Abstract
Objectives
There is controversy regarding the benefits of N-acetylcysteine (NAC) in acute kidney injury (AKI). This study was to compare three commonly used regimens and explore which regimen is best for the protection of AKI.
Design
Prospective experimental study.
Setting
University research laboratory.
Interventions
AKI was induced with folic acid (FA) intra-peritoneal injection in mice. Mice in pretreatment were treated with subcutaneous injection of NAC prior to the FA injection. Mice in posttreatment were treated with NAC after FA. Mice in pre +posttreatment were treated with NAC prior to FA and after FA. Placebo mice received vehicle only using the pre + posttreatment protocol. 14 healthy animals were given NAC to evaluate for toxicity and other 24 mice subjected to FA were sacrificed for kidney histology and analysis for oxidative injury. The same studies were also carried out in milder AKI (lower FA) model.
Measurements and Main Results
Plasma concentrations of creatinine, cystatin C and reduced glutathione (GSH) were measured. Survival time was assessed up to 7 days. The survival rates in NAC pretreatment mice were significantly better (73.33% vs. 46.67%, P<0.04) and AKI was significantly less compared to placebo. However, mice with posttreatment exhibited significantly worse survival and more severe AKI. Histological findings were consistent with functional parameters. GSH levels decreased less in NAC pretreatment but also increased beginning on day2 compared to placebo (11.5 vs 8.1 μg/ml, p<0.05). GSH levels did not increase in NAC posttreatment. However, three different NAC interventions neither significantly improved nor worsened renal function in the milder AKI model.
Conclusion
NAC pretreatment was effective in reducing the incidence and severity of AKI as well as in increasing survival. However, NAC posttreatment worsened FA toxicity. Only pretreatment was effective in increasing GSH. These data may help explain the variation from clinical studies of NAC use.
Keywords: N-acetylcysteine, Renal, Oxidative, Glutathione, oxidative stress, acute kidney injury
Acute kidney injury (AKI) is common and is associated with increased hospital mortality (1, 2, 3). As many as 67% of critically ill patients have evidence of AKI by RIFLE criteria (4, 5, 6). The cause of AKI in critically ill patients is often multi-factorial (3). Between 45 and 70% of all AKI is associated with sepsis (2, 6, 7, 8). Nephrotoxic drugs and contrast materials are implicated in 19% to 25% (2, 9). Drugs excreted by the kidney may exert direct toxic effects to the renal tubules, or induce inflammation in the renal interstitium in acute interstitial nephritis (10).
Given the apparent effect of AKI on mortality, it is important to prevent or hasten the resolution of even the mildest forms of AKI. However, there are very few interventions that have been shown to consistently prevent AKI. Measures such as ensuring adequate hydration, maintenance of adequate circulating blood volume and mean arterial pressure, and avoidance of nephrotoxins are still the mainstay of prevention (11). Until now, there is no effective pharmacological strategy that can be used to prevent or to treat AKI.
Several studies support the involvement of reactive oxygen species (ROS) in the development of various forms of AKI (12). Neutralization of these metabolites by natural antioxidants could reduce toxicity. N-acetylcysteine (NAC) has been successfully used in various animal models of AKI (13, 14, 15). NAC, the acetylated variant of amino acid cysteine, is an excellent source of sulfhydryl goups, and increases glutathione synthesis, promoting detoxification and acting directly as free radical scavenger. Among the available pharmacologic options, NAC has perhaps the strongest evidence in prevention of AKI (11).
However, NAC could also cause harm because it can be pro-oxidative in certain settings (19, 20). In vitro, NAC can increase hydroxyl radical generation when free iron is available by reducing ferric iron to its catalytic, active Fe 2+ form. Indeed, while some clinical trials demonstrated protective effects of NAC, others have not (16, 17) and differences in treatment protocols could underlie the disparities in results obtained (18). Indeed many centers do not use NAC and further studies are necessary to demonstrate whether there may be any benefits of therapy in various settings. It is possible that NAC may be beneficial in selected groups of patients, doses (17) or specific prescribed regimens while it is ineffective or even harmful in others (18).
We hypothesized that different treatment regimens for NAC would be associated with different efficacy in limiting AKI following FA exposure. Therefore, this study compared the three commonly used regimens (pretreatment, posttreatment, and combined pre and posttreatment), and explored which regimen is best for the protection of AKI.
Material and Methods
Animals
Outbred male CD-1 mice (Charles Rivers, 6 to 8 weeks old, weight 28 to 32g) were housed in an air-conditioned room with 12 h light–dark cycles, constant temperature (21 ± 2°C), relative humidity (60–65%) and free access to water and food. All procedures were performed with the approval of the University of Pittsburgh Animal Care and Use Committee and in accordance with NIH guidelines.
Experimental Models
This study consists of two experimental models based on the severity of AKI. In the first model (severe), mice were randomly assigned to four groups. AKI was induced with 350mg/kg (Sigma Aldrich, St. Louis, MO) of folic acid (FA) intra-peritoneal injection. Mice in the pretreatment group were treated with subcutaneous injection of 300 mg/kg of NAC (Sigma Aldrich, St. Louis, MO) at 24 and 6 hours prior to the FA injection (n=15). Mice in posttreatment group were treated with subcutaneous injection of 300 mg/kg of NAC at 6 and 24 hours after FA injection (n=12). Mice in pre +posttreatment group were treated with 300 mg/kg of NAC at 24 and 6 hours prior to the FA injection and at 6, 24, and 48 hours after FA injection (n=12). The dose and timing of NAC administration were based on and modified from studies showing benefit with NAC (13, 14, 15). In a fourth group the same amounts of normal saline were given subcutaneously at the same time points as the pre + posttreatment group (n=15).
