Abstract
Racial/ethnic disparities in breast cancer incidence may contain important evidence for understanding and control of the disease. Monitoring the incidence trends of breast cancer by race/ethnicity allows identification of high risk groups and development of targeted prevention programs.
Using population-based cancer registry data from the Los Angeles Cancer Surveillance Program, we examined the invasive female breast cancer incidence trends among the diverse racial/ethnic populations in Los Angeles County, California, from 1972 to 2007. Age-adjusted incidence rates (AAIR) and age-specific incidence rates (ASIR) were calculated and examined respectively for non-Hispanic (NH) white, black, Hispanic, Chinese, Filipina, Japanese, and Korean women by calendar year and time period.
Rising trends of AAIR were found in all racial/ethnic groups during the 1980s and 1990s. The breast cancer risk increased more substantially in Japanese and Filipinas than in Chinese and Koreans. During 2000–2007, the trends of AAIR declined significantly among NH white women and slightly in blacks, remained unchanged for Hispanics, and continued to rise significantly among all Asian subgroups. The patterns of ASIR by race/ethnicity changed dramatically over time. By 2000–2007, younger Hispanic women had the lowest breast cancer risk, replacing the Chinese and Koreans who formerly had the lowest risk.
Rapidly increasing breast cancer incidence trends among Asian-Americans underline the importance of behavioral and lifestyle changes as a result of acculturation on the development of the disease. The unique trends of breast cancer incidence by race/ethnicity suggest the need for targeted breast cancer control programs for different racial/ethnic populations.
Keywords: Breast cancer, Incidence, Race/Ethnicity, Trends
Introduction
Despite the recent decline in breast cancer incidence rates among women in the U.S. following the early termination of the Women's Health Initiative trial of estrogen-progestin therapy in 2002,1–9 breast cancer remains the most common cancer type for women in all racial/ethnic populations in the U.S.10 Yet, breast cancer is characterized by marked differences in incidence rates by race/ethnicity. Historically, women of Asian and Pacific Islander descent have had the lowest risk as compared to whites, blacks, and Hispanics.10 However, profound heterogeneities in breast cancer incidence rates and trends by ethnicity within Asian-Americans have been reported, with Japanese and Filipina showing much higher and rapidly rising incidence rates than Chinese and Korean women.11–15 The ethnic differences in breast cancer risk within Asian-American women are of particular interest and carry important messages for breast cancer research and control efforts. With typically lower breast cancer rates in their native countries, the immigrants' breast cancer incidence trends reflect an apparent environmental impact, including lifestyle and behavioral changes, on the development of breast cancer.
However, cancer surveillance for ethnic subgroups is limited by the lack of official annual population estimates for these populations to provide denominator data for calculating incidence rates. As a result, data on long-term breast cancer incidence trends among Asian subgroups are rare. Following our earlier report on rapidly rising breast cancer incidence rates among Asian-American women in Los Angeles County during 1988–1997,11 we now report our recent surveillance analyses of breast cancer incidence over a 36-year period in the County. We used data from the Los Angeles Cancer Surveillance Program (CSP) from 1972 to 2007 with detailed racial/ethnic classification of cancer cases and corresponding annual population estimates to provide a more complete and current description of breast cancer incidence trends in the diverse racial/ethnic populations of Los Angeles County.
With its large and racially/ethnically diverse population and the long history and high quality of cancer surveillance, Los Angeles County, California offers unique opportunities for cancer epidemiologic studies. According to the 2006–2008 American Community Survey 3-year estimates, of its nearly 10 million residents, 2.8 million are non-Hispanic white, 2.1 million Hispanic white, 0.9 million black, 0.4 million Chinese, 0.3 million Filipino, 0.1 million Japanese, and 0.2 million Korean.16 About 36% of the County's total population is foreign born, of which 74% entered the U.S. after 1980.16 In Los Angeles County, the overall proportion of foreign born is highest in Asians (68%), followed by Hispanics (44%), non-Hispanic whites (17%), and lowest in blacks (6%).16 Within Asians, the proportion of foreign born varied by ethnicity and age.
The CSP is the population-based cancer registry for Los Angeles County and identifies and obtains information on all new cancer diagnoses made among residents of the County. It is a regional registry of the statewide California Cancer Registry (CCR) and one of the largest of the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) registries. The CSP has complete cancer incidence data for the County since 1972. To date, the CSP master file contains over one million records and some 35,000 incident cases are added annually.
Material and Methods
Cancer Case Identification
From the CSP database, female breast cancer cases were identified according to the International Classification of Disease for Oncology, 3rd Edition (ICD-O-3)17 site codes C50.0–C50.9 with invasive tumor behavior. From 1972–2007 a total of 163,448 invasive breast cancer cases were identified by year of diagnosis, sex, age at diagnosis, and race/ethnicity, based on the demographic and diagnostic information routinely reported to the CSP from hospitals following the CCR and SEER reporting guidelines.18 For analytical purposes, we further grouped the racial/ethnic information into the following mutually exclusive categories: non-Hispanic (NH) white, black, Hispanic, Chinese, Japanese, Filipina, Korean, and other. Included in the “other” group are American Indians, Pacific Islanders, other Asians and not otherwise specified (NOS) individuals. Because the majority of the Hispanic population in Los Angeles County is of Mexican descent and considered to be white, the Hispanic group is represented by the Hispanic whites in the County. Due to its great heterogeneity, the “other” group (1.5% of total invasive breast cancer cases) was excluded from the analyses. The rest of seven racial/ethnic groups were included in the analyses with a total of 160,935 primary cases of invasive breast cancer during 1972–2007. Identification of Hispanic status used available sources of information, such as hospital reported Hispanic origin, Spanish surname list, birthplace, and followed the North American Association of Central Cancer Registries Hispanic/Latino Identification Algorithm (NHIA).19 Based on year of diagnosis and age at diagnosis, the individual records were also grouped into 4 time periods (1972–79, 1980–89, 1990–99, 2000–07) and 4 age groups (<45, 45–54, 55–64, 65+) to approximate the pre-menopausal, peri-menopausal, and post-menopausal stages for women.
