Abstract
Tenascins regulate cell interaction with the surrounding pericellular matrix. Within bone, tenascin C and W influence osteoblast adhesion and differentiation, although little is known about the regulation of tenascin expression. In this study we examined the effect of osteogenic differentiation, BMP and Wnt growth factors, and mechanical loading on tenascin expression in osteogenic cells. Osteogenic differentiation increased Tenascin C (TnC), and decreased Tenascin W (TnW), expression. Both growth factors and mechanical loading increased both TnC and TnW expression, albeit via distinct signaling mechanisms. Both BMP-2 and Wnt5a induction of tenascin expression were mediated by MAP kinases. These data establish a role for BMP, Wnts, and mechanical loading in the regulation of tenascin expression in osteoblasts.
Keywords: Tenascin, Bone morphogenetic protein, Wnt, MAPK, Osteoblast, Osteocyte, Mechanotransduction
INTRODUCTION
The skeletal system is critical for structural support and mineral homeostasis, while also providing a protected environment conducive to hematopoiesis. To fulfill these multiple functions, bones adapt to a variety of signals, including systemic hormones and localized biophysical forces. These forces are generated during the loading and unloading of long bones, and include oscillatory flow of interstitial fluid, substrate strain, and streaming potentials [Robling et al., 2006]. These signals trigger mechanosensitive cells resident within the skeleton, such as mineral depositing-osteoblasts, to activate a variety of signal transduction pathways that alter growth factor release, gene expression, and mineral deposition in order to minimize tissue strain [Robling et al., 2006]. The organic portion of bone extracellular matrix is composed of both large structural proteins and smaller matricellular proteins, which modulate cell behavior and cell surface interactions through direct and indirect mechanisms.
Tenascins are matricellular glycoproteins that are highly expressed during tissue development and remodeling. Tenascin expression is increased under a plethora of pathological conditions including skin wounds, atherosclerosis, asthma, and cancer [Chiquet-Ehrismann and Chiquet, 2003]. Of the four vertebrate tenascins—C, W, R, and Xb—a function in the skeleton has been previously assigned to tenascins C and W. Tenascin C is widely, although transiently, expressed during embryogenesis and organogenesis [Chiquet-Ehrismann, 2004]. Tenascin W is also transiently expressed during development, but is largely restricted to the musculoskeletal system. In adult bone both tenascin C and W localize to the periosteum, while tenascin C is also found in the endosteum [Mackie et al., 1987; Scherberich et al., 2004]. Expression of both tenascins C and W increase during fracture healing [Kilian et al., 2008; Kimura et al., 2007]. Functionally, tenascins C and W are implicated in osteoblast differentiation and proliferation [Mackie and Ramsey, 1996; Meloty-Kapella et al., 2008]. Tenascin X expression is observed embryonically within the mandible [Bristow et al., 2005; Kurihara and Sato, 2004], and, in the adult, tenascin X is described in muscle and loose connective tissue. Its deletion is implicated in the connective tissue disorder Ehlers-Danlos syndrome [Burch et al., 1997]. The expression of tenascin R or Xb have not been widely described in bone. The distinct expression pattern of each member of the tenascin family suggests possible unique transcriptional regulation, yet the reported control of tenascins in bone, as well as the body, remains poorly defined.
The osteoblast-derived proteins and molecules that demonstrate anabolic effects upon the skeleton do so by increasing proliferation and matrix production and by decreasing apoptosis. Such factors include ATP [Orriss et al., 2010], prostaglandins [Pilbeam et al., 2002], bone morphogenetic proteins (BMPs) [Wan and Cao, 2005] and Wnt glycoproteins [Westendorf et al., 2004]. BMPs were recognized in the 1960s for their osteoinductive effect [Urist, 1965], and BMP-2 and BMP-7 are currently in clinical use for spinal fusion and non-unions [Axelrad and Einhorn, 2009]. Similarly, Wnt signaling through cognate Lrp5 or Lrp6 receptors, is implicated in bone formation [Gong et al., 2001]. Both BMP and Wnt signaling drive the embryologic development of bone [Wozney, 1992], are increased during fracture repair [Marsell and Einhorn, 2009; Secreto et al., 2009], and are activated by mechanical loading of bone cells both in vitro and in vivo [Hens et al., 2005; Kido et al., 2010; Lau et al., 2006]. Further, these pathways have been implicated in tenascin regulation in other tissues [Cohen et al., 2009; Scherberich et al., 2005].
Because tenascins are differentially expressed in the skeleton during various phases (e.g., embryogenesis, fracture repair, and post-natal homeostasis), and because tenascin expression is influenced in other tissues by factors known to influence bone cell behavior, we examined tenascin expression in response to osteogenic differentiation, growth factors, and biophysical signaling. BMP-2, Wnt5a, and fluid shear stress each induced the expression of tenascins C and W, albeit via distinct signaling pathways involving de novo protein synthesis, phospholipase C, and MAP kinases. These data indicate that tenascin C and W are regulated by multiple distinct mechanisms in osteoblasts.
MATERIALS AND METHODS
Reagents
Chemical inhibitors of MEK1/2 (U0126), p38 (SB 203580), JNK (JNK Inhibitor II), phospholipase C (U-73122), and calcineurin (cyclosporin A, CsA) were purchased from EMD Biosciences. Cycloheximide (CHX) was purchased from Sigma, BMP-2 was from Peprotech, and Wnts were from R&D Systems. All antagonists were added one hour prior to the beginning of each experiment, and were present in experimental media.