In the second model (mild), AKI was induced with 250mg/kg of FA intra-peritoneal injection. The same groups (n= 12 each) with the same interventions as the severe model were carried out. Finally, in a third experiment, the same dose of NAC as the pre +posttreatment group was administered to 14 mice without FA injection to evaluate the direct toxicity of NAC (6 were euthanized for tissue oxidative analysis as control after all NAC doses were given).
From all animals, we drew blood (0.1ml/per time) from greater saphenous vein 4 days prior to FA injection as baseline and on days 1, 2, 3 and 7 after FA injection. Plasma was collected after centrifugation at 1500 g for 10 minutes. All samples were stored at -80°C until assay. Survival time was assessed up to 7 days.
In order to evaluate kidney histology, another experiment consisting of 24 mice with the same interventions (pretreatment, posttreatment, pre +posttreatment and placebo, n=6 each) was carried out. These mice were euthanized on day 2 after FA injection for histological observation and tissue oxidative analysis.
Assessment of renal function
Plasma concentrations of creatinine (Cr) and cystatin C were measured to evaluate glomerular function. Plasma Cr was measured with a creatinine enzymatic assay kit (BioVision Technologies, Mountain View, CA). Cystatin C was measured with enzyme-linked immunosorbent assay kit (BioVendor, Candler, NC). The severity of AKI was assessed using RIFLE criteria based on the change in plasma Cr. The criteria is: R, Risk (Cr increases>50%); I, Injury (Cr increases>100%); F, Failure (Cr increases>200%).
Analysis of oxidative injury
Reduced glutathione (GSH) was determined by Glutathione Colorimetric Detection Kit (BioVision, Technologies, Mountain View, CA). Proteins were first removed from plasma samples by using 5-Sulfosalicylic acid. GSH and 5,5′-Dithiobis (2-nitrobenzoic Acid) react to generate 2-nitro-5-thiobenzoic acid which has a yellow color. The GSH concentrations from blood and kidney tissue were determined by measuring absorbance at 405nm.
Malondialdehyde (MDA) contents from kidney tissue were measured with the thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical Company, Ann Arbor, MI). The MDA-thiobarbituric acid adduct formed by the reaction of MDA and thiobarbituric acid under high temperature (90-100° C) and acidic conditions is measured colorimetrically at 530-540 nm.
Evaluation of kidney histology
Kidney sections were fixed in 10% neutral buffered formalin, dehydrated in graded anhydrous absolute ethanol, and embedded in paraffin. Histological sections (5μm) of kidney were stained with hematoxylin, eosin and periodic acid Schiff. We considered the morphological changes indicating acute tubular necrosis (ATN) as the loss of membrane and nuclear integrity, the loss of brush border, the vacuolization of tubular epithelial cells, and the presence of intra-tubular debris.
Statistical analyses
All numeric data with normal distribution were expressed as mean ± standard deviation (SD). Mean differences among the four groups and among different time points were analyzed by analysis of variance for repeated measures. When significant differences among groups and time points were found, pair–wise comparisons were used with Bonferroni adjustment. The primary tests of interest are the comparisons between NAC-treated groups and the placebo-treated group at each time point. Numeric data without normal distribution (data for renal oxidative parameters, survival-days) were expressed as median (ranges) and compared with Kruskal-Wallis test. Categorical data (RIFLE criterion) were expressed as percentage and were compared with Chi-square tests. Survival time was assessed by Kaplan-Meier method (log rank test) and overall survival in each group was compared using Fisher's exact test. All analyses were conducted using the SPSS statistical software package (SPSS for Windows; Chicago, IL, USA). P-values below 0.05 were considered to be statistically significant.
Results
NAC pretreatment improved survival, while posttreatment worsened survival
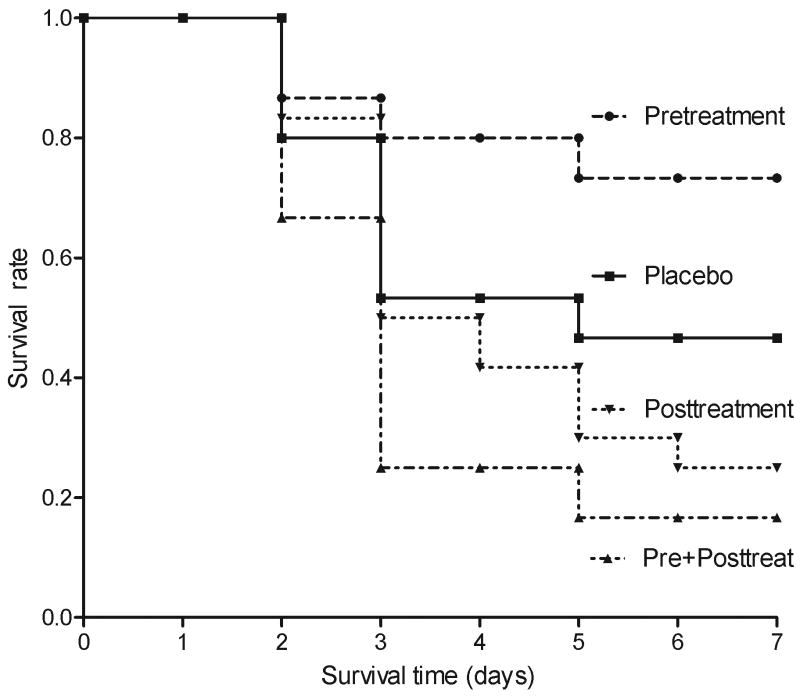
Survival was evaluated for 7 days. Pretreatment with NAC resulted in significantly better survival compared to placebo-treated animals (log rank test, p=0.03, Fig 1) with a greater 7-day survival rate (73.33% vs 46.67%, P=0.04) and a longer median survival time (6.13, vs 4.63 days, P=0.03, Fig. 1A). In contrast, mice treated with posttreatment did not show any difference in survival and mice treated before and after FA actually exhibited significantly lower survival rates (16.67% vs 46.67%, P=0.01) compared to placebo. There were no deaths or other apparent adverse effects among animals receiving five days of NAC in the absence of FA.