Annual Population Estimates
Because of the lack of official annual population estimates by detailed race/ethnicity, the CSP has developed its own annual population estimates by age, sex, and race/ethnicity for Los Angeles County to facilitate cancer surveillance and research. The CSP estimates were based on the age-sex-specific population counts for Los Angeles County from the 1970, 1980, 1990, and 2000 censuses with matching racial/ethnic categories as used in the cancer cases. Linear interpolation between census years was used to estimate the intercensal annual populations. Due to the multiracial reporting scheme, there were two counts for a given racial/ethnic group in the 2000 census: “race alone” representing the minimum and “in combination with other race” representing the maximum. The simple average between the minimum and maximum counts was used in counting the 2000 population for a given racial/ethnic group. For 2001–2007 estimates, we adopted the race-age-sex-specific annual population growth rates estimated by the National Center for Health Statistics (NCHS)20 for Los Angeles County for the NH Whites, blacks, and Hispanics. For the Chinese, Japanese, Filinipo, and Korean populations we continued their individual linear growth trends of 1990–2000 into 2001–2002. For 2003–2007, based on the slowdown in growth trends indicated by the NCHS estimates for Asian and Pacific Islanders combined, we reduced the extrapolated annual growth by half for each of these four Asian subgroups.
Statistical Analysis
Annual age-adjusted incidence rates (AAIR) per 100,000 population were obtained for each of the racial/ethnic groups by direct standardization to the 2000 US Standard Population. Age-specific incidence rates (ASIR) per 100,000 by race/ethnicity were calculated for ages <45, 45–54, 55–64, and 65+ for the time periods of 1972–1979, 1980–1989, 1990–1999, and 2000–2007. A Log-linear Poisson regression model was used to examine the multivariate effects of time, age, and race/ethnicity on breast cancer incidence rates, using the GENMOD procedure in the statistical software SAS version 9.2 (SAS Institute Inc, Cary, NC). Joinpoint Regression Program version 3.4.3 (National Cancer Institute) was employed to describe changes in incidence trends and estimate the annual percent change (APC) during 1972–2007 by race/ethnicity as well as the racial/ethnic-specific average annual percent change (AAPC) of AAIR within each of the four time periods. AAPC is a summary measure of a trend over a pre-specified fixed period of time, which uses a single number to describe the average trend over a period of multiple years. AAPC is a weighted average of the joinpoint APCs with the weights equal to the length of the APC intervals included, thus would be more stable compared to the APC calculated by fitting a log linear regression line in dealing with variability in the underlying data.
Results
Trends of age-adjusted incidence rates (AAIR)
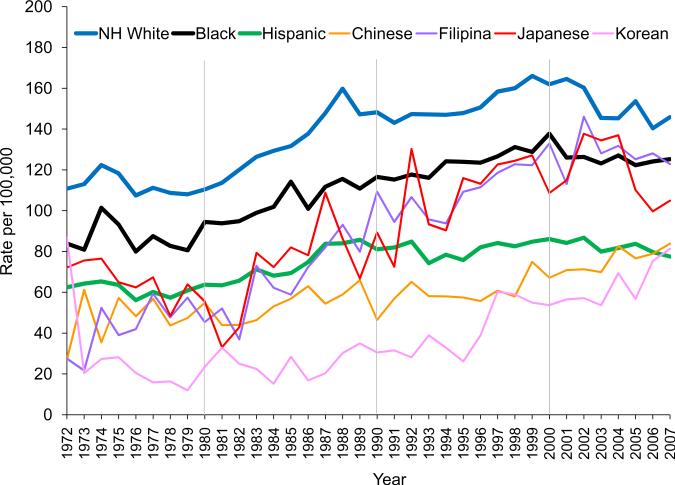
After some fluctuations in the 1970s, the AAIR of invasive breast cancer continued to rise throughout the 1980s and 1990s among women of all races/ethnicities in Los Angeles County, with no sign of decline until the 2000s (Figure 1). The AAIR (per 100,000) for NH white women rose from 110.8 in 1972 to 166.0 in 1999, dropping to 145.8 in 2007. For black women, their AAIR was 83.9 in 1972, 128.8 in 1999 and 125.2 in 2007. The AAIR for Hispanic women climbed from 62.4 in 1972 to 84.8 in 1999 and down to 77.5 in 2007. The AAIRs of Japanese and Filipinas increased much more rapidly than the Chinese and Koreans throughout the 1980s and 1990s, closely matching those of the blacks and fast-approaching those of the NH whites, and surpassed the Hispanics in early 1990s. By 2007, the AAIR for Chinese was 83.9, 81.3 for Koreans, and 77.5 for Hispanics - for the first time, the County's lowest AAIR of breast cancer was found in Hispanic women (Figure 1).
Figure 1.