Cell culture
Murine pre-osteoblastic cells (MC3T3-E1; provided by Norman J. Karin, Pacific Northwest National Laboratory) were seeded on tissue culture-treated plastic at a density of 10,000 cell/cm2 in α-MEM supplemented with 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin (P/S) for all static experiments. Cells were maintained in a standard humidified incubator at 37°C with 5% CO2. Media was replaced with α-MEM supplemented with 2% FBS and 1% P/S overnight prior to performing all experiments. For osteogenic differentiation, cells were cultured in the presence of osteogenic media (standard media supplemented with 50 μg/mL ascorbic acid-2-phosphate and 5mM β-glycerol phosphate).
Oscillatory fluid flow
MC3T3-E1 cells were seeded at a density of 5,000 cell/cm2 onto 7.5 × 3.8 cm2 glass slides in α-MEM supplemented with 10% FBS, and 1% P/S. Cells were cultured in a standard humidified incubator at 37°C with 5% CO2. Media was replaced with α-MEM supplemented with 2% FBS and 1% P/S overnight prior to the application of fluid flow. Cell-seeded slides were placed into a custom-made parallel plate flow chamber, modified from that described by Frangos et al. [Frangos et al., 1988], immediately prior to flow experiments. Flow chambers were maintained in a humidified incubator at 37°C throughout the flow period. Fluid flow was delivered using 500 μL Hamilton glass syringes mounted into a mechanical loading device (TestBench, Bose) as previously described [Jacobs et al., 1998]; this system generates sinusoidal oscillatory fluid flow at a frequency of 1 Hertz which produces a peak shear stress of 15dynes/cm2. The flow rate was confirmed with an in-line ultrasonic flow meter (Transonic Systems). Flow medium (αMEM+2% FBS+1% P/S) was supplemented with 10mM HEPES and 50 μg/mL gentamicin. Slides were removed from flow chambers after two hour exposure and placed in tissue culture dishes with α-MEM supplemented with 2% FBS, and 1% P/S for the indicated post-flow incubation time.
Quantitative PCR
RNA was isolated from mid-diaphysial sections of murine femurs (aged 14–16 weeks) according to Genetos et al. [Genetos, 2010]. Alternately, RNA was isolated and purified using RNeasy Mini Kit per the manufacturer’s instructions (Qiagen). Total RNA was reverse-transcribed with QuantiTect Reverse Transcription Kit (Qiagen), which includes a genomic DNA digestion step. Quantitative PCR was performed using QuantiFast PCR Master Mix (Qiagen) on a Mastercycler Realplex2 (Eppendorf). Proprietary primers and probes for tenascins C (TnC), W (TnN), R (TnR), and Xb (TnXb) and Rpl13 were purchased from Applied Biosystems. Amplification conditions were 95°C for three minutes followed by 40 cycles of three seconds at 95°C and 60°C for 30 seconds. The ribosomal gene Rpl13 was used to normalize samples for comparison. Gene expression was calculated relative to Rpl13 (2−ΔCt), and was occasionally further normalized to matched control (2−ΔΔCt) [Schmittgen and Livak, 2008].
Western blot
Protein was collected in RIPA (0.1% Triton X-100, 10mMTris pH 8, 1mMEDTA, 200 nM Na3VO4,) with HALT protease and phosphatase inhibitors (Pierce). Protein concentration was determined using the DC Protein Assay (Biorad) according to the manufacturer’s instructions. Equal amounts of protein were loaded in each well and run out on a 10% SDS gel before transfer to a nitrocellulose membrane. Membranes were probed with primary antibody overnight at 4°C and an appropriate secondary antibody (1:1000) at room temperature for one hour prior to development with enhanced chemiluminescent substrate (Denville). Anti-mouse and anti-rabbit secondary antibodies were purchased from Jackson laboratories. Primary antibodies against p-JNK (1:1000), JNK (1:1000), p38 (1:500) and P-p38 (1:1000) were purchased from Cell Signaling while p-ERK1/2 (1:1000) and ERK ½ (1:1000) antibodies were purchased from Santa Cruz Biotechnology.
Statistical analysis
Each data set is the result of a minimum of three independent experiments. Unless otherwise indicated, data were normalized to vehicle control samples in the absence of growth factor. Data were analyzed by Student’s t-test, one-way, or two-way ANOVA. Dunnet or Tukey’s post-hoc tests were performed when significant differences were detected by ANOVA. Statistical significance was considered for p < 0.05.
