti-HA antibody (1:1000; F7, Santa Cruz Biotechnology, Santa Cruz, CA) to detect PDGFB expression or with an antibody for IGFBP2 (1:1000; C-18, Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies, including goat anti-mouse IgG (1:2500; Pierce, Rockford, IL) and donkey anti-goat IgG (1:2500; Santa Cruz Biotechnology, Santa Cruz, CA), were used for detection. The blots were developed with the ECL Plus Detection Kit (GE Healthcare, Piscataway, NJ) following the manufacturer’s protocol.
In vivo somatic cell transfer in transgenic mice
Creation of the transgenic Ntv-a mouse has been previously described 29. The mice are mixtures of the following strains: C57BL/6, BALB/C, FVB/N, and CD1. To transfer genes via RCAS vectors, we injected DF-1 producer cells transfected with a particular RCAS vector (1×105 cells in 1 to 2 μL of PBS) into the right frontal brain lobe of Ntv-a mice from an entry point just anterior to the coronal suture of the skull using a 10 μl gas-tight Hamilton syringe. We injected mice within 24 to 72 hours after birth because the population of Nestin+ cells producing TVA receptors diminishes progressively with time. In the injection sets consisting of two vectors, equal numbers of DF-1 cells were injected. The mice were sacrificed 90 days after injection or sooner if they demonstrated symptoms related to tumor burden (such as hydrocephalus). The brains were fixed in formalin, embedded in paraffin, sectioned for immunohistochemical analysis, and analyzed for tumor formation. Histological verification of tumor formation and determination of low- or high-grade type was performed by a neuropathologist (GNF). High-grade tumors were differentiated by the presence of microvascular proliferation, brisk mitotic activity, and foci of necrosis. The animal experiments described in this research were approved by The Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center (Protocol No. 08-06-11632).
Immunohistochemistry
Mouse brains were paraffin embedded and 4 μm sections were used for immunohistochemical analysis. The ThermoScientific PTModule (Thermo Fisher Scientific, Fremont, CA) with citrate buffer was used for antigen retrieval. Staining was performed using the Lab Vision Immunohistochemical Autostainer 360 (Thermo Fisher Scientific, Fremont, CA). Immunoreactive staining was visualized using an avidin-biotin complex technique with diaminobenzidine (Invitrogen, Carlsbad, CA) as the chromogenic substrate and hematoxylin as the counterstain. Human Bcl-2 expressed by RCAS was detected in tumor sections using a primary monoclonal antibody(Mab100) that specifically reacts with human Bcl-2 but does not cross react with endogenous mouse Bcl-2 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). To detect endogenous Bcl-2 expression, we used an antibody that reacts with mouse (and human) Bcl-2 (MabC2), (1:100, Santa Cruz Biotechnology, Santa Cruz, CA). Spleens harvested from Ntv-a mice were used as control tissue for Bcl-2 reactivity. Bcl-2 (with either MabC2 or Mab100) immunoreactivity was assessed in three tumors per injection set. To detect apoptosis, we used a primary antibody for cleaved caspase 3 (1:1000, Cell Signaling Technology, Beverly, MA). This antibody specifically detects the large fragment of activated caspase-3, and is an indicator of both mitochondrially-mediated (intrinsic) and death receptor-mediated (extrinsic) apoptosis. To analyze the extent of mitotic activity as an indicator of cellular proliferation, we used an antibody to phosphohistone H3 (pHH3;1:1000 dilution; Millipore, Temecula, CA). Mitotic activity was confirmed in three AO induced by RCAS-PDGFB+ RCAS-Bcl-2 using the Ki-67 antibody (Ki-67; 1:100 dilution Abcam Cambridge, MA)
Mitotic index
To detect and quantify mitotic activity in the different injection sets we immunostained formalin-fixed, paraffin-embedded, tumor-bearing tissue sections with an antibody against pHH3. We counted the number of positively stained cells in the area of highest tumor cell density and mitotic activity in ten non-overlapping high-power microscopic fields (at 400X magnification) from five different tumor-bearing brains from each injection set. The mitotic index was calculated as the number of positive cells divided by the number of total cells in each field. The median number of cells counted was 1795 (range 616 – 3003).
Apoptotic assay
We detected and quantified apoptosis in the different injection sets by immunostaining formalin-fixed, paraffin-embedded, tumor-bearing tissue sections with an antibody against cleaved caspase-3. We counted the number of positively stained cells in the area of highest tumor cell density and apoptotic activity in ten non-overlapping high-power microscopic fields (at 400X magnification) from three different tumor-bearing brains from each injection set. The apoptotic index was calculated as the number of positive cells divided by the number of total cells in each field. The median number of cells counted was 1951 (range 1015–4040).