Trends of annual age-adjusted (2000 U.S. Standard) incidence rates of invasive breast cancer among women by race/ethnicity, Los Angeles County, CA, 1972–2007
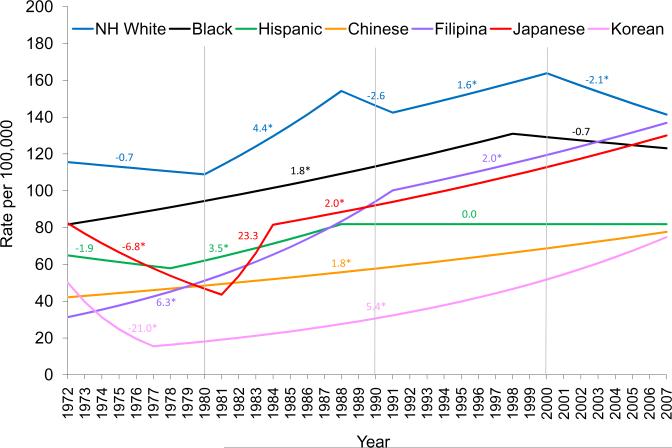
Joinpoint analyses of the AAIR trends revealed extended increases of statistical significance in breast cancer risk among all Asian subgroups since the 1980s (Figure 2). In contrast, the AAIR for Hispanic women remained unchanged since the late 1980s. Starting in 2000, NH whites experienced a decline of breast cancer risk with a statistically significant APC of −2.1, as blacks also saw a slight decrease in their AAIR at the same time (APC −0.7) (Figure 2).
Figure 2.
Joinpoint analyses of annual percent change (APC) of age-adjusted (2000 U.S. Standard) incidence rates by race/ethnicity, invasive breast cancer among women, Los Angeles County, CA, 1972–2007
Trends of age-specific incidence rates (ASIR)
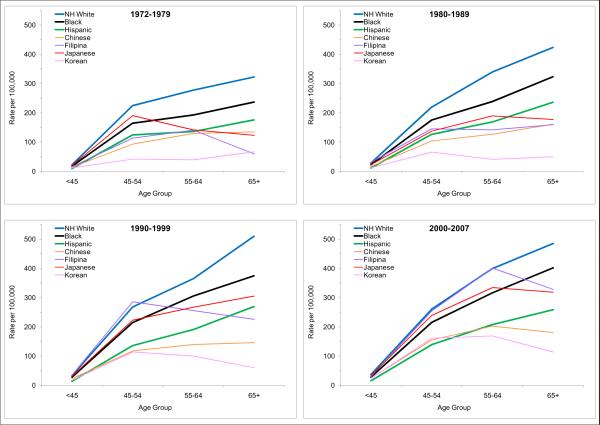
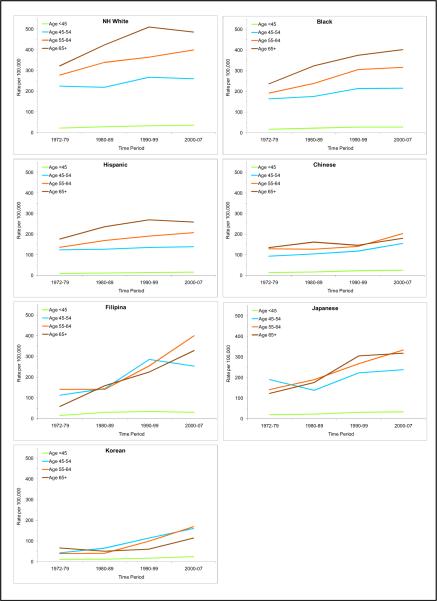
There was a clearly positive relationship between age and incidence rates among the NH whites, blacks, and Hispanics, i.e., the older the age, the higher the breast cancer risk. (Table 1 and Figures 3 and 4). The differences in incidence rates between age groups in these non-Asian groups appeared to be rather consistent over the four time periods (Table 1 and Figure 4). However, the age-incidence association is not as clear among the Asians. While Asian women under age 45 experienced the lowest risk of breast cancer, Asian women aged 65 years and older did not always have the highest incidence rates. In fact, during 2000–07, Asian women aged 55–64 of all ethnicities had the highest incidence rates compared to other age groups of Asian origin (Table 1 and Figures 3 and 4).
Table 1.
Age-specific case counts and incidence rates (ASIR) with 95% confidence intervals (CI) and average annual percent change (AAPC) by by race/ethnicity and time period, invasive breast cancer among women, Los Angeles County, CA, 1972–2007
| Race/Ethnicity | Time Period | Age Group |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 |
45–54 |
55–64 |
65+ |
|||||||||||
| Case | ASIR | 95% CI | Case | ASIR | 95% CI | Case | ASIR | 95% CI | Case | ASIR | 95% CI | AAPC§ | ||
| NH White | ||||||||||||||
| 1972–79 | 2554 | 21.8 | 20.9–22.6 | 5307 | 224.3 | 218.3–230.3 | 6091 | 277.4 | 270.5–284.4 | 9262 | 322.7 | 316.1–329.2 | −0.7 | |
| 1980–89 | 3450 | 28.0 | 27.1–28.9 | 5106 | 219.2 | 213.2–225.2 | 7895 | 339.2 | 331.7–346.6 | 15138 | 423.3 | 416.5–430.0 | 3.6* | |
| 1990–99 | 3251 | 33.1 | 31.9–34.2 | 5902 | 267.5 | 260.7–274.3 | 6388 | 364.6 | 355.6–373.5 | 16579 | 510.1 | 502.3–517.8 | 1.1 | |
| 2000–07 | 2297 | 36.3 | 34.8–37.8 | 4920 | 260.1 | 252.8–267.3 | 5772 | 399.0 | 388.7–409.3 | 11402 | 485.3 | 476.4–494.2 | −2.1* | |
| Black | ||||||||||||||
| 1972–79 | 493 | 17.3 | 15.8–18.9 | 570 | 163.9 | 150.4–177.3 | 504 | 192.1 | 175.3–208.9 | 592 | 236.5 | 217.4–255.5 | 1.8* | |
| 1980–89 | 845 | 22.3 | 20.8–23.8 | 854 | 174.9 | 163.2–186.7 | 892 | 238.6 | 223.0–254.3 | 1414 | 323.2 | 306.3–340.0 | 1.8* | |
| 1990–99 | 1023 | 27.7 | 26.0–29.4 | 1240 | 214.1 | 202.2–226.0 | 1237 | 305.5 | 288.5–322.6 | 1942 | 374.7 | 358.0–391.3 | 1.6* | |