RESULTS
Tenascin Expression
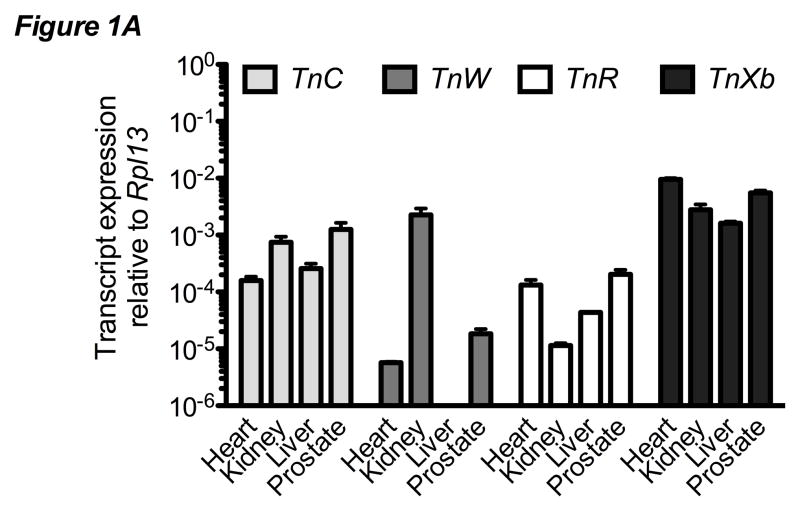
Tenascin expression was examined in a variety of tissues by qPCR. Expression of TnC, TnW, TnR, and TnXb was observed at varying levels in samples from adult murine heart, kidney, liver and prostate (Figure 1A). TnW expression was observed in heart, kidney, and prostate, but not in the liver. Similarly, there was differential expression of each tenascin isoform in adult murine femurs from mice strains (CD-1 versus C57BL/6) and clonal cells of varying osteoblastic phenotype (pre-osteoblastic MC3T3-E1 versus osteocyte-like MLO-Y4) (Figure 1B). TnC and TnW were detected in each tissue or cell sample. TnR and TnXb transcripts were occasionally detected at low levels but expression was not confirmed at the protein level. For all skeletal-derived samples, TnC and TnW were consistently expressed at higher levels than were TnR and TnXb. Similarly, tenascin expression was higher in femur-derived RNA than in clonal cell lines.
Figure 1. Tenascin expression in murine bone.
A) qPCR of tenascin expression in a range of murine tissues. Data are expressed relative to control gene Rpl13. Bars represent mean ± SEM n≥3. B) qPCR analysis of tenascin expression in the femur from two strains of mice, pre-osteoblastic MC3T3-E1 cells, and osteocyte-like MLO-Y4 cells. Data are expressed relative to control gene Rpl13. Bars represent mean ± SEM n≥3 C) qPCR analysis of TnC in MC3T3-E1 cells after 0, 7, 14 or 21 days of osteogenic differentiation. Data are normalized to control gene Rpl13, then to Day 0. Bars represent mean ± SEM n≥8 D) qPCR analysis of TnW expression in MC3T3-E1 cells after 0, 7, 14 or 21 days of osteogenic differentiation. Data are normalized to the control gene Rpl13 and then to Day 0. ** indicates p < 0.01 compared to Day 0. Bars represent mean ± SEM n≥8
Tenascin expression was also examined during the course of in vitro osteogenic differentiation. MC3T3-E1 cells were cultured with osteogenic media for 0, 7, 14 and 21 days. By day 14 TnC transcript was significantly increased compared to expression on day 0 (Figure 1C). TnW transcript levels decreased over time and were significantly decreased from day 0 at all time points examined (Figure 1D). These data illustrate that TnC and TnW transcripts are present in the MC3T3-E1 pre-osteoblast cell line and are differentially expressed during the course of osteoblastic differentiation.
BMP Regulation of Tenascin Expression
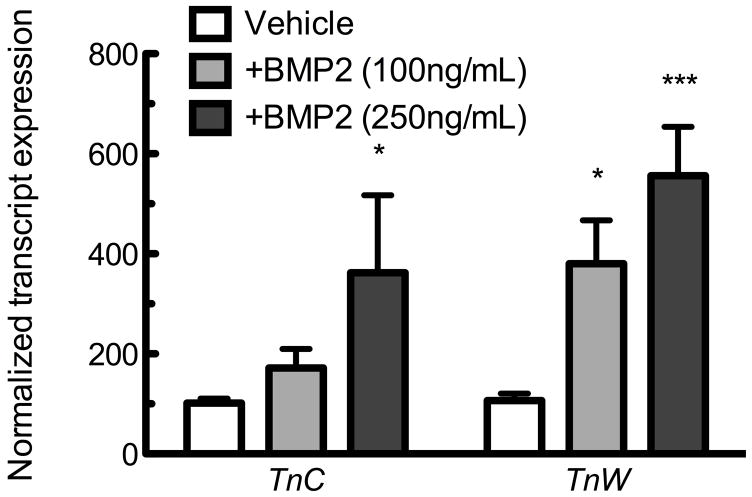
Tenascins C and W are localized within the periosteum, where BMP-2 plays a role in differentiation of osteoprogenitors [Kimura et al., 2007; Wan and Cao, 2005; Zhao, 2003], thus we chose to examine whether BMP signaling influenced expression of TnC or TnW. MC3T3-E1 pre-osteoblastic cells were exposed to BMP-2 at 0, 100, or 250ng/mL for 6 hours. qPCR revealed an inductive effect of BMP-2 upon TnC and TnW expression (Figure 2A). 100ng/mL BMP-2 induced TnW expression, and 250ng/mL BMP-2 significantly increased both TnC and TnW compared to treatment-matched controls.
Figure 2. BMP-2 induces tenascin expression.