Statistical analysis
The chi-square test was used to compare the tumor formation rates between the different injection sets. The Kaplan-Meier method was used to estimate the time to symptomatic tumor development among the different groups. For this analysis, the mice were observed from the day of RCAS vector injection to the day of symptomatic tumor development or for 90 days, whichever took place first. The log rank test was used to compare the various distributions. To compare the mitotic and apoptotic indices between the various injection sets, mixed model repeated measures analyses were performed with logit transformation of the indices. The Bonferroni method was used to adjust for the multiple pairwise comparisons. In all analyses, a P-value ≤ 0.05 was considered significant. Statistical analyses were performed using the SPSS 17.0 software package (Chicago, IL).
Results
To determine whether ectopic expression of Bcl-2 cooperates with PDGFB to enhance oligodendroglioma formation, we injected 33 newborn Ntv-a mice with RCAS-PDGFB + RCAS-Bcl-2. We compared this group to a cohort of 26 mice injected with RCAS-PDGFB alone. Combined expression of PDGFB and Bcl-2 in Ntv-a mice resulted in a significantly higher incidence of tumors relative to independent expression of PDGFB. Tumors were identified in 27 of 33 (82%) mice coinjected with RCAS-PDGFB + RCAS-Bcl-2, whereas injection of RCAS-PDGFB alone resulted in 11 tumors in 26 mice (42%) (chi-square test, P=0.002). In the mice injected with RCAS-PDGFB + RCAS-Bcl-2, high-grade tumors consistent with AO were observed in 20 mice (61%) (Figures 1A–C). These tumors displayed the requisite histological features of anaplasia including brisk mitotic activity, microvascular proliferation, and in 14 mice (42%), prominent areas of necrosis. Low-grade tumors (lacking mitotic figures, microvascular proliferation, or necrosis) consistent with grade II oligodendroglioma, were seen in 7 mice (21%). Bcl-2 expression induced by RCAS-Bcl-2 was verified in tumor-bearing sections by immunohistochemical staining for a human Bcl-2 specific antibody Mab100 but does not detect endogenous mouse Bcl-2 (Figure 1D). Among the 26 mice injected with RCAS-PDGFB alone, AOs formed in five (19%), a rate significantly lower than that observed in mice injected with RCAS-PDGFB + RCAS-Bcl-2 (chi square test, P=0.001). Grade II oligodendrogliomas were induced in six (23%) mice (Figures 2A and B). Necrosis was identified in only three (12%) mice injected with RCAS-PDGFB alone. We injected 19 mice with RCAS-Bcl-2 alone to determine its capacity to form tumors. None of these mice developed any sign or symptom of neurological morbidity during the 90-day observation period, and no tumors were detected in any of the mice at necropsy.
Figure 1. RCAS-Bcl-2 promotes malignancy in the RCAS-PDGFB-dependent model of oligodendroglioma.

A. Hematoxylin and eosin-stained coronal section of whole mouse brain after injection with RCAS-PDGFB and RCAS-Bcl-2 resulting in anaplastic oligodendroglioma (AO). The tumor (indicated by Tu) has formed in the right forebrain and invades the right frontal lobe.
B. Microscopic view (400X) of AO induced by expression of PDGFB and Bcl-2 (hematoxylin and eosin staining). Arrows indicate mitotic figures (scale bar = 100μm). Inset shows detailed view of mitotic figure (1000X).
C. Microscopic view (200X) of AO induced by PDGFB and Bcl-2 (hematoxylin and eosin staining). Arrows indicate area of microvascular proliferation. Arrowheads indicate pseudopalisading cells surrounding areas of necrosis. (scale bar = 100μm)
D. Microscopic view (400X) of immunohistochemical staining of Bcl-2 in AO induced by PDGFB and Bcl-2 verifying expression of Bcl-2. Arrows indicate positive cells.(scale bar = 100μm).
Figure 2. RCAS-PDGFB alone predominantly results in low-grade oligodendroglioma.

A. Hematoxylin and eosin-stained coronal section of whole mouse brain after injection of RCAS-PDGFB resulting in oligodendroglioma. Arrow indicates tumor in the right frontal lobe.
B. Microscopic view (400X) of low-grade oligodendroglioma induced by PDGFB expression alone. These tumors feature areas of moderately increased cellularity but lack necrosis, microvascular proliferation, and frequent mitoses (scale bar = 100μm).