| 2000–07 | 751 | 27.8 | 25.8–29.8 | 1194 | 215.7 | 203.4–227.9 | 1185 | 316.2 | 298.2–334.3 | 1826 | 401.7 | 383.2–420.1 | −0.7 | |
| Hispanic | ||||||||||||||
| 1972–79 | 442 | 9.4 | 8.5–10.3 | 551 | 124.2 | 113.8–134.5 | 389 | 135.8 | 122.3–149.3 | 442 | 175.9 | 159.5–192.3 | −1.1 | |
| 1980–89 | 1049 | 11.1 | 10.5–11.8 | 1003 | 126.0 | 118.2–133.8 | 905 | 168.9 | 157.9–179.9 | 1184 | 235.7 | 222.3–249.2 | 3.1* | |
| 1990–99 | 1909 | 13.3 | 12.7–13.9 | 1947 | 135.5 | 129.5–141.6 | 1650 | 190.5 | 181.3–199.7 | 2432 | 269.3 | 258.6–280.0 | 0.0 | |
| 2000–07 | 2027 | 15.0 | 14.4–15.7 | 2465 | 138.9 | 133.4–144.4 | 2060 | 206.9 | 198.0–215.8 | 2656 | 258.3 | 248.5–268.1 | 0.0 | |
| Chinese | ||||||||||||||
| 1972–79 | 28 | 13.1 | 8.2–17.9 | 24 | 93.1 | 55.9–130.3 | 24 | 128.8 | 77.3–180.3 | 24 | 134.4 | 80.7–188.2 | 1.8* | |
| 1980–89 | 100 | 16.2 | 13.0–19.3 | 79 | 103.8 | 80.9–126.7 | 75 | 126.5 | 97.9–155.2 | 107 | 160.9 | 130.4–191.4 | 1.8* | |
| 1990–99 | 229 | 22.0 | 19.1–24.8 | 231 | 117.4 | 102.3–132.6 | 161 | 140.0 | 118.4–161.6 | 222 | 145.4 | 126.3–164.6 | 1.8* | |
| 2000–07 | 252 | 24.9 | 21.8–28.0 | 420 | 154.6 | 139.8–169.4 | 259 | 202.4 | 177.7–227.0 | 337 | 180.0 | 160.8–199.2 | 1.8* | |
| Filipina | ||||||||||||||
| 1972–79 | 35 | 15.5 | 10.4–20.6 | 27 | 113.2 | 70.5–155.9 | 26 | 141.0 | 86.8–195.2 | 9 | 59.3 | 20.6–98.0 | 6.3* | |
| 1980–89 | 187 | 30.4 | 26.0–34.7 | 126 | 144.9 | 119.6–170.2 | 80 | 141.3 | 110.4–172.3 | 97 | 159.0 | 127.4–190.7 | 6.3* | |
| 1990–99 | 304 | 34.1 | 30.2–37.9 | 529 | 285.5 | 261.2–309.9 | 287 | 255.1 | 225.6–284.7 | 289 | 225.3 | 199.3–251.3 | 2.4* | |
| 2000–07 | 235 | 30.2 | 26.3–34.0 | 552 | 253.7 | 232.5–274.8 | 532 | 400.5 | 366.5–434.6 | 486 | 328.2 | 299.0–357.3 | 2.0* | |
| Japanese | ||||||||||||||
| 1972–79 | 61 | 19.7 | 14.7–24.6 | 134 | 190.5 | 158.2–222.7 | 59 | 141.6 | 105.4–177.7 | 46 | 123.2 | 87.6–158.8 | −6.8* | |
| 1980–89 | 86 | 21.8 | 17.2–26.4 | 120 | 137.4 | 112.8–162.0 | 156 | 189.5 | 159.8–219.3 | 124 | 176.6 | 145.5–207.7 | 7.6 | |
| 1990–99 | 120 | 30.9 | 25.4–36.4 | 187 | 222.8 | 190.8–254.7 | 214 | 267.0 | 231.3–302.8 | 353 | 304.9 | 273.0–336.7 | 2.0* | |
| 2000–07 | 93 | 33.3 | 26.5–40.1 | 186 | 238.6 | 204.3–272.9 | 160 | 333.8 | 282.1–385.5 | 393 | 318.4 | 286.9–349.8 | 2.0* | |
| Korean | ||||||||||||||
| 1972–79 | 14 | 11.2 | 5.3–17.1 | 5 | 41.9 | 5.2–78.7 | 3 | 38.9 | 0.0–82.9 | 5 | 66.3 | 8.2–124.4 | −14.2* | |
| 1980–89 | 46 | 11.7 | 8.3–15.1 | 36 | 65.2 | 43.9–86.5 | 14 | 40.7 | 19.0.4–62 | 18 | 49.8 | 26.8–72.8 | 5.4* | |
| 1990–99 | 99 | 16.6 | 13.3–19.9 | 131 | 114.3 | 94.7–133.9 | 74 | 99.5 | 76.9–122.2 | 49 | 60.4 | 43.5–77.3 | 5.4* | |
| 2000–07 | 131 | 23.7 | 19.6–27.7 | 204 | 160.5 | 138.5–182.5 | 145 | 168.8 | 141.4–196.3 | 109 | 114.3 | 92.8–135.7 | 5.4* | |
Estimated by joinpoint regression based on annual age-adjusted incidence rates during the time period.
Indicates statistical significance with 95% probability.
Figure 3.
Patterns of age-specific incidence rates of invasive breast cancer among women by race/ethnicity and time period, Los Angeles County, CA, 1972–2007
Figure 4.