Figure 1. Effects of NAC on one week survival in FA-induced AKI.
Mice were received NAC pretreatment (n=15), Posttreatment (n=12), Pre+posttreatment (n=12) or saline placebo treatment (n=15).
Survival time (days) was observed from FA injection.
p<0.05, Pretreatment vs placebo; P<0.01, Pre+posttreatment vs Placebo.
NAC pretreatment alleviated FA-induced AKI, while posttreatment worsened AKI
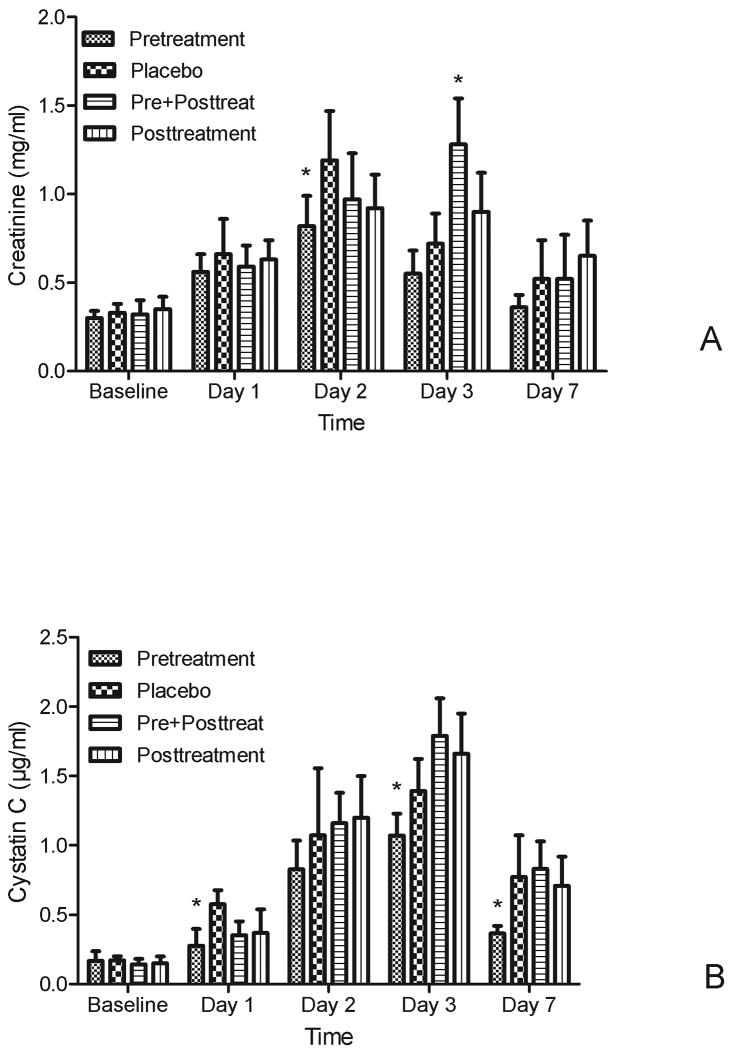
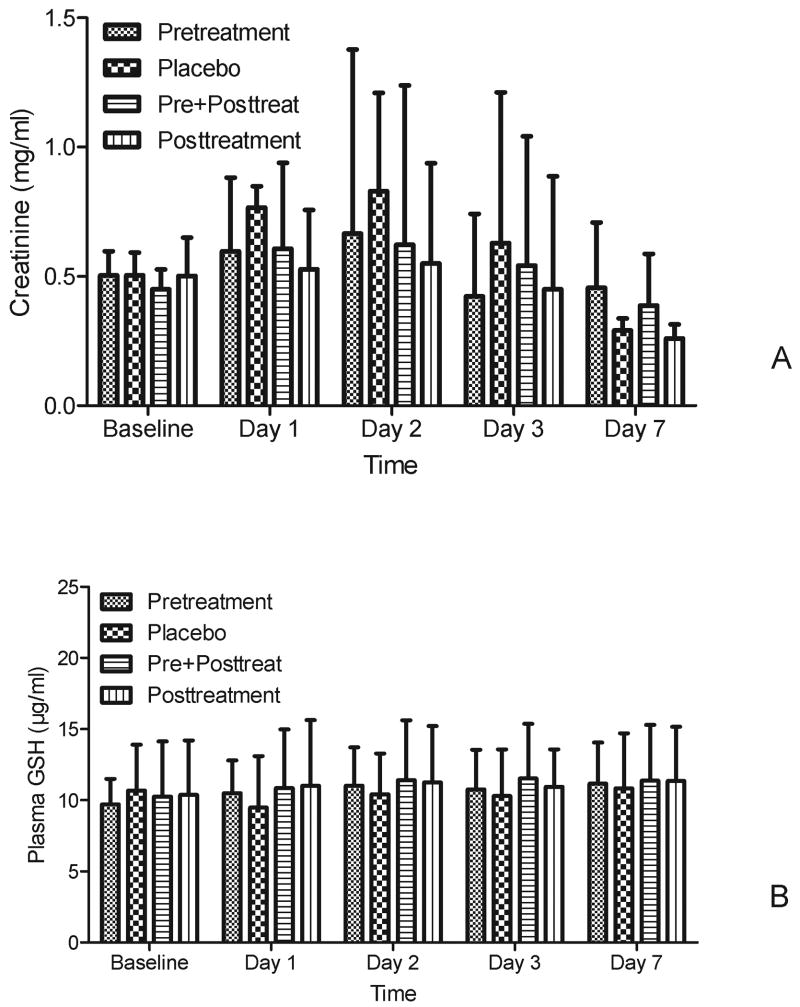
In placebo treated animals exposed to FA plasma Cr increased early (1 day) after exposure and reached a peak at day 2, and then recovered to baseline by day 7 (Fig 2A). The pretreatment group exhibited significant protection (0.82 vs 1.19 mg/dl on day 2, p=0.01), compared to placebo while the posttreatment group did not show this effect. Pre+posttreatment with NAC significantly worsened the Cr (1.28 vs 0.72 mg/dl on day 3, p=0.01). Cystatin C changes were similar to those of Cr. NAC pretreatment significantly attenuated the increased cystatin C on days 1, 3 and 7 (p<0.05, Fig 2B).