The median latency to symptomatic tumor development for mice injected with RCAS-PDGFB and RCAS-Bcl-2 was 57 days (range 21–90 days). In contrast, the median latency to symptomatic tumor development for mice injected with RCAS-PDGFB alone was 90 days (range 25–90 days). All mice sacrificed prior to the 90 day endpoint in either of these injection sets harbored tumors. The difference in tumor latency between these injection sets was statistically significant (log rank test, P = 0.003) (Figure 3A). As a comparison group for symptomatic tumor formation, we injected RCAS-PDGFB and RCAS-IGFBP2 into a cohort of Ntv-a mice (N=30). AOs formed by RCAS-PDGFB + RCAS-IGFBP2 are phenotypically indistinguishable from those formed from overexpression of RCAS-PDGFB + RCAS-Bcl-2. We found that symptomatic tumor latency in this group was similar to the group injected with RCAS-PDGFB + RCAS-Bcl-2 (log rank test, P=0.18, Figure 3B).
Figure 3. RCAS-Bcl-2 decreases the latency of symptomatic tumor formation.
A. Kaplan-Meier curves comparing the tumor latency of mice injected with RCAS-PDGFB and RCAS-Bcl-2 (solid line) with those of mice injected with RCAS-PDGFB alone (dashed line). The difference is statistically significant (log rank test, P = 0.003).
B. Kaplan-Meier curves comparing the tumor latency of mice injected with RCAS-PDGFB and RCAS-Bcl-2 (dashed line) with those of mice injected with RCAS-PDGFB and RCAS-IGFBP2 (solid line). The difference was not statistically significant (log rank test, P=0.18)
Tumor cell proliferation was detected and quantified by immunostaining tumor-bearing brains for mitotic figures using an antibody against pHH3. The use of pHH3 has been validated as a reliable method for determining the mitotic index in gliomas30. We compared the number of pHH3-positive cells in both low- and high-grade tumors generated by RCAS-PDGFB alone and in high-grade tumors produced by RCAS-PDGFB in combination with RCAS-Bcl-2. We also compared this mitotic index with that seen in high-grade tumors induced by combined expression of RCAS-PDGFB + RCAS-IGFBP2 (Figures 4A–D). IGFPB2 promotes the invasiveness and migration of glioma cells31, but also increases mitotic activity consistent with its stimulation of more malignant tumors, and it thus served as an additional control for tumor cell proliferation. In this way, we were able to compare tumor cell proliferation in another PDGFB-dependent model of AO. The median mitotic index in tumors generated by RCAS-PDGFB + RCAS-Bcl-2 was 8.1% (range 1.9% to 22%). For low-grade and high-grade tumorsinduced by RCAS-PDGFB alone, the median mitotic indices were 0.9% (range 0.3% to 13%) and 1.8% (range 0% to 9.8%), respectively. For high-grade tumors formed by RCAS-PDGFB + RCAS-IGFBP2, the median mitotic index was 2.1% (range 0% to 8.8%). Tumors formed by RCAS-PDGFB + RCAS-Bcl-2 had a significantly higher mitotic index than tumors formed by all other injection sets (P<0.001 for the three pairwise comparisons). The high mitotic rate observed with PDGFB+Bcl-2 was verified by staining three specimens with Ki-67 (see Supplemental Figure A).
Figure 4. RCAS-Bcl-2 increases cellular proliferation in tumors.
Microscopic view of anaplastic oligodendroglioma (AO) (200X, inset 1000X) after immunohistochemical staining for phosphohistone H3 (brown cells). Mitotic activity was greater in all AOs compared with low-grade oligodendroglioma, but significantly higher in tumors induced by RCAS-Bcl-2 and RCAS-PDGFB (scale bar = 200 μm).
A. Oligodendroglioma induced by PDGFB demonstrating minimal staining (positive cells indicated by arrows).
B. AO induced by RCAS-PDGFB alone.
C. AO induced by RCAS-PDGFB and RCAS-IGFBP2.
D. AO induced by RCAS-PDGFB and RCAS-Bcl-2.
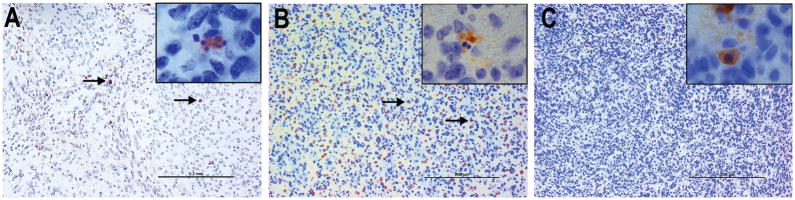
To determine if a suppressive effect on apoptosis was induced by and specific to Bcl-2 overexpression, we compared the apoptotic indices by analyzing Caspase 3 cleavage by immunohistochemistry in AO-bearing brains generated by RCAS-PDGFB + RCAS-Bcl-2 with those generated by RCAS-PDGFB expression alone and by RCAS-PDGFB + RCAS-IGFBP2 (Figures 5A–C). The median apoptotic index of high-grade tumors formed by RCAS-PDGFB + RCAS-Bcl-2 was 0.4% (range 0% to 10.9%). The apoptotic index of high-grade tumors formed by PDGFB alone was 6.7% (range 0.8% to 22%). The apoptotic index of high-grade tumors formed by RCAS-PDGFB + RCAS-IGFBP2 was 4.8% (range 0.2% to 25%). We found that the apoptotic index was significantly lower in the high-grade tumors formed by RCAS-PDGFB + RCAS-Bcl-2 than in those formed by either RCAS-PDGFB alone or the combination of RCAS-PDGFB and RCAS-IGFBP2 (P<0.001 in both cases). The apoptotic index was not significantly different between high-grade tumors formed by RCAS-PDGFB alone and RCAS-PDGFB + RCAS-IGFBP2 (P=0.11), consistent with predominantly mitogenic effects of PDGFB and IGFBP2.