Trends of age-specific incidence rates of invasive breast cancer among women by race/ethnicity, Los Angeles County, CA, 1972–2007
During 2000–07, breast cancer incidence rates for Filipinas younger than 65 years were nearly identical to those for NH whites (30.2 in Filipina vs. 36.3 in NH whites for ages <45, 253.7 vs. 260.1 for ages 55–54, 400.5 vs. 399.0 for ages 55–64) (Table 1 and Figure 3). The ASIR of Japanese for these age groups also closely followed those of the Filipinas (33.3, 238.6, and 333.8 respectively) and exceeded those of the blacks (27.8, 215.7, and 316.2 respectively). The ASIR of younger age groups (<45 and 45–54) of Chinese and Koreans also surpassed those of the Hispanics (Table 1 and Figure 3).
The AAPCs in Table 1 clearly demonstrate that throughout the 1980s and 1990s breast cancer AAIR increased in all racial/ethnic groups examined, although the magnitude of the increase varied by race/ethnicity. The Asian subgroups displayed consistent and statistically significant increases in AAIRs and such trends continued into the 2000s. In contrast to the Asians, during 2000–2007 the NH whites experienced a statistically significant decline of AAIR of −2.1% per year. Black women also appeared to experience a similar but non-significant drop in AAIR at the same time. Despite the changes in AAIR in other racial//ethnic groups, Hispanics showed a stable level of breast cancer risk since the 1990s.
Multivariate regression analyses were performed to consider the effects of time period, age group, and race/ethnicity on breast cancer risk. The results shown in Table 2 further confirmed the above observations with AAIRs and ASIRs. Controlling for age and race/ethnicity, compare to breast cancer risk during 1972–79, risk was 21% higher during 1980–1989, 42% higher during 1990–1999, and 46% higher during 2000–2007 (Table 2). Compared to women in the age group <45 (reference group), the risk of developing breast cancer was 7.57 times higher for ages 45–54, 10.91 time higher for ages 55–64, and 13.93 times higher for ages 65+. Adjusting for time periods and age groups, NH white has the highest breast cancer risk during 1972–2007, followed by blacks (78%), Filipinas (76%), Japanese (68%), Hispanics (49%), Chinese (45%), and Koreans (34%).
Table 2.
Multivariate analyses of the effect of time, age, and race/ethnicity on invasive breast cancer risk among women, Los Angeles County, CA, 1972–2007
| Risk factor | RR | 95% CI | P value |
|---|---|---|---|
| Time period | |||
| 1972–79 | 1.00 | ||
| 1980–89 | 1.21 | 1.19–1.22 | <.0001 |
| 1990–99 | 1.42 | 1.40–1.44 | <.0001 |
| 2000–07 | 1.46 | 1.44–1.48 | <.0001 |
| Age group | |||
| <45 | 1.00 | ||
| 45–54 | 8.57 | 8.43–8.72 | <.0001 |
| 55–64 | 11.91 | 11.71–12.12 | <.0001 |
| 65+ | 14.93 | 14.70–15.16 | <.0001 |
| Race/Ethnicity | |||
| NH White | 1.00 | ||
| Black | 0.78 | 0.77–0.79 | <.0001 |
| Hispanic | 0.49 | 0.48–0.50 | <.0001 |
| Chinese | 0.45 | 0.44–0.47 | <.0001 |
| Japanese | 0.68 | 0.65–0.70 | <.0001 |
| Filipina | 0.76 | 0.73–0.78 | <.0001 |
| Korean | 0.34 | 0.32–0.36 | <.0001 |
RR: relative risk; CI: confidence interval.
Discussion
Our analyses demonstrated the disparities and changes in breast cancer incidence trends by race/ethnicity in Los Angeles County. While the NH white women continued to have the highest breast cancer risk, the risk among Filipinas and Japanese are fast-approaching. After a persistent rise in AAIR throughout the 1980s and 1990s in all racial/ethnic groups examined, breast cancer incidence rates began to decline during 2000–2007 significantly for NH whites, slightly for blacks, and remained stable for Hispanics. Statistical analyses showed continued growing trends with statistical significance for all Asian subgroups.
Breast cancer incidence rates increased gradually over time in Asian countries, including Japan, Philippines, China (including mainland China, Taiwan, and Hong Kong), and the Republic of Korea.21–22 Although the breast cancer incidence rates in Asia are currently not as high as those of the Asian-American women, they are expected to rise rapidly for the coming decades. Much of the variations in breast cancer risk in Asia is thought to be attributable to the country-specific differences in the prevalence of risk factors and westernization of lifestyles. Nationwide data in the U.S. also reported similar declines in invasive breast cancer incidence rates in NH white, black, and Hispanic women, respectively since 1999.7 The recent downward trends in invasive breast cancer incidence rates in the U.S. were reportedly limited to postmenopausal women with estrogen/progestin receptor positive tumors. Reduction in the prescription and use of menopausal hormone therapy (HT)2–6 and saturation in mammogram screening7–9 were thought to be largely responsible for the recent decline in invasive breast cancer incidence rates.
Our ability to examine breast cancer incidence trends by individual Asian ethnic groups not only confirmed the previous reports of rapidly rising breast cancer risk among Japanese and Filipinas as compared to the Chinese and Korean women,11–15 but also revealed the persistence of such alarming trends among these groups in recent years. In sharp contrast to the consistently and rapidly rising breast cancer risk among Asians, Hispanic women successfully maintained a stable risk level for the past 20 years. Since both the Hispanics and Asians are major immigrant populations in Los Angeles, their markedly different breast cancer risks warrant further investigations into the acculturation experiences and exposure to breast cancer risk factors in both groups.