Figure 2. Effects of NAC on renal function in FA-induced AKI.
A. Effect s of NAC on plasma creatinine (mean ±SD, mg/dl),
B. Effect s of NAC on plasma cystatin C (mean ±SD, μg/ml)
Both plasma creatinine and cystatin C showed significant differences over time (p < 0.05) and among groups (p < 0.05). For pair-wise comparisons, * p<0.05 vs placebo in the same day.
NAC pretreatment mitigated the FA-induced oxidative injury, while posttreatment did not
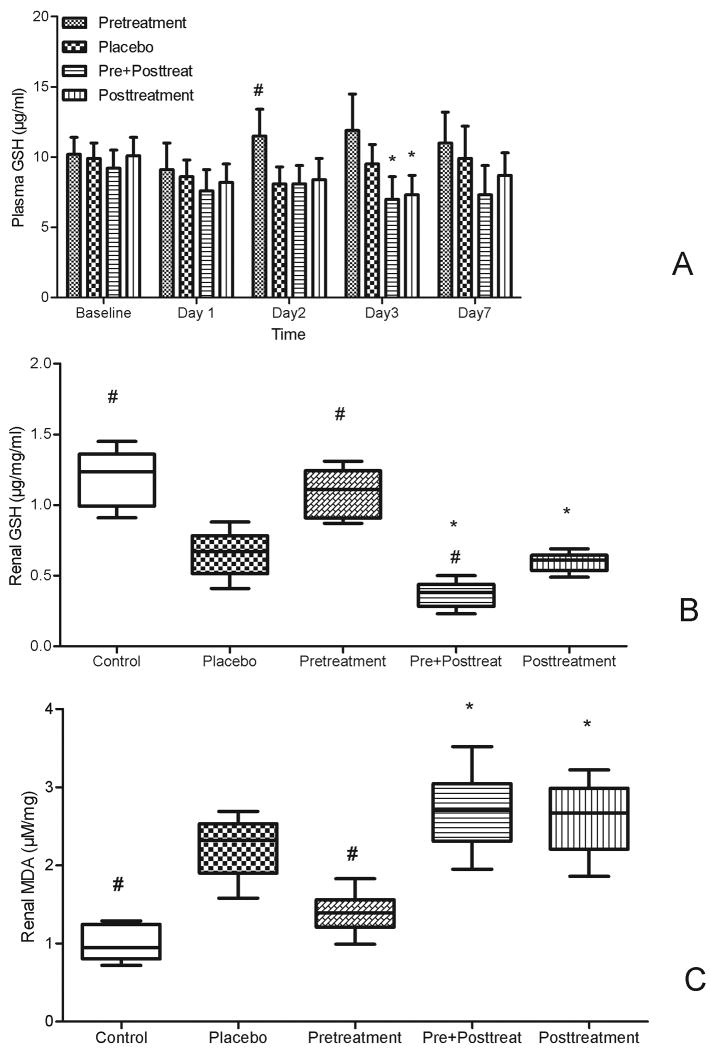
Plasma GSH decreased sharply at day 1 after FA injection. The GSH in pretreatment mice began to recover and increase from day 2 compared to placebo (11.5 vs 8.1 μg/ml, p=0.04, Fig. 3A). However, the GSH in both posttreatment groups continued to decrease, and was even lower than that in the placebo group on day 3 (7.3 vs 9.5 μg/ml, Fig.3A). Renal GSH contents decreased and renal MDA contents increased at day 2 after FA injection compared to normal control. NAC pretreatment increased renal GSH and decreased MDA contents (Fig 3B & 3C), while both pre+posttreatment and posttreatment had the trend to worsen oxidative injury.