Figure 5. RCAS-Bcl-2 decreases the apoptotic index in anaplastic oligodendroglioma.
Microscopic view (200X, inset 1000X) of AO after immunohistochemical staining for cleaved caspase-3 to determine the apoptotic index. The apoptotic index was significantly lower in high-grade tumors induced by RCAS-PDGFB and RCAS-Bcl2 compared with those induced by RCAS-PDGFB and RCAS-IGFBP2. (scale bar = 200μm)
A. Tumor induced by RCAS-PDGFB alone showing positive staining for cleaved caspase-3 (positive cells indicated by arrows) (inset 1000X ).
B. Microscopic view of tumor induced by RCAS-PDGFB and RCAS-IGFBP2 showing more pronounced staining for cleaved caspase-3 (positive cells indicated by arrows) (inset 1000X).
C. Microscopic view of tumor induced by RCAS-PDGFB and RCAS-Bcl-2 demonstrating largely negative staining.
To determine the effect of blocking apoptosis in PDGFB-driven tumors we probed tumor-sections by immunohistochemistry with two antibodies; one that detects both human andmouse Bcl-2 (MabC2), and one that detects only human Bcl-2 (Mab100). We used spleens harvested from Ntv-a mice as control tissue for mouse and human Bcl-2 antibodies. As expected, murine spleens demonstrated negative staining for human Bcl-2 (Mab100) (Figure 6A). Homogeneous staining was observed throughout the spleens stained for endogenous Bcl-2 (MabC2) (Figure 6B). We did not detect ectopic Bcl-2 expression in low-grade tumors induced by RCAS-PDGFB and RCAS-Bcl-2 (See Supplemental Figure B). Abundant expression of Bcl-2 was observed in high-grade tumors particularly around areas of necrosis with both Bcl-2 antibodies although the low-grade areas of these tumors did not demonstrate Bcl-2 expression (Figures 6C and D). High-grade tumors induced by RCAS-PDGFB alone did not demonstrate staining with either Bcl-2 antibody (Figures 6E and F).
Figure 6. Endogenous and Ectopic Bcl-2 expression in low-grade and anaplastic oligodendrogliomas (scale bar = 50 μm).
A. Mouse spleen demonstrating negative staining for human Bcl-2 as detected by monoclonal antibody Mab100 (which is specific for human Bcl-2).
B. Mouse spleen demonstrating homogeneous positive staining throughout verifying that monoclonal antibody MabC2 (which detects mouse and human Bcl-2) detects endogenous Bcl-2.
C. AO induced by RCAS-PDGFB and RCAS-Bcl-2 probed by Mab100 demonstrating positive staining throughout, particularly around areas of necrosis.
D. AO induced by RCAS-PDGFB and RCAS-Bcl-2 probed by MabC2 demonstrating positive staining throughout, particularly around areas of necrosis.
E. AO induced by RCAS-PDGFB alone probed by Mab100 demonstrating a lack of Bcl-2 expression.
F. AO induced by RCAS-PDGFB alone probed by MabC2 demonstrating a lack of Bcl-2 expression.
Discussion
The initiation, maintenance, and progression of cancer require the combination of signaling programs that promote cellular proliferation with those that suppress apoptosis (reviewed in 32). The first anti-apoptotic gene discovered, Bcl-2, has been implicated in the formation and progression of a variety of cancers including gliomas5. Several studies have correlated increased expression of Bcl-2 (and its related family members) with poorer survival in patients with glioma 23, 33, 34. Further, a direct correlation between Bcl-2 expression and histologic grade in human glioma specimens has been described 21, 35, 36. To verify that the suppression of apoptosis plays a significant role in glioma formation and progression, we used a murine model to determine the functional impact of Bcl-2 expression in vivo. Here, we show that the anti-apoptotic effect of Bcl-2 significantly enhances the tumor formation rate in a PDGFB-dependent mouse model of oligodendroglioma. We also show that Bcl-2 increases tumor grade and decreases tumor-free survival when coexpressed with PDGFB.