A) TnC and TnW expression in MC3T3-E1 cells in response to a 6 hour exposure to BMP-2 as measured by qPCR. * indicates p < 0.05 or *** indicates p≤0.001 compared to control. Bars represent mean ± SEM n≥8 B) TnC and TnW expression induced by BMP-2 (250 ng/mL) in the presence or absence of CHX (10 μg/mL). CHX was applied to cells one hour prior to the 6 hour application of BMP-2. * indicates p < 0.05 compared to vehicle control. The effects of CHX and BMP-2 were not additive when analyzed by 2-way ANOVA. All data are normalized to control gene Rpl13, and then to the vehicle control. Bars represent mean ± SEM n≥9
We next examined whether de novo protein synthesis was required for induction of TnC or TnW by BMP-2. Super-induction of TnC was observed with 10 μg/mL CHX in the presence or absence of 250ng/mL BMP-2. Similar super-induction was observed when using another protein synthesis inhibitor, emetime (data not shown). A significant interaction between growth factor and CHX treatment indicated that these effects are not additive. In contrast to TnC, we observed no super-induction in response to CHX (Figure 2B) or emetine (data not shown). CHX pre-treatment prevented BMP-2 induced TnW expression (Figure 2B), indicating that BMP-2-induced tenascin W requires de novo protein synthesis. Thus BMP-2 induces both TnC and TnW, via a differential requirement for protein synthesis.
MAP kinases mediate BMP-2 induced tenascin transcript
To further elucidate the mechanism behind BMP-2 induced tenascin expression, we examined the role of MAPK signaling in BMP-2-induced tenascins. Cells were pretreated with individual MAPK inhibitors for one hour prior to BMP exposure; the efficacy of each of these inhibitors has been previously demonstrated in osteoblasts [Guicheux et al., 2003; Kozawa et al., 2001; Patil et al., 2004]. BMP-2-induced TnC response persisted in the presence of the MEK1/2 inhibitor U0126, but was abrogated by pre-treatment with inhibitors of p38 (SB203580) or JNK (JNKi II). BMP-2-induced TnW transcript was induced in the presence of all three MAPK inhibitors (Figure 3B), although it was significantly reduced in cells in which p38 was inhibited. These data suggest that BMP-2-induced TnC is dependent upon p38 and JNK, whereas TnW induction is partially p38-dependent.
Figure 3. The differential role of MAP kinases in BMP-2-induced tenascin expression.
TnC (A) and TnW (B) expression induced by BMP-2 (250 ng/mL) in the presence of inhibitors for the MAP kinases MEK 1/2 (U0126, 10 μM), p38 (SB203580, 10 μM), and JNK (JNKi II, 20 μM). All inhibitors were applied for one hour prior to the addition of BMP-2. qPCR was performed after 6hrs. * indicates p < 0.05 compared to solvent matched control. a indicates p < 0.05 compared to BMP-2-treated vehicle control. All data are normalized to control gene Rpl13, then to the solvent-matched control. Bars represent mean ± SEM n≥9.
Wnt Regulation of Tenascin Expression
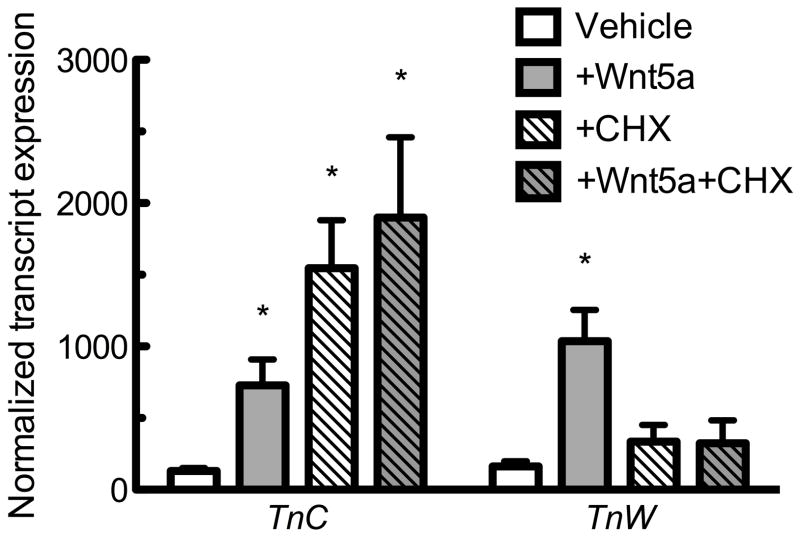
Similar to BMPs, Wnts demonstrate powerful effects upon the osteogenic differentiation of MSCs [Westendorf et al., 2004]. We next examined the influence of both non-canonical and canonical Wnts upon expression of TnC and TnW. A non-canonical Wnt, Wnt5a, significantly increased both TnC and TnW transcript after six hours (Figure 4A) and this was maintained through 24 hours of Wnt5a treatment (data not show). A canonical Wnt, Wnt3a, demonstrated trends toward induction of both TnC and TnW after a six hour exposure (Figure 4B). After 24hrs there was a significant increase in both TnC and TnW, in response to Wnt3a (Figure 4C).
Figure 4. Wnt induces tenascin expression.
TnC and TnW induction by Wnt3a at 6 hours (A) and Wnt5a at 6 hours (B) and 24 hours (C) as measured by qPCR. * indicates p< 0.05 compared to control. TnC and TnW expression induced by Wnt5a (250 ng/mL) in the presence or absence of CHX (10 μg/ml) (D). CHX was applied to cells one hour prior to a six hour exposure to Wnt5a. * indicates p < 0.05 compared to vehicle control; The effects of CHX and BMP-2 were not additive when analyzed by 2-ANOVA. All data are normalized to control gene Rpl13, then to the vehicle control. Bars represent mean ± SEM. n≥8.
We next examined the role of de novo protein synthesis in Wnt5a-induced TnC and TnW expression. TnC transcript was induced by the protein synthesis inhibitor CHX in the absence or presence of Wnt5a (Figure 4D); there was a significant interaction between growth factor treatment and CHX treatment suggesting the effects were not additive. Conversely, CHX blocked Wnt5a-induced TnW expression (Figure 4D). These data indicate that Wnt5a, similar to BMP-2, utilizes distinct signaling pathways in order to increase TnC and TnW expression.