Figure 3. Effect s of NAC on oxidative injury.
A. The plasma GSH changes with time in different groups (mean ±SD, μg/ml, n=12-15)
B. The renal GSH contents after 2 days of FA injection in different groups (median with range, μg/mg/ml,n=6).
C. The renal MDA contents after 2 days of FA injection in different groups; (median with range, μM/mg,n=6).
Control: group without FA injection
#p<0.05 vs placebo; * p<0.05 vs pretreatment group
NAC pretreatment improved FA-induced kidney histology, while posttreatment did not
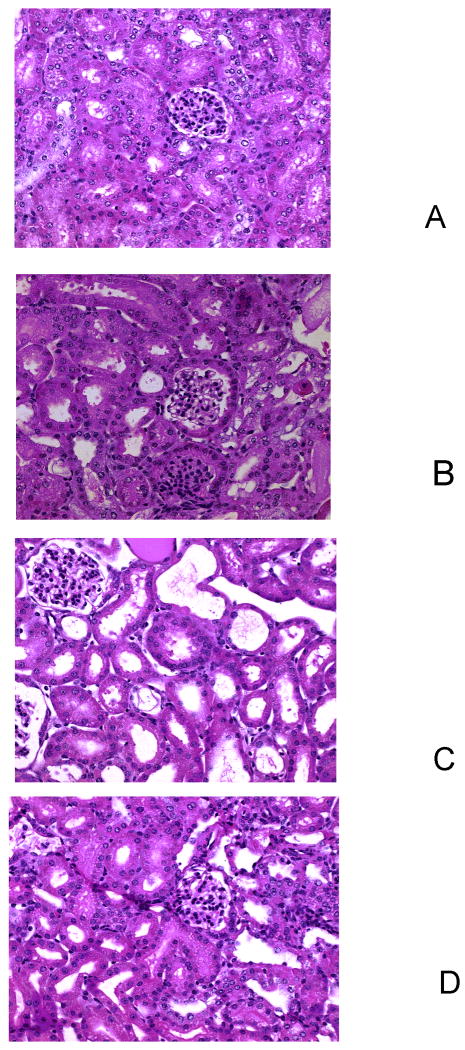
Fig 4 shows the kidney histology under light microscopy. Renal tubules in all mice showed loss of brush border, vaculation of cells and acute tubular necrosis 2 days after FA injection. These pathological changes were attenuated in the pretreatment group (Fig 4A) but were actually worse in the posttreatment group (Fig 4C).
Figure 4. Effect s of NAC on kidney histology.
Histological sections (5μm) of kidney were stained with hematoxylin, eosin and periodic acid Schiff.
A: Pretreatment
B: Placebo treatment
C: Pre+posttreatment
D: Posttreatment
NAC pretreatment improved survival, which was related to the reduced oxidative injury and subsequently reduced AKI severity
The plasma GSH contents at day 2 of FA injection categorized as AKI RIFLE-R from all groups were significantly higher than those categorized as RIFLE-I and RIFLE-F (11.4 vs 8.8, p=0.01; 11.4 vs 8.0 μg/ml, p=0.01; respectively, Fig.5A). On the other hand, there was a lower percentage of RIFLE-F AKI in the pretreatment group (32%) compared to other groups (67-72%) (Fig 5C). Fig. 5B shows mortality by RIFLE strata. Mortality increased from 17% (2/12)with RIFLE-R to 44% (8/18)with RIFLE-I to 85%(17/20) with RIFLE-F.
Figure 5. The relationship between oxidative injury, AKI severity and survival.
A. The relationship between plasma GSH and AKI severity. The comparison in GSH contents after 2 days of FA injection among the NO-AKI, AKI RIFLE-R, RIFLE -I, and RIFLE-F animals. #: p<0.01, vs AKI-R
B. The relationship between AKI severity and survival. Survival patterns by RIFLE strata. #:P<0.001 vs AKI-R(Chi-square).
C. RIFLE percentage among different groups. R: Risk (Cr increases>50%); I: Injury (Cr increases>100%); F: Failure (Cr increases>200%). * p<0.05 vs placebo.
Both NAC pretreatment and posttreatment neither improved nor worsened renal function in the milder AKI model
Finally, in order to explore whether the NAC-induced paradoxical oxidative effects seen in our severe AKI model are related to the severity of injury, we also studied the effect of NAC in a mild AKI model. A lower dose of FA (250mg/kg) induced mild temporary AKI. In this model, all of the various NAC regimens demonstrated trends to reduced AKI, but the differences were not statistically significant (Fig 6). Neither pre+posttreatment nor posttreatment showed harm in this model.
Figure 6. Effects of NAC on renal function and oxidative injury in mild AKI.