Our results support a correlation between Bcl-2 expression and higher tumor grade as reported in clinical studies 20, 36 and in vitro studies showing that ectopic Bcl-2 expression in glioma cell lines promotes a more malignant phenotype 27. The exact mechanism by which Bcl-2 exerts its tumor-enhancing effect in our study is difficult to surmise because of the complexity of Bcl-2’s functions and structure which impact multiple cell-death pathways including apoptosis, autophagy, and necrosis 37 and it is possible that other anti-apoptotic members of the Bcl-2 gene family (including Bcl-XL, A1, Mcl-1, and Bcl-W, reviewed in 38) may display the same effect on tumor formation and malignant progression observed with Bcl-2 overexpression. Nonetheless, we were able to validate the effect of apoptotic suppression mediated through Bcl-2 on tumor formation by quantifying the expression of cleaved caspase-3 in tumor-bearing brainsections. This is a sensitive method for assessing apoptosis because expression of cleaved caspase-3 is an early and probably universal signaling event in apoptosis 39. Cleaved caspase-3 expression was significantly lower in AOs induced by PDGFB and Bcl-2 than in high-grade tumors induced by PDGFB alone or in combination with IGFBP2, confirming the anti-apoptotic effect of Bcl-2 expression. Cellular proliferation (as measured by pHH3 expression) was significantly higher in tumors generated by PDGFB and Bcl-2 than in those induced by PDGFB alone or PDGFB and IGFBP2 (not surprisingly, mitotic activity was also significantly higher in grade III tumors than grade II tumors). This data supports the contention that Bcl-2 facilitates increased tumor cell proliferation (by PDGFB expression) by uncoupling apoptosis from proliferation. Further, our results suggest that the increased tumor formation rate and higher incidence of the AO phenotype observed in our study are due to the strong anti-apoptotic effect.
The PDGFB-dependent RCAS/Ntv-a based model of glioma has been used by several investigators to evaluate the functional consequences of gene overexpression on tumor formation in vivo. In a study using the RCAS/Ntv-a system, IGFBP2 was overexpressed in combination with PDGFB and was shown to enhance progression to AO in Ntv-a mice 12. Similar to our result with Bcl-2, IGFBP2 was not tumorigenic by itself. AOs formed more frequently in our study (61% for RCAS-PDGFB + RCAS-Bcl-2 compared with 38% for RCAS-PDGFB+RCAS-IGFBP2), although the symptom-free survival times for these two groups were similar which may be due to additional effects conferred by IGFBP2 on tumor cells including the promotion of migration and invasion31 (although more recent evidence suggests that Bcl-2 promote these as well40). The comparative decrease in apoptotic activity in high-grade tumors induced by PDGFB+Bcl-2 relative to PDGFB+IGFBP2 may explain this difference in high-grade tumorformation rates and indicates that PDGFB or IGFBP2 induced apoptosis is overcome by co-expression of Bcl-2.
The finding that Bcl-2 overexpression promotes the histologic features of AO is consistent with the observation that other members of the Bcl-2 family stimulate formation of necrosis in glioma while suppressing apoptosis. Bcl-2-like12 (Bcl-2L12), a member of the Bcl-2 family, is a potent inhibitor of apoptosis in glioma41, 42. Its downstream effector, αB-Crystallin appears to possess oncogenic properties which include inducing cell migration and invasion through activation of the MAPK kinase/ERK pathway 41, 43. Bcl-2L12 exhibits unique mechanisms to inhibit caspases 3 and 7, which shift the tumor cell from undergoing an apoptotic death to a necrotic one. In our study, necrosis was more common in tumors formed by coexpression of Bcl-2 and PDGFB than by PDGFB alone, correlating with the increased incidence of anaplastic tumors. Although overexpression of PDGFB in Ntv-a mice can induce anaplastic tumors, they are infrequent and range from 0% to 29% after injection 9, 10, 12, 15. Shih et al. observed regions of necrosis in 8 of 28 (29%) tumor-bearing mice injected with RCAS-PDGFB 9. Consistent with their results, we observed necrosis in 3 of 11 (27%) tumor-bearing mice injected with RCAS-PDGFB. However, foci of necrosis were present in 14 (52%) tumor-bearing mice injected with RCAS-PDGFB + RCAS-Bcl-2, and Bcl-2 staining was more prominent in areas of tumor surrounding necrosis. The necrotic phenotype is relevant in glioma because it is a hallmark of high-grade lesions, is associated with poor survival, and mitigation of its formation may be a requirement of an effective therapeutic strategy 44, 45.