MAP kinase signaling is required for Wnt5a-induced tenascin transcript
Non-canonical Wnts, such as Wnt5a, activate the MAPK family in other cell types [Ma and Wang, 2007; Yamanaka et al., 2002]. In order to confirm this activation in osteoblasts we measured the phosphorylation of ERK 1/2, p38, and JNK after exposure to 250ng/mL Wnt5a for 5–120 minutes. Wnt5a transiently increased the phosphorylated forms of both ERK1/2 (Tyr 204) and p38 (Thr180/Tyr182) (Figure 5A), while the phosphorylated form of JNK (Thr183/Tyr185) was not detected (data not shown). Next, we examine whether Wnt5a induction of TnC or TnW was dependent upon MAPK signaling. Wnt5a induction of TnC remained intact in the presence of each MAPK inhibitor (Figure 5B); there was a trend for attenuated TnC expression in cells treated with Wnt5a in the presence of U0126, although this did not achieve statistical significance. The Wnt5a-induced increase in TnW was prevented in cells treated with SB203580 (Figure 5C), while U0126 or JNKi II did not affect Wnt5a induction of TnW..
Figure 5. Wnt5a induces tenascin C and W transcripts through distinct mechanisms.

A) Wnt5a (250ng/mL) transiently induces ERK 1/2 and p38 phosphorylation. TnC (B) and TnW (C) expression induced by Wnt5a (250ng/mL) in the presence of inhibitors for the MAP kinases MEK 1/2 (U0126, 10 μM), p38 (SB203580, 10 μM), and JNK (JNKi II, 20μM). All inhibitors were applied for one hour prior to the 6 hour exposure to Wnt5a. * indicates p < 0.05 compared to solvent-matched static. a indicates p < 0.05 compared to Wnt5a treated vehicle control. All data are normalized to control gene Rpl13 and then to the solvent-matched control. Bars represent mean ± SEM. n≥7.
PLC and NFAT in Wnt5a regulation of tenascins
The requirement for phospholipase C (PLC) in Wnt5a-induced tenascin expression was examined using the PLC inhibitor U73122. U73122 inhibited Wnt5a-induced TnC but had no effect on Wnt5a-induced TnW (Figure 6A). These findings suggest that Wnt5a-induced TnC, but not TnW, is mediated by a PLC-dependent mechanism. We examined whether Wnt5a-induced tenascin expression was mediated by activation of the transcription factor nuclear factor of activated T cells (NFAT), as has been shown in other cell types [Ma and Wang, 2007]. Inhibition of the NFAT-activating phosphatase calcineurin with the immunosuppressant cyclosporin A (5 μg/mL) demonstrated no attenuation of Wnt5a-induced TnC and TnW expression (Figure 6B).
Figure 6. Role of NFAT and PLC in Wnt5a-induced tenascin expression.
ATnC and TnW induction by Wnt5a in the presence of a phospholipase C inhibitor (U73122, 10 μM). * indicates p < 0.05 compared to treatment-matched control; ** indicates p < 0.01 compared to solvent-matched static. B) TnC and TnW expression induction by Wnt5a in the presence of a cyclosporine A (5 μg/mL). * indicates p < 0.05 compared to vehicle control *** indicates p < 0.001 compared to solvent-matched control. All data are normalized to control gene Rpl13, and to the solvent-matched control. Bars represent mean ± SEM. n≥14.
Mechanical Induction of Tenascin Expression
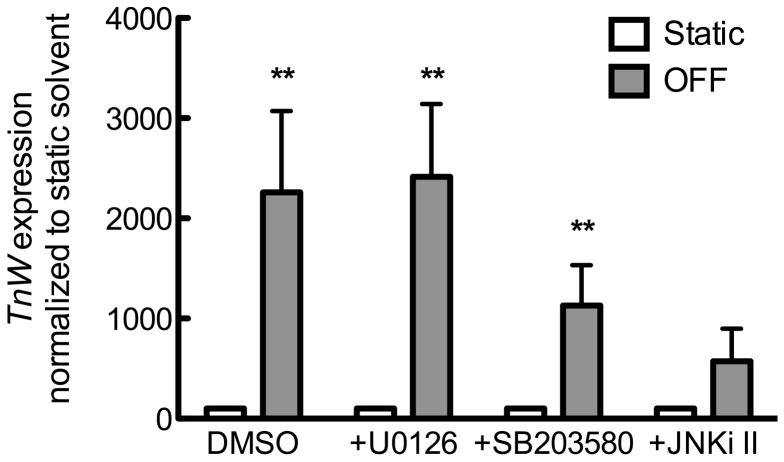
Both BMP signaling and non-canonical Wnt signaling are induced by fluid flow across osteoblastic or mesenchymal cells [Lau et al., 2006; Arnsdorf, 2009]. Thus, we next sought the effect of oscillatory fluid flow on tenascin expression. MC3T3-E1 cells were exposed to oscillatory fluid flow at 15 dynes/cm2 or maintained under static conditions for two hours. RNA was subsequently isolated 0, 6, 12, or 24 hours thereafter. TnC and TnW expression transiently increased following oscillatory fluid flow. TnC demonstrated a significant increase in expression, compared to time-matched static samples, at 6 or 12 hours post-flow, but returned toward baseline levels after 24 hours (Figure 7A). TnW expression was significantly increased 12 hours after oscillatory fluid flow (Figure 7A).