A: Effects of NAC on plasma creatinine (mean ±SD, mg/dl, n=12)
B: Effects of NAC on plasma GSH (mean ±SD, μg/dl, n=12)
Discussion
Our results demonstrate that NAC pretreatment is effective in reducing the severity of AKI as well as increasing survival time in experimental nephrotoxicity, while NAC posttreatment was not effective, and evened worsened AKI. The protective effect of NAC was apparently related to the replenishing depleted GSH, while the harmful effect might be induced by NAC's pro-oxidative actions in the setting of injury and inflammation (19, 20). Our results also show that oxidative injury plays an important role in FA-induced AKI, but that NAC is only effective for prevention not treatment. This study has thus confirmed and extended the observation that NAC is only effective in preventing AKI induced by nephrotoxic substances, but worsens AKI when given for treatment, at least under some conditions (19, 20, 21). While our results cannot be directly translated into clinical practice, they do raise important cautionary notes regarding the use of NAC in the clinical setting.
In this study, we administered intra-peritoneal FA to induce AKI. FA is a water-soluble vitamin that precipitates in the acidic urine within the tubules. In high concentration this may result in epithelial hypoxia, renal tubular necrosis, renal failure, and even death after several hours or days (22). In the first few days after injury, epithelial proliferation leads to epithelial regeneration of some tubules and gradually leads to renal recovery (22,23,24). This model may mimic clinically relevant AKI secondary to various toxins, medications or contrast media. However, we also chose FA because it has no systemic effects that would confound assessment of oxidative stress. Our results may not be relevant to sepsis-induced AKI or AKI caused by any number of other insults (e.g. ischemia/reperfusion). A dose of 250mg/kg of FA has been used for chronic kidney injury (22) or the first hit model together with the second hit (23,24). This dose induces only mild kidney injury (figure 6). Using this lower dose we could not find any significant changes even with high dose NAC treatment. Thus, we chose a higher dose of FA (350mg/kg) to induce much more severe AKI, and observed significant changes with NAC treatment.
Subcutaneous injection of 300 mg/kg of NAC was used in this study, which is greater than that commonly used in humans. However, the dose was based on and modified from studies showing benefit with NAC (13, 14, 15). Di Giorno demonstrated a dose-dependent (40-300 mg/kg peritoneal injection) renal protection. 300mg/kg of NAC showed a better renal function than other doses (14). The ideal concentration for NAC to induce anti-inflammatory and anti-oxidative effects is 2-10mM (25, 26).In a human volunteer study, 200mg NAC intravenous injection only lead to peak NAC plasma concentration 0.07mM (27). However Sprong et al calculated the intraperitoneal injection at concentrations of 200 to 1,000 mg/kg of NAC in rats led to peak NAC plasma concentrations of about 3-15 mM (20). In humans the dose of IV NAC used for prevention of contrast-induced AKI has been as high as 1200mg every 12 hours for 48hours (total dose 6000mg) (28). This dose would be expected to achieve plasma concentrations of about 4-6mM. Thus, our dosing strategy is in line with the high dose regimens being used in some studies of contrast-induced AKI. We administered NAC subcutaneously rather than intraperitoneally to avoid the possible formation of complexes with FA, as subcutaneous administration has also been reported to induce protective effects in animals (29) and humans (30).
Our results are compatible with our recent findings in patients undergoing liver transplant (31), in which we found that pre + posttreatment with NAC was not effective in reducing the incidence of AKI. Importantly, we also found that NAC was effective in increasing GSH in only about 50% of patients and that and fewer patients developed AKI when GSH levels were increased. Our results in this study were similar in that NAC appeared to be protective when GSH levels were increased.
Other groups have also found mixed results with NAC for preventing AKI. Most clinical studies have been done in the setting of contrast nephropathy. Data from meta-analyses are also conflicting and show significant heterogeneity between studies. Seven of 13 recently published meta-analyses concluded that NAC is beneficial for preventing contrast-induced nephropathy. Differences in study design, patient populations, treatment protocols, and primary outcomes may explain this heterogeneity. The protective effects of NAC may be related to timing, dosing, and the severity of AKI (16, 17, 18, 31, 32). Moreover, a recent large retrospective cohort study of 7977 patients with contrast exposures performed at the Mayo Clinic suggested that there was a trend toward decreased incidence of nephropathy in cases where administration of NAC was performed on two consecutive days (one day before and the day of contrast administration). In those cases where NAC was administered in other regimens, there was a trend toward increased incidence of renal injury (18). While these results are only trends, the signal is quite consistent with our results.