Independent expression of Bcl-2 did not result in tumor formation. Similarly, a study evaluating the effect of Bcl-2 expression in an RCAS/Ntv-a-based, Sonic hedgehog (SHH)-dependent model system of medulloblastoma showed that while coexpression of Bcl-2 with SHH resulted in a higher tumor formation rate it was not sufficient to form tumors independently28. In that study, independent expression of Bcl-2 resulted in non-cancerous but definite histologic changes in the cerebellum. We were unable to identify any histologic abnormalities after Bcl-2 expression in the forebrains of Ntv-a mice. This may be a consequence of the differences in the microenvironment of the cerebellum and forebrain. The granule neuron precursor, a progenitor cell unique to the cerebellum and considered the cell of origin of some types of medulloblastoma, may be more vulnerable to ectopic Bcl-2 overexpression than the glioneuronal precursors in the forebrain.
Targeting Bcl-2 may provide a therapeutic benefit by countering its anti-apoptotic effects46. The inhibition of Bcl-2 using anti-sense constructs results in tumor cell death in vitro 47, 48. Small molecule inhibitors of Bcl-2, known as BH3-mimetics, have also been shown to reactivate tumor necrosis factor-related apoptosis inducing ligand (TRAIL)49. Inducing cell death with TRAIL may be an effective approach against malignant gliomas, which are otherwise resistant to apoptosis. Another small molecule inhibitor of several members of the Bcl-2 family, ABT-737, was recently shown to induce apoptosis in glioma cell lines and to increase survival time in mice with intracranially implanted glioma cells 50. Our results indicate that Bcl-2 strongly inhibits apoptotic cell death in glioma, supporting the strategy of suppressing Bcl-2 signaling. The profound negative effect of Bcl-2 signaling on symptom free survival of the mice in our study also increases its appeal as a therapeutic target.
In conclusion, we have shown that the anti-apoptotic gene Bcl-2 enhances tumor formation in a PDGFB-dependent model system of oligodendroglioma. We find that Bcl-2promotes the AO phenotype through its anti-apoptotic effect. Our results confirm and extend observations from clinical studies that increased Bcl-2 expression correlates with poorer survival.
Supplementary Material
Acknowledgments
TAD, YY and GR performed experiments; DWF and WZ provided key reagents. DWF, DS, GNF, TAD, and WZ, GR, designed the research and analyzed data; TAD, DWF, DS, WZ, GNF and GR wrote the paper. We are grateful to David Wildrick, PhD for editorial assistance.
Footnotes
Conflict of Interest:
The project described was supported by Award Number P50CA127001 (GR) from the National Cancer Institute and Award Number CA108622 (DWF) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The other authors listed declare no conflicts of interest.
Contributor Information
Tiffany Doucette, Department of Neurosurgery, The University of Texas M.D. Anderson Cancer, Center, 1515 Holcombe Blvd, Houston, Texas 77030, Phone: (713) 792 2400, Fax: (713) 794 4950
Gregory N. Fuller, Department of Pathology The University of Texas M.D. Anderson Cancer, Center, 1515 Holcombe Blvd, Houston, Texas 77030, Phone: (713) 792 2400, Fax: (713) 794 4950
Yuhui Yang, Department of Neurosurgery, The University of Texas M.D. Anderson Cancer, Center, 1515 Holcombe Blvd, Houston, Texas 77030, Phone: (713) 792 2400, Fax: (713) 794 4950
Dima Suki, Department of Neurosurgery, The University of Texas M.D. Anderson Cancer, Center, 1515 Holcombe Blvd, Houston, Texas 77030, Phone: (713) 792 2400, Fax: (713) 794 4950.