A clear distinction in the association between age and breast cancer risk was documented in our findings between Asian and non-Asian women. As breast cancer risk consistently increases with age among non-Asians, after menopause the older Asian women did not necessarily have higher risk as compare to the younger women; by 2000–2007 all Asian ethnicities showed declining risk after age 65. The risk of developing breast cancer is known to increase rapidly with age until menopause then slows, indicating the involvement of reproductive hormones in breast cancer etiology.23 Among Asian women, breast cancer risk plateaus after menopause.24–25 The differences in age-specific patterns in incidence rates between Asians and non-Asians have been attributed to a combination of multiple factors that favored the Asians in terms of breast cancer risk, including late age at menarche, low body weight, low premenopausal ovarian estrogen and progesterone serum levels, and low postmenopausal estrogen levels.23,26–27 Our analyses of ASIR documented that over time the Filipinas and Japanese have moved away from the traditional Asian patterns of ASIR approaching that of the NH whites, as the Chinese and Koreans appear to be following the same trend. Since our ASIR data are cross-sectional, the age-specific patterns may also reflect a cohort effect. Previous studies have shown that breast cancer risk increases steadily with more recent birth cohorts.22
Studies of immigrants have shown that women who migrate to the U.S. from Asia experience substantial increases in breast cancer risk after living in the U.S. a decade or more and the risk among their offspring approaching that of U.S. born women.28–30 These observations underline the environmental and behavioral influences through acculturation on the development of breast cancer. The process of acculturation involves changes in lifestyle, including adoption of a western diet and sedentary living are believed to lead to a variety of chronic diseases, including breast cancer.31–32
The positive association between breast cancer risk and western diet that is high in meat and dairy products and sweets has been well established by many studies.33–37 Asian women who adopted the Western diet pattern had higher breast cancer risk as compared to those with a healthy traditional diet pattern that is high in vegetable, fruit, soy, and fish consumption.38–39 To date, fewer studies, particularly prospective cohort studies on dietary factors have included premenopausal women. Thus the associations between dietary components and breast cancer risk among premenopausal women have yet to be established. The effects of diet on the development of breast cancer are likely achieved through body weight/size and circulating blood hormone concentrations in postmenopausal women.33,40 Body size as a risk factor is inversely associated with breast cancer risk in premenopausal women, but positively associated in postmenopausal women.40 Studies of dietary acculturation among Japanese Americans showed clear generational differences with the younger generation more accustomed to western dietary patterns.41 Longer U.S. residency has been linked to overweight or obese among foreign-born Asian Americans.42 Physical activity has been found to be a means of reducing breast cancer risk.43 Physical activity that is sustained over lifetime has the greatest benefit. However activities done later in life or specifically after menopause have a larger impact than activities before menopause.44 While vigorous activities have the greatest benefit on breast cancer reduction, activities that are of moderate intensity were also found to confer a sizeable decrease in breast cancer risk.44 Although physical activity helps to reduce breast cancer risk, Asian Americans were found to have significantly lower levels of physical activities.45
It is important to note that each of the four Asian ethnic groups included in the study has its own unique cultural practices, dietary habits, immigration history, socioeconomic status, genetic makeup, and so on that may influence breast cancer risk. For example, Filipino- and Japanese-Americans generally have higher educational levels, lower poverty rates, and higher proportions of people with social security income and ability to speak English “very well”, as compared to their Chinese and Korean counterparts.46–47 Consequently, Filipina and Japanese women had higher rates of participation in Pap screening, mammography, and health insurance coverage than the Chinese and Korean women in California.14 Filipina-Americans tend to have higher body mass index (BMI) than other Asian-American women,14,48 which likely increases their risk for developing breast cancer.40
The possible limitations of our study relate to the fact that the analyses depended on the accurate and reliable racial/ethnic classification of cancer cases and annual population estimates. Over-counting cancer cases by racial/ethnic misclassification or under-estimating the underlying population will produce artifactually increased incidence rates. As in all population-based cancer registries in the U.S., the CSP collects the racial/ethnic information based on medical charts and administrative records. There have been reports evaluating the quality of race and Hispanic origin classifications in population-based cancer registry data.49–50 The overall quality was found to be excellent on race and moderate to substantial on Hispanic ethnicity among the SEER registries.49 Misclassification was very minimal for NH Whites, Blacks, Hispanics, Japanese, and Filipinos, while underestimates were found for Chinese by 16%, according to one study conducted in northern California.50 Being one of the SEER registries, in addition to following all the SEER reporting and coding rules, the CSP has two distinct advantages in this regard: 1) It has historically actively collected pathology reports on all microscopically confirmed cases diminishing the risk of underreporting, and 2) it performs visual editing for quality control on demographics (including race/ethnicity) for 100% of cases.
We would like to note the methodological difference in the racial/ethnic identification between the cancer patients and the population at risk. As described above, patient demographic information in the registry database was abstracted from the medical records that were not always self-identified. The estimates of the population at risk by race/ethnicity were based on self-reported census data. This less than ideal difference in the sources of racial/ethnic data is part of the nature of registry-based studies.
It is difficult to assess the quality of the annual population estimates by age, sex, and race/ethnicity without a large-scaled actual enumeration. Different sources of data and different estimation methodology may result in discrepancies in numbers. Our postcensal estimates of 2001–2007 compares well overall with the official population estimates for Los Angeles County by either the American Community Survey or the National Center for Health Statistics in collaboration with the Census Bureau. Furthermore, as our findings confirmed the known trends and patterns among the major racial/ethnic categories, we believe that any possible misclassifications of race/ethnicity or errors in population estimates are minimal and should not bias the overall observations.