Figure 7. Mechanical load induces tenascin expression.
A) TnC and TnW induced after a two hour exposure to 15 dynes/cm2 oscillatory fluid flow. * indicates p < 0.05 compared to time-matched static control. All data are first normalized to the control gene Rpl13 and then to the time-matched static control. TnC (B) and TnW (C) induction 12 hours post exposure to two hours of oscillatory fluid flow in the presence of inhibitors for the MAP kinases MEK 1/2 (U0126, 10 μM), p38 (SB203580, 10 μM), and JNK (JNKi II, 10 μM). * indicates p < 0.05 compared to solvent-matched static. All data are normalized to control gene Rpl13, then to the solvent-matched control. Bars represent mean ± SEM. n≥5.
We, and others, have previously demonstrated that MAP kinase activation is required for oscillatory fluid flow induced changes in expression matrix proteins such as osteopontin [You et al., 2001] and collagen 1 [Wu et al., 2006]. To test whether the same is true for oscillatory fluid flow-induced TnC or TnW, MC3T3-E1 osteoblasts were exposed to oscillatory fluid flow for two hours in the presence of individual MAPK antagonists, and RNA was collected 12 hours later. Activation of p38, but not MEK1/2 or JNK, was required for oscillatory fluid flow induction of TnC, whereas TnW induction required JNK, but not p38 or MEK1/2 (Figure 7B).
DISCUSSION
Tenascins modulate osteoblast differentiation and proliferation, and are implicated in skeletal-associated pathologies including breast cancer, giant cell tumors of bone, and osteoarthritis [Hasegawa et al., 2004; Pazzaglia et al., 2010; Yoshida et al., 1995]. Tenascins also play an important role in osteoclast adhesion and fracture repair as illustrated by inappropriate resorption and excessive bone production during fracture repair in the TnC knockout mouse [Alford and Hankenson, 2006]. In vivo adult tenascin expression is limited to the bone surface and does not extend into the cortex [Mackie et al., 1987; Scherberich et al., 2004]. Consistent with these observations we observed increased transcript levels for both TnC and TnW in the osteoblastic cell line, MC3T3-E1, compared to the osteocytic cell line, MLO-Y4 (Figure 1A). Tenasin C [Mackie and Ramsey, 1996] and W [Meloty-Kapella et al., 2008] are known to induce osteoblastic differentiation, yet the levels of endogenous expression during osteoblastic differentiation are unclear. In the current study we observed decreasing TnW levels during the course of osteoblastic differentiation (Figure 1C). This contrasts with earlier reports of increasing TnW expression in clonal myoblast and osteoprogenitor lineages [Mikura et al., 2009; Scherberich et al., 2004]. This discrepancy is possibly due to the less mature nature of the C2C12 and Kusa-A1 cell lines, or the use of BMP-2 to induce osteogenic differentiation, as both this study and others have shown that BMP-2 itself induce TnW [Scherberich et al., 2005]. In our study, TnC levels increased during osteogenic differentiation until day 14, and decreased thereafter (Figure 1C). The decreasing trend in tenascin expression by day 21 is consistent with decreased tenascin expression as cells further differentiate towards osteocytes. Thus we believe our in vitro data accurately reflect in vivo observations, and also establish the MC3T3-E1 pre-osteoblastic cell line as a viable model for the study of osteoblastic tenascin expression.
While several studies have established the importance of tenascins in regulating osteoblast adhesion and ECM interactions, relatively little is known about the regulation of skeletal tenascin expression. This study found a variety of physiologic stimuli including BMPs, Wnts, and mechanical stimulation induced increases in osteoblast expression of TnC and TnW through distinct signaling mechanisms. BMPs are some of the earliest and most well described osteoinductive proteins. BMP-2 has implications developmentally [Wan and Cao, 2005] and clinically [Axelrad and Einhorn, 2009]. We found that BMP-2 induces TnC and TnW (Figure 2A). A previous report [Guicheux et al., 2003] found that BMP-2 treatment of MC3T3-E1 cells induced activation of p38, JNK, and to a much lesser extent, ERK1/2. Consistent with these findings the MEK 1/2 inhibitor, immediately upstream of ERK 1/2, did not affect BMP-2 induced TnC or TnW expression in our study. In our hands inhibition of p38 prevented BMP-2 induced TnC and TnW, while Jnk inhibition only prevented the induction of TnC (Figure 2B). These findings greatly expand on a previous study by [Scherberich et al., 2005] which identified p38 as an important mediator of BMP-2 induced TnW in fibroblasts. The independent regulation of tenascin C by JNK provides a potential explanation for the distinct but overlapping expression patterns of tenascin C and W. Based on these findings we conclude that BMP-2 induced TnC expression is driven by both p38 and JNK signaling while BMP-2 induced TnW is driven by only the p38 branch of MAPK signaling (Figure 8). We have not ruled out additional contribution by the traditional BMP activation of Smad signaling. Previous work suggests that while TGF-β-induced Smad signaling drives TnC expression [Jinnin et al., 2004], Smad1/5/8 activation in pulmonary artery smooth muscle cells is inhibitory to TnC [Ihida-Stansbury et al., 2006]. The role of Smads in tenascin W expression has yet to be examined. However, that BMP-2-induced TnW expression required de novo protein synthesis (detailed below) suggests that Smads do not directly induce TnW transcription. While the role of Smad signaling in tenascin expression is an important area of future research, this study highlights the contribution of MAPK signaling in non-canonical BMP regulation of tenascin expression in osteoblastic cells.