Wei Zhang, Department of Pathology, The University of Texas M.D. Anderson Cancer, Center, 1515 Holcombe Blvd, Houston, Texas 77030, Phone: (713) 792 2400, Fax: (713) 794 4950
Daniel W. Fults, Department of Neurosurgery, The University of Utah, 175 North Medical Drive East, Building 550 Room 5228, Salt Lake City, Utah 84132, Phone: 801-581-6908, FAX: 801-581-4385
Ganesh Rao, Department of Neurosurgery, The University of Texas M. D. Anderson Cancer, Center, 1515 Holcombe Blvd, Houston, Texas 77030, Phone: (713) 792 2400, Fax: (713) 794 4950
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaeckle KA, Ballman KV, Rao RD, Jenkins RB, Buckner JC. Currentstrategies in treatment of oligodendroglioma: evolution of molecular signatures of response. J Clin Oncol. 2006;24:1246–52. doi: 10.1200/JCO.2005.04.9874. [DOI] [PubMed] [Google Scholar]
- 3.Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D, Buckner J, Fink K, Souhami L, Laperierre N, Mehta M, Curran W. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–14. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 4.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 5.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 6.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139–47. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 8.Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64:4783–9. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 10.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–25. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appolloni I, Calzolari F, Tutucci E, Caviglia S, Terrile M, Corte G, Malatesta P. PDGF-B induces a homogeneous class of oligodendrogliomas from embryonic neural progenitors. Int J Cancer. 2009;124:2251–9. doi: 10.1002/ijc.24206. [DOI] [PubMed] [Google Scholar]
- 12.Dunlap SM, Celestino J, Wang H, Jiang R, Holland EC, Fuller GN, Zhang W. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc Natl Acad Sci U S A. 2007;104:11736–41. doi: 10.1073/pnas.0703145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukushima T, Tezuka T, Shimomura T, Nakano S, Kataoka H. Silencing of insulin-like growth factor-binding protein-2 in human glioblastoma cells reduces both invasiveness and expression of progression-associated gene CD24. J Biol Chem. 2007;282:18634–44. doi: 10.1074/jbc.M609567200. [DOI] [PubMed] [Google Scholar]
- 14.Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T, Holland EC. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327–37. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tchougounova E, Kastemar M, Brasater D, Holland EC, Westermark B, Uhrbom L. Loss of Arf causes tumor progression of PDGFB-induced oligodendroglioma. Oncogene. 2007;26:6289–96. doi: 10.1038/sj.onc.1210455. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 17.Ikegaki N, Katsumata M, Minna J, Tsujimoto Y. Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res. 1994;54:6–8. [PubMed] [Google Scholar]
- 18.Monni O, Joensuu H, Franssila K, Klefstrom J, Alitalo K, Knuutila S. BCL2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood. 1997;90:1168–74. [PubMed] [Google Scholar]
- 19.Andrews GA, Xi S, Pomerantz RG, Lin CJ, Gooding WE, Wentzel AL, Wu L, Sidransky D, Grandis JR. Mutation of p53 in head and necksquamous cell carcinoma correlates with Bcl-2 expression and increased susceptibility to cisplatin-induced apoptosis. Head Neck. 2004;26:870–7. doi: 10.1002/hed.20029. [DOI] [PubMed] [Google Scholar]
- 20.Strik H, Deininger M, Streffer J, Grote E, Wickboldt J, Dichgans J, Weller M, Meyermann R. BCL-2 family protein expression in initial and recurrent glioblastomas: modulation by radiochemotherapy. J Neurol Neurosurg Psychiatry. 1999;67:763–8. doi: 10.1136/jnnp.67.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krajewski S, Krajewska M, Ehrmann J, Sikorska M, Lach B, Chatten J, Reed JC. Immunohistochemical analysis of Bcl-2, Bcl-X, Mcl-1, and Bax in tumors of central and peripheral nervous system origin. Am J Pathol. 1997;150:805–14. [PMC free article] [PubMed] [Google Scholar]
- 22.Stegh AH, Chin L, Louis DN, DePinho RA. What drives intense apoptosis resistance and propensity for necrosis in glioblastoma? A role for Bcl2L12 as a multifunctional cell death regulator. Cell Cycle. 2008;7:2833–9. doi: 10.4161/cc.7.18.6759. [DOI] [PubMed] [Google Scholar]
- 23.McDonald FE, Ironside JW, Gregor A, Wyatt B, Stewart M, Rye R, Adams J, Potts HW. The prognostic influence of bcl-2 in malignant glioma. Br J Cancer. 2002;86:1899–904. doi: 10.1038/sj.bjc.6600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–7. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 25.Holland EC, Li Y, Celestino J, Dai C, Schaefer L, Sawaya RA, Fuller GN. Astrocytes give rise to oligodendrogliomas and astrocytomas after gene transfer of polyoma virus middle T antigen in vivo. Am J Pathol. 2000;157:1031–7. doi: 10.1016/S0002-9440(10)64615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao G, Pedone CA, Coffin CM, Holland EC, Fults DW. c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia. 2003;5:198–204. doi: 10.1016/S1476-5586(03)80052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wick W, Wagner S, Kerkau S, Dichgans J, Tonn JC, Weller M. BCL-2 promotes migration and invasiveness of human glioma cells. FEBS Lett. 1998;440:419–24. doi: 10.1016/s0014-5793(98)01494-x. [DOI] [PubMed] [Google Scholar]