Conclusions
Breast cancer is a complex disease and subject to behavioral and lifestyle influences. Despite increasing awareness and commitment towards reducing breast cancer risk, the unabated rise of incidence rates among some of the racial/ethnic groups and certain age groups are of particular concern. In contrast to the fast growing incidence trends among most Asian-Americans, breast cancer risk among Hispanic women remained largely unchanged since the 1990s. With over one third of the County's population being first generation immigrants, it appears that ethnically unique characteristics and acculturation processes impact breast cancer risk. Our analyses demonstrate the variations in breast cancer incidence trends and patterns by race/ethnicity to facilitate culturally appropriate programs in cancer control efforts.
Acknowledgements
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract HHSN261201000035C awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
The authors also would like to thank Dr. Thomas Taylor and Ms. Yaping Wang for their statistical input, and all cancer registrars whose hard work and dedication make studies like this possible.
Footnotes
This paper reveals the dramatic disparities and changes in female breast cancer incidence rates by detailed race/ethnicity over 36 years of 1972–2007. The varied breast cancer trends among different racial/ethnic populations, especially the rapid increase in risk among Asian-American women, provide evidence for the importance of modifiable factors in the development of breast cancer.
References
- 1.Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA. The decrease in breast cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 3.Kerlikowske K, Miglioretti DL, Buist DSM, Walker R, Carney PA. Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. J Natl Cancer Inst. 2007;99:1335–1339. doi: 10.1093/jnci/djm111. [DOI] [PubMed] [Google Scholar]
- 4.Robbins AS, Clarke CA. Regional changes in hormone therapy use and breast cancer incidence in California from 2001 to 2004. J Clin Oncol. 2007;25:3437–3439. doi: 10.1200/JCO.2007.11.4132. [DOI] [PubMed] [Google Scholar]
- 5.Clarke CA, Glaser SL. Declines in breast cancer after the WHI: apparent impact of hormone therapy. Cancer Causes Control. 2007;18(8):847–852. doi: 10.1007/s10552-007-9029-1. [DOI] [PubMed] [Google Scholar]
- 6.Clarke CA, Glaser SL, Uratsu SL, Selby JV, Kushi LH, Herrington LJ. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24:49e–50e. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 7.Stewart SL, Sabatino SA, Foster SL, Richardson LC. Decline in breast cancer incidence—United States, 1999–2003. MMWR Morb Mortal Wkly Rep. 2007;56(22):549–553. [PubMed] [Google Scholar]
- 8.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among US women. Breast Cancer Res. 2007;9(3):R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995–2004. Cancer Epidemiol Biomarkers Prev. 2007;16:2773–2780. doi: 10.1158/1055-9965.EPI-07-0546. [DOI] [PubMed] [Google Scholar]
- 10.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, et al. Annual Report to the Nation on the Status of Cancer, 1975–2006, Featuring Colorectal Cancer Trends and Impact of Interventions (Risk Factors, Screening, and Treatment) to Reduce Future Rates. Cancer. 2009;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deapen D, Liu L, Perkins C, Bernstein L, Ross RK. Rapidly rising breast cancer incidence rates among Asian-American women. Int J Cancer. 2002;99:747–750. doi: 10.1002/ijc.10415. [DOI] [PubMed] [Google Scholar]
- 12.Kwong SL, Chen MS, Snipes KP, Bal DG, Wright WE. Asian Subgroups and Cancer Incidence and Mortality Rates in California. Cancer. 2005;104:2975–2981. doi: 10.1002/cncr.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keegan THM, Gomez SL, Clarke CA, Chan JK, Glaser SL. Recent trends in breast cancer incidence among 6 Asian groups in the Greater Bay Area of Northern California. Int J Cancer. 2007;120:1324–1329. doi: 10.1002/ijc.22432. [DOI] [PubMed] [Google Scholar]
- 14.McCracken M, Olsen M, Chen MS, Jr., Jemal A, Thun M, Cokkinides V, Deapen D, Ward E. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57:190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- 15.Miller BA, Chu KC, Hankey BF, Ries LAG. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19:227–56. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Census Bureau [accessed on 6/10/10];2006–2008 American Community Survey 3-Year Estimates, Detailed Tables. Available online: http://factfinder.census.gov/servlet/DTGeoSearchByListServlet?ds_name=ACS_2008_3YR_ G00_&_lang=en&_ts=283890324234,
- 17.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S. International Classification of Diseases for Oncology. 3rd Edition World Health Organization; Geneva: 2000. pp. 58–59. [Google Scholar]
- 18.California Cancer Registry . Cancer Reporting in California: Abstracting and Coding Procedures for Hospitals. Ninth Edition Volume I. Jun, 2009. Data Standards and Data Dictionary. [Google Scholar]
- 19.NAACCR Latino Research Work Group . NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2] North American Association of Central Cancer Registries; Springfield (IL): Jul, 2009. [Google Scholar]
- 20.United States Department of Health and Human Services (US DHHS) Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) July 1st resident population by state, county, age, sex, bridged-race, and Hispanic origin, on CDC WONDER On-line Database. Bridged-Race Population Estimates, United States. Vintage 2007: years 2000–2007, October 2008 online database, based on the September 5, 2008 data release. Available online: http://wonder.cdc.gov/Bridged-Race-v2007.HTML. [Google Scholar]