Figure 8. Working model of osteoblast tenascin induction.

A) BMP-2 induces both Smad and MAPK signaling which can drive tenascin expression. We found that p38 and JNK drive TnC expression. We propose p38 and Smads drive TnW expression through a protein intermediate, represented by X. B) Wnt5a induces TnW through p38 and requires a protein intermediate, represented here by Y. We believe that Wnt5a induced TnC occurs through a PLC-dependent pathway independent from NFAT. C) Oscillatory fluid flow induces MAPK signaling, and JNK mediated the induction of TnW, while p38 mediated the TnC response.
Wnts are also critical regulators of bone density. Both canonical Wnt co-receptors Lrp5/6 and the non-canonical Wnt co-receptor Ror2 regulate bone mass [Gong et al., 2001; Liu et al., 2007]. Thus we were interested in their role in the regulation of TnC and TnW. Both canonical (Wnt3a) and non-canonical (Wnt5a) Wnts increased TnC and TnW (Figure 4A–C), although induction of expression was more rapid (6 versus 24 hours) and greater in Wnt5a- versus Wnt3a-treated cells. Similar to BMP-2, Wnt5a induces p38 activation (Figure 5A), which is a critical step in Wnt5a-induced TnW expression, as inhibition of p38 abolished the Wnt5a induction of TnW. Conversely, Wnt5a-induction of TnC was independent of MAPK signaling (Figure 5). Wnt5a stimulates phospholipase C (PLC) through a G-protein-dependent mechanism [Kühl et al., 2000]. We found that inhibition of PLC abolished the induction of TnC by Wnt5a (Figure 6A). This suggests that while TnW is induced by Wnt5a through a p38-dependent mechanism, TnC induction requires activation of PLC. One down-stream transcription factor from this Wnt5a/PLC pathway, NFAT, was investigated, but inhibition of NFAT signaling did not block TnC induction (Figure 6B). Thus we believe TnC is induced by a PLC-dependent pathway that is independent of NFAT. One candidate is the transcription factor ATF2 which is activated by Wnt5a [Ma and Wang, 2007] signaling and important in skeletal growth [Luvalle et al., 2003].
In order to target growth factor pathways to regulate tenascins, it would be important to understand if growth factor- induced tenascin expression is a direct effect of the growth hormone or mediated by a protein intermediate. The increases in TnW by either BMP-2 or Wnt5a was blocked by protein synthesis inhibitors, indicating that induction of TnW requires a protein intermediate rather than being a direct target of the growth factors. TnC, but not TnW, was induced by protein synthesis inhibitors alone (Figure 2B and 4D). TnC super-induction in response to CHX was also observed in chick dermal fibroblasts [Chiquet et al., 2004; Jinnin et al., 2006]. TnC super-induction likely results from the absence of an inhibitory protein which normally functions to decrease TnC transcript; inhibition of protein synthesis would, in turn, prevent expression of this repressor, thereby inducing tenascin expression. Ghatnekar and Trojanowska recently demonstrated that constitutively-expressed GATA-6 represses basal and TGF-β1-induced TnC expression in fibroblasts [Ghatnekar and Trojanowska, 2008]. Our data suggests that TnC is regulated by a labile repressor, although its induction could still require protein synthesis. We did not observe enhanced transcript expression in the presence of growth factors and CHX, possibly because the induction in response to CHX was greater than to growth factor alone. Understanding endogenous inhibitors of tenascin expression could provide another promising target for intervention and induction of tenascin expression.
As the skeleton experiences constant mechanical loading in vivo it is important to understand the role of mechanical load on tenascin expression. Mechanical load could potentially modulate tenascin expression directly or through the local release of a variety of growth factors. TnC and TnW were both induced by the application of oscillatory fluid flow to simulate the shear stress experienced by bone cells during locomotion (Figure 7A). These data support a role for fluid flow as a mechanism to explain previously reported increases in tenascin expression in loaded murine long bone [Webb et al., 1997]. Previous reports also support a role for mechanical regulation of TnC expression in fibroblasts and myocytes [Chiquet et al., 2004; Fluck et al., 2000; Mikic et al., 2000; Yamamoto et al., 1999]. To our knowledge this is the first report of mechanically-induced TnW expression. Previous reports illustrate the capacity of shear stress to induce MAPK signaling in osteoblasts [Wu et al., 2006; You et al., 2001]. We found that p38 inhibition decreased the magnitude of mechanically-induced TnC (Figure 7B). A similar trend was observed in TnW, although it did not reach statistical significance (Figure 7C). JNK also plays a role in mechanically induced TnW (Figure 7C). These data support a differential role for the MAP kinases in creating distinct expression mechanisms for the two tenascins.
Consistent with our hypothesis that growth factors and local biophysical signals would regulate tenascin expression in osteoblasts, this study found that TnC and TnW were induced by BMP-2, Wnt5a, and oscillatory fluid flow. Figure 8 presents a working model for regulation of tenascin expression by these various stimuli. TnC and TnW were distinctly regulated by each stimulus, which is consistent with the overlapping but unique expression patterns observed in vivo. Further, Each isoform of tenascin had multiple signaling pathways capable of inducing its expression. The variety of signaling pathways that converge upon TnC and TnW expression supports the notion of a vital role for tenascins in the vertebrate skeleton. Future studies are needed to understand the role of tenascin in mediating some of the long-term effects of these stimuli on osteoblast behavior. Tenascins are known to induce osteoblast differentiation, but whether BMP-2-induced TnC is required for BMP-2-driven osteogenesis has yet to be investigated. Based on the findings of this study we can conclude that these physiologic stimuli regularly adjust tenascin expression. Future studies are needed to examine the role of these stimuli in tenascin regulation in vivo as well as the clinical implications of this increase in tenascin expression.