- 28.McCall TD, Pedone CA, Fults DW. Apoptosis suppression by somatic cell transfer of Bcl-2 promotes Sonic hedgehog-dependent medulloblastoma formation in mice. Cancer Res. 2007;67:5179–85. doi: 10.1158/0008-5472.CAN-06-4177. [DOI] [PubMed] [Google Scholar]
- 29.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95:1218–23. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colman H, Giannini C, Huang L, Gonzalez J, Hess K, Bruner J, Fuller G, Langford L, Pelloski C, Aaron J, Burger P, Aldape K. Assessment andprognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. Am J Surg Pathol. 2006;30:657–64. doi: 10.1097/01.pas.0000202048.28203.25. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Shen W, Huang H, Hu L, Ramdas L, Zhou YH, Liao WS, Fuller GN, Zhang W. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003;63:4315–21. [PubMed] [Google Scholar]
- 32.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 33.Rieger L, Weller M, Bornemann A, Schabet M, Dichgans J, Meyermann R. BCL-2 family protein expression in human malignant glioma: a clinical-pathological correlative study. J Neurol Sci. 1998;155:68–75. doi: 10.1016/s0022-510x(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 34.Deininger MH, Weller M, Streffer J, Meyermann R. Antiapoptotic Bcl-2 family protein expression increases with progression of oligodendroglioma. Cancer. 1999;86:1832–9. doi: 10.1002/(sici)1097-0142(19991101)86:9<1832::aid-cncr27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 35.Hara A, Hirose Y, Yoshimi N, Tanaka T, Mori H. Expression of Bax and bcl-2 proteins, regulators of programmed cell death, in human brain tumors. Neurol Res. 1997;19:623–8. doi: 10.1080/01616412.1997.11740871. [DOI] [PubMed] [Google Scholar]
- 36.Weller M, Malipiero U, Aguzzi A, Reed JC, Fontana A. Protooncogene bcl-2 gene transfer abrogates Fas/APO-1 antibody-mediated apoptosis of human malignant glioma cells and confers resistance to chemotherapeutic drugs and therapeutic irradiation. J Clin Invest. 1995;95:2633–43. doi: 10.1172/JCI117965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed JC. Bcl-2-family proteins and hematologic malignancies: history and future prospects. Blood. 2008;111:3322–30. doi: 10.1182/blood-2007-09-078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 39.Arai M, Sasaki A, Saito N, Nakazato Y. Immunohistochemical analysis of cleaved caspase-3 detects high level of apoptosis frequently in diffuse large B-cell lymphomas of the central nervous system. Pathol Int. 2005;55:122–9. doi: 10.1111/j.1440-1827.2005.01808.x. [DOI] [PubMed] [Google Scholar]
- 40.Wick W, Wild-Bode C, Frank B, Weller M. BCL-2-induced glioma cell invasiveness depends on furin-like proteases. J Neurochem. 2004;91:1275–83. doi: 10.1111/j.1471-4159.2004.02806.x. [DOI] [PubMed] [Google Scholar]
- 41.Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, Louis DN, Chin L, DePinho RA. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci U S A. 2008;105:10703–8. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stegh AH, Kim H, Bachoo RM, Forloney KL, Zhang J, Schulze H, Park K, Hannon GJ, Yuan J, Louis DN, DePinho RA, Chin L. Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev. 2007;21:98–111. doi: 10.1101/gad.1480007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO, Perou CM, et al. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–70. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker FG, 2nd, Davis RL, Chang SM, Prados MD. Necrosis as a prognostic factor in glioblastoma multiforme. Cancer. 1996;77:1161–6. doi: 10.1002/(sici)1097-0142(19960315)77:6<1161::aid-cncr24>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 45.Raza SM, Lang FF, Aggarwal BB, Fuller GN, Wildrick DM, Sawaya R. Necrosis and glioblastoma: a friend or a foe? A review and a hypothesis. Neurosurgery. 2002;51:2–12. doi: 10.1097/00006123-200207000-00002. discussion -3. [DOI] [PubMed] [Google Scholar]
- 46.Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Z, Zheng X, Rich KM. Down-regulation of Bcl-2 and Bcl-xL expression with bispecific antisense treatment in glioblastoma cell lines induce cell death. J Neurochem. 2003;84:273–81. doi: 10.1046/j.1471-4159.2003.01522.x. [DOI] [PubMed] [Google Scholar]
- 48.Julien T, Frankel B, Longo S, Kyle M, Gibson S, Shillitoe E, Ryken T. Antisense-mediated inhibition of the bcl-2 gene induces apoptosis in human malignant glioma. Surg Neurol. 2000;53:360–8. doi: 10.1016/s0090-3019(00)00178-6. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 49.Hetschko H, Voss V, Horn S, Seifert V, Prehn JH, Kogel D. Pharmacological inhibition of Bcl-2 family members reactivates TRAIL-induced apoptosis in malignant glioma. J Neurooncol. 2008;86:265–72. doi: 10.1007/s11060-007-9472-6. [DOI] [PubMed] [Google Scholar]
- 50.Tagscherer KE, Fassl A, Campos B, Farhadi M, Kraemer A, Bock BC, Macher-Goeppinger S, Radlwimmer B, Wiestler OD, Herold-Mende C, Roth W. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene. 2008;27:6646–56. doi: 10.1038/onc.2008.259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.