- 21.Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, Nishino Y, Sobue T, Chen CJ, You SL, Mirasol-Lumague MR, Law SCK, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21:1777–1785. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 22.Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol. 2005;34:405–412. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 23.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. `Hormonal' risk factors, `breast tissue age' and the age-incidence of breast cancer. Nature. 1983;303:767–70. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 24.Pisani P. Breast cancer: geographic variation and risk factors. J Environ Pathol Toxicol Oncol. 1992;11:313–6. [PubMed] [Google Scholar]
- 25.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer incidence in five continents. Vol. VII. IARC Press; Lyon: 2002. IARC Sci Publ No. 155. [Google Scholar]
- 26.Key TJA, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24:29–43. doi: 10.1016/0277-5379(88)90173-3. [DOI] [PubMed] [Google Scholar]
- 27.Wu A, Pike MC. Dietary soy protein and hormonal status in females. Am J Clin Nutr. 1995;62:151–152. doi: 10.1093/ajcn/62.1.151. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85(22):1819–27. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 29.Stanford JL, Herrinton LJ, Schwartz SM, Weiss NS. Breast cancer incidence in Asian migrants to the United States and their descendants. Epidemiology. 1995;6:181–3. doi: 10.1097/00001648-199503000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Gomez SL, Quach T, Horn-Ross PL, Pham JT, Cockburn M, Chang ET, Keegan THM, Glaser SL, Clarke CA. Hidden breast cancer disparities in Asian women: Disaggregating incidence rates by ethnicity and migrant status. Am J Public Health. doi: 10.2105/AJPH.2009.163931. Published online ahead of print February 10, 2010: e1-e7. doi:10.2105/AJPH.2009.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services . Physical activity and health: A report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- 32.U.S. Department of Health and Human Services . The Surgeon General's call to action to prevent and decrease overweight and obesity. U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; Rockville, MD: 2001. [PubMed] [Google Scholar]
- 33.Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC. Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr. 2009;89(4):1145–54. doi: 10.3945/ajcn.2008.26915. [DOI] [PubMed] [Google Scholar]
- 34.Cho E, Chen WY, Hunter DJ, Stampfer MJ, Colditz GA, Hankinson SE, Willett WC. Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med. 2006;166:2253–9. doi: 10.1001/archinte.166.20.2253. [DOI] [PubMed] [Google Scholar]
- 35.Thiebaut AC, Kipnis V, Chang SC, Subar AF, Thompson FE, Rosenberg PS, Hollenbeck AR, Leitzmann M, Schatzkin A. Dietary fat and postmenopausal invasive breast cancer in the National Institutes of Health-AARP Diet and Health Study cohort. J Natl Cancer Inst. 2007;99:451–62. doi: 10.1093/jnci/djk094. [DOI] [PubMed] [Google Scholar]
- 36.Sieri S, Krogh V, Ferrari P, Berrino F, Pala V, Thiébaut ACM, Tjønneland A, Olsen A, Overvad K, Jakobsen MU, Clavel-Chapelon F, Chajes V, et al. Dietary fat and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88:1304–12. doi: 10.3945/ajcn.2008.26090. [DOI] [PubMed] [Google Scholar]
- 37.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JAE, Parker LM, Paskett E, Phillips L, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–42. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 38.Hirose K, Matsuo K, Iwata H, Tajima K. Dietary patterns and the risk of breast cancer in Japanese women. Cancer Sci. 2007;98:1431–8. doi: 10.1111/j.1349-7006.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui X, Dai Q, Tseng M, Shu XO, Gao YT, Zheng W. Dietary patterns and breast cancer risk in the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2007;16(7):1443–8. doi: 10.1158/1055-9965.EPI-07-0059. [DOI] [PubMed] [Google Scholar]
- 40.Wu AH, Yu MC, Tseng CC, Pike MC. Body size, hormone therapy and risk of breast cancer in Asian-American women. Int J Cancer. 2007 Feb 15;120(4):844–52. doi: 10.1002/ijc.22387. [DOI] [PubMed] [Google Scholar]
- 41.Pierce BL, Austin MA, Crane PK, Retzlaff BM, Fish B, Hutter CM, Leonetti DL, Fujimoto WY. Measuring dietary acculturation in Japanese Americans with the use of confirmatory factor analysis of food-frequency data. Am J Clin Nutr. 2007;86:496–503. doi: 10.1093/ajcn/86.2.496. [DOI] [PubMed] [Google Scholar]
- 42.Lauderdale DS, Rathouz PJ. Body mass index in a US national sample of Asian Americans: effects of nativity, years since immigration and socioeconomic status. Int J Obes Relat Metab Disord. 2000;24:1188–94. doi: 10.1038/sj.ijo.0801365. [DOI] [PubMed] [Google Scholar]
- 43.Yang D, Bernstein L, Wu AH. Physical activity and breast cancer risk among Asian-American women in Los Angeles: a case-control study. Cancer. 2003 May 15;97(10):2565–75. doi: 10.1002/cncr.11364. [DOI] [PubMed] [Google Scholar]
- 44.Friedenreich CM. The role of physical activity in breast cancer etiology. Semin Oncol. 2010;37:297–302. doi: 10.1053/j.seminoncol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Kandula NR, Lauderdale DS. Leisure time, non-leisure time, and occupational physical activity in Asian Americans. Ann Epidemiol. 2005;15:257–265. doi: 10.1016/j.annepidem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Min PG. Asian Americans: Contemporary Trends and Issues. 2nd ed. Sage Publications, Inc.; Thousand Oaks, CA: 1995. [Google Scholar]
- 47.U.S. Census Bureau 2007 American Community Survey 1-Year Estimates. Selected Population Profile. Available online: http://factfinder.census.gov/servlet/IPGeoSearchByListServlet?ds_name=ACS_2007_1YR_ G00_&_lang=en&_ts=294937822576.
- 48.Yates A, Edman J, Aruguete M. Ethnic differences in BMI and body/self-dissatisfaction among whites, Asian subgroups, Pacific Islanders, and African-Americans. Society for Adolescent Medicine. 2004;34:300–307. doi: 10.1016/j.jadohealth.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Clegg LX, Reichman M, Hankey BF, Miller BA, Lin YD, Johnson NJ, Schwartz SM, Bernstein L, Chen VW, Goodman MT, Gomez SL, Graff JJ, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18:177–187. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 50.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–781. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]