If NAC causes increased injury to the kidney when given after a nephrotoxic insult, the mechanism is unclear. In our study, GSH levels in posttreatment did not improve after being reduced by FA. This observation could suggest further oxidation of GSH by oxidative stress induced by NAC as well as by FA. This effect of NAC is supported by the considerable literature reporting that low-molecular-weight thiols are pro-oxidants as well as antioxidants (33). Pro-oxidant activity is the result of transition metal-dependent auto-oxidation yielding O−2·, H2O2, and the reduced form of the transition metal, which may behave as a catalyst in free radical reactions. Under normal physiological circumstances, metals are bound to circulating proteins and are rendered redox-inactive (33, 34). However, levels of free metal ions, including iron, may be elevated in a variety of clinical conditions (including inflammation, sepsis, and cardio-pulmonary bypass) and become redox-active (35, 36). Because this auto-oxidation only occurs during stress, it could explain why posttreatment worsened AKI. Molnar et al. have suggested that the initiation of NAC treatment >24 hrs after hospital admission may potentially be harmful (37) in critical illness. Furthermore, the more severe the AKI is, the higher levels of the free iron are. It may explain why NAC was not effective in severe AKI in previous studies (16, 21). In severe sepsis, NAC treatment even aggravated organ failure (38). Data from our mild AKI model showed both post and pre +posttreatment with NAC neither worsen AKI nor changed oxidative injury. This suggested that severity of injury played an important role in the progression of auto-oxidation. We also speculate that a relatively high NAC dose can actually worsen oxidative stress. Before day 1, both the group with pretreatment and the group with pre and posttreatment received the same intervention. The group with pre and posttreatment received three more doses of NAC from day 1 to day 3. These additional doses may have caused harm. Laisalmi-Kokki showed the potentially detrimental effects of NAC on renal function in knee arthroplasty when a loading dose of NAC was infused in a short time (19). In a rodent endotoxin model, low doses of NAC protected against oxidative stress by directly scavenging oxygen radicals. On the contrary, higher doses decreased animal survival and increased oxidative stress by auto-oxidation of NAC sulphydryl groups (20). The concentration-dependent reduction of ferricytochrome-c by NAC was also reported in this study. In a human model of acute muscle injury induced by eccentric exercise, Childs et al. (39) have shown that combination of vitamin C and NAC possesses pro-oxidative properties. In our study, this increased dose could not be attributed to a direct toxic effect of NAC itself, since there were no deaths among animals receiving the five days of NAC in mice without FA. However, this high cumulative dose of NAC might have resulted in a paradoxically higher oxidative stress in animals treated with FA. Once again, it indicated that this dose-dependent auto-oxidation only occurred in severe injury.
There are limitations to our study. First, we did not measure plasma NAC or its metabolites, so we were not able to evaluate the potential correlations of NAC concentration on AKI. Second, pretreatment with NAC did not prevent AKI but rather reduced its severity. However, AKI was sustained for several days after the intervention and there appeared to be significant differences between groups in terms of RIFLE class, Cr and cystatin C over time. Indeed it has been suggested that Cystatin C may be a more accurate measure of renal function particularly in the presence of NAC (40, 41). Finally, FA is an unusual cause of AKI in humans. However, FA bares similarities to other nephrotoxins and has the added benefit of having no confounding systemic effects. Fourth, intravenous or intraperitoneal administration of NAC is presumably superior to oral use, as the former have rapid onsets of effect, complete bioavailability, and higher peak serum NAC levels. Here we administered NAC subcutaneously rather than intraperitoneally to avoid the possible formation of complexes with FA.
In summary, our data indicate that the renal protective effects of NAC in the setting of nephrotoxic (FA-induced) AKI are limited to pretreatment. When given after onset of AKI and in high concentrations, NAC did not decrease oxidative stress and even worsened FA toxicity. Further studies are needed to determine the exact mechanism of this effect and whether it is applicable to humans with contrast-induced and other forms of AKI. Nevertheless, given the controversial nature of the existing evidence for NAC, evidence of potential harm, seen in this study, should prompt a reevaluation of NAC particularly when used a posttreatment regimen.
Acknowledgments
Support: This study was supported by a Seed grant from Department of Critical Care Medicine, University of Pittsburgh School of Medicine (ZP) and in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK070910 and the National Hearth Lung and Blood Institute (NHLBI) R01HL080926. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDDK, NHLBI, or the National Institutes of Health.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Liangos O, Wald R, O'Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagshaw SM, George C, Dinu I, et al. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 5.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 6.Neveu H, Kleinknecht D, Brivet F, Loirat P, Landais P. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 7.Silvester W, Bellomo R, Cole L. Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med. 2001;29:1910–1915. doi: 10.1097/00003246-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta RL, Pascual MT, Soroko S, et al. Program to Improve Care in Acute Renal Disease. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 10.Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med. 2008;36(4 Suppl):S216–223. doi: 10.1097/CCM.0b013e318168e375. [DOI] [PubMed] [Google Scholar]
- 11.Venkataraman R. Can we prevent acute kidney injury? Crit Care Med. 2008;36(4 Suppl):S166–171. doi: 10.1097/CCM.0b013e318168c74a. [DOI] [PubMed] [Google Scholar]
- 12.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109:665–678. doi: 10.1016/s0002-9343(00)00612-4. 1. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Grigoryev DN, Crow MT, et al. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–285. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- 14.Di Giorno C, Pinheiro HS, Heinke T, et al. Beneficial effect of N-acetyl-cysteine on renal injury triggered by ischemia and reperfusion. Transplant Proc. 2006;38:2774–2776. doi: 10.1016/j.transproceed.2006.08.178. [DOI] [PubMed] [Google Scholar]
- 15.Ali BH, Al-Salam S, Al-Husseini I, et al. Comparative protective effect of N-acetyl cysteine and tetramethylpyrazine in rats with gentamicin nephrotoxicity. J Appl Toxicol. 2009;29:302–307. doi: 10.1002/jat.1409. [DOI] [PubMed] [Google Scholar]
- 16.Liu R, Nair D, Ix J, et al. N-acetylcysteine for the prevention of contrast-induced nephropathy. A systematic review and meta-analysis. J Gen Intern Med. 2005;20:193–200. doi: 10.1111/j.1525-1497.2005.30323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. doi: 10.1056/NEJMoa054209. 29. [DOI] [PubMed] [Google Scholar]
- 18.From AM, Bartholmai BJ, Williams AW, et al. Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at mayo clinic. Clin J Am Soc Nephrol. 2008;3:10–18. doi: 10.2215/CJN.03100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laisalmi-Kokki M, Personen E, Kokki H, et al. Potentially detrimental effects of N-acetylcysteine on renal function in knee arthroplasty. Free Radic Res. 2009;43:691–696. doi: 10.1080/10715760902998206. [DOI] [PubMed] [Google Scholar]
- 20.Sprong RC, Winkelhuyzen-Janssen AM, Aarsman CJ, et al. Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1283–1293. doi: 10.1164/ajrccm.157.4.9508063. [DOI] [PubMed] [Google Scholar]
- 21.Castini D, Lucreziotti S, Bosotti L, et al. Prevention of Contrast-induced Nephropathy: A Single Center Randomized Study. Clin Cardiol. 2010;33:E63–E68. doi: 10.1002/clc.20576. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullin EM, Bonar RA, Kane RD, et al. Reduction of folic acid-induced acute tubular injury by diuresis: an experimental model. Exp Mol Pathol. 1976;25:99–105. doi: 10.1016/0014-4800(76)90020-4. [DOI] [PubMed] [Google Scholar]
- 23.Yuan HT, Li XZ, Pitera JE, et al. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol. 2003;163:2289–2301. doi: 10.1016/s0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long DA, Woolf AS, Suda T, et al. Increased renal angiopoietin-1 expression in folic acid-induced nephrotoxicity in mice. J Am Soc Nephrol. 2001;12:2721–2731. doi: 10.1681/ASN.V12122721. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Navath RS, Romero R, et al. Anti-inflammatory and anti-oxidant activity of anionic dendrimer-N-acetyl cysteine conjugates in activated microglial cells. Int J Pharm. 2009;377:159–168. doi: 10.1016/j.ijpharm.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wuyts WA, Vanaudenaerde BM, Dupont LJ, et al. N-acetylcysteine inhibits interleukin-17-induced interleukin-8 production from human airway smooth muscle cells: a possible role for anti-oxidative treatment in chronic lung rejection? J Heart Lung Transplant. 2004;23:122–127. doi: 10.1016/s1053-2498(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 27.Cotgreave IA, Moldéus P. Methodologies for the analysis of reduced and oxidized N-acetylcysteine in biological systems. Biopharm Drug Dispos. 1987;8:365–375. doi: 10.1002/bdd.2510080407. [DOI] [PubMed] [Google Scholar]
- 28.Thiele H, Hildebrand L, Schirdewahn C, et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol. 2010;55:2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 29.Muñnoz AM, Rey P, Soto-Otero R, et al. Systemic administration of N-acetylcysteine protects dopaminergic neurons against 6-hydroxydopamine-induced degeneration. J Neurosci Res. 2004;76:551–562. doi: 10.1002/jnr.20107. 15. [DOI] [PubMed] [Google Scholar]
- 30.Orrell RW, Lane JM, Ross MA. Antioxidant treatment for amyotrophic lateral sclerosis / motor neuron disease. Cochrane Database Syst Rev. 2004;18 doi: 10.1002/14651858.CD002829.pub2. CD002829. [DOI] [PubMed] [Google Scholar]
- 31.Hilmi IA, Peng Z, Planinsic RM, et al. N-acetylcysteine does not prevent hepatorenal ischaemia-reperfusion injury in patients undergoing orthotopic liver transplantation. Nephrol Dial Transplant. 2010;25:2328–2333. doi: 10.1093/ndt/gfq077. [DOI] [PubMed] [Google Scholar]
- 32.Millea PJ. N-acetylcysteine: multiple clinical applications. Am Fam Physician. 2009;80:265–269. [PubMed] [Google Scholar]
- 33.Park JW, Floyd RA. Generation of strand breaks and formation of 8-hydroxy-2′-deoxyguanosine in DNA by a thiol/Fe3+/O2-catalyzed oxidation system. Arch Biochem Biophys. 1994;312:285–291. doi: 10.1006/abbi.1994.1311. [DOI] [PubMed] [Google Scholar]
- 34.Winterbourn CC. Hydroxyl radical production in body fluids. Roles of metal ions, ascorbate and superoxide. Biochem J. 1981;198:125–131. doi: 10.1042/bj1980125. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 36.Biemond P, van Eijk HG, Swaak AJ, et al. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984;73:1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molnár Z, Shearer E, Lowe D. N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo-controlled study. Crit Care Med. 1999;27:1100–1104. doi: 10.1097/00003246-199906000-00028. [DOI] [PubMed] [Google Scholar]
- 38.Spapen HD, Diltoer MW, Nguyen DN, et al. Effects of N-acetylcysteine on microalbuminuria and organ failure in acute severe sepsis: results of a pilot study. Chest. 2005;127:1413–1419. doi: 10.1378/chest.127.4.1413. [DOI] [PubMed] [Google Scholar]
- 39.Childs A, Jacobs C, Kaminski T, et al. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans. Free Radic Biol Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. 15. [DOI] [PubMed] [Google Scholar]
- 40.Kimmel M, Butscheid M, Brenner S, et al. Improved estimation of glomerular filtration rate by serum cystatin C in preventing contrast induced nephropathy by N-acetylcysteine or zinc--preliminary results. Nephrol Dial Transplant. 2008;23:1241–1245. doi: 10.1093/ndt/gfm785. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann U, Fischereder M, Krüger B, et al. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407–410. doi: 10.1097/01.asn.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]