Acknowledgments
Grant sponsor: NIH NIA AG22305 (CEY) and NIAMS R057547 (DCG).
The authors would like to thank Kevin Ip for his assistance in sample collection and RNA isolation. This work was supported by the UC Davis Clinical and Translational Science Center T32 Predoctoral Clinical Research Training Program.
References
- Alford AI, Hankenson KD. Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS ONE. 2009;4:e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad TW, Einhorn TA. Bone morphogenetic proteins in orthopaedic surgery. Cytokine & Growth Factor Reviews. 2009;20:481–488. doi: 10.1016/j.cytogfr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Bristow J, Carey W, Egging D, Schalkwijk J. Tenascin-X, collagen, elastin, and the Ehlers–Danlos syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2005;139C:24–30. doi: 10.1002/ajmg.c.30071. [DOI] [PubMed] [Google Scholar]
- Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J. Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet. 1997;17:104–8. doi: 10.1038/ng0997-104. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Tenascins. The International Journal of Biochemistry & Cell Biology. 2004;36:986–990. doi: 10.1016/j.biocel.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. The Journal of Pathology. 2003;200:488–499. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Sarasa-Renedo A, Tunç-Civelek V. Induction of tenascin-C by cyclic tensile strain versus growth factors: distinct contributions by Rho/ROCK and MAPK signaling pathways. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2004;1693:193–204. doi: 10.1016/j.bbamcr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119:2538–49. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M, Tunc-Civelek V, Chiquet M. Rapid and reciprocal regulation of tenascin-C and tenascin-Y expression by loading of skeletal muscle. J Cell Sci. 2000;113:3583–3591. doi: 10.1242/jcs.113.20.3583. [DOI] [PubMed] [Google Scholar]
- Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng. 1988;32:1053–60. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- Genetos DC, Wong A, Watari S, Yellowley CE. Hypoxia Increases Annexin A2 Expression in Osteoblastic Cells via VEGF and ERK. Bone. 2010;47:1013–1019. doi: 10.1016/j.bone.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatnekar A, Trojanowska M. GATA-6 is a novel transcriptional repressor of the human Tenascin-C gene expression in fibroblasts. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2008;1779:145–151. doi: 10.1016/j.bbagrm.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GCM, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard M-J, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL Receptor-Related Protein 5 (LRP5) Affects Bone Accrual and Eye Development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 Mitogen-Activated Protein Kinase and c-Jun-NH2-Terminal Kinase by BMP-2 and Their Implication in the Stimulation of Osteoblastic Cell Differentiation. Journal of Bone and Mineral Research. 2003;18:2060–2068. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Hirata H, Sudo A, Kato K, Kawase D, Kinoshita N, Yoshida T, Uchida A. Tenascin-C concentration in synovial fluid correlates with radiographic progression of knee osteoarthritis. The Journal of Rheumatology. 2004;31:2021–2026. [PubMed] [Google Scholar]
- Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. TOPGAL Mice Show That the Canonical Wnt Signaling Pathway Is Active During Bone Development and Growth and Is Activated by Mechanical Loading In Vitro. Journal of Bone and Mineral Research. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- Ihida-Stansbury K, McKean DM, Lane KB, Loyd JE, Wheeler LA, Morrell NW, Jones PL. Tenascin-C is induced by mutated BMP type II receptors in familial forms of pulmonary arterial hypertension. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2006;291:L694–L702. doi: 10.1152/ajplung.00119.2006. [DOI] [PubMed] [Google Scholar]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential Effect of Steady versus Oscillating Flow on Bone Cells. Journal of Biomechanics. 1998;31:969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Tenascin-C upregulation by transforming growth factor-[beta] in human dermal fibroblasts involves Smad3, Sp1, and Ets1. Oncogene. 2004;23:1656–1667. doi: 10.1038/sj.onc.1207064. [DOI] [PubMed] [Google Scholar]
- Jinnin M, Ihn H, Asano Y, Yamane K, Trojanowska M, Tamaki K. Upregulation of Tenascin-C Expression by IL-13 in Human Dermal Fibroblasts via the Phosphoinositide 3-kinase//Akt and the Protein Kinase C Signaling Pathways. J Invest Dermatol. 2006;126:551–560. doi: 10.1038/sj.jid.5700090. [DOI] [PubMed] [Google Scholar]
- Kido S, Kuriwaka-Kido R, Umino-Miyatani Y, Endo I, Inoue D, Taniguchi H, Inoue Y, Imamura T, Matsumoto T. Mechanical Stress Activates Smad Pathway through PKC to Enhance Interleukin-11 Gene Transcription in Osteoblasts. PLoS ONE. 2010;5:e13090. doi: 10.1371/journal.pone.0013090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian O, Dahse R, Alt V, Zardi L, Hentschel J, Schnettler R, Kosmehl H. mRNA Expression and Protein Distribution of Fibronectin Splice Variants and High-Molecular Weight Tenascin-C in Different Phases of Human Fracture Healing. Calcified Tissue International. 2008;83:101–111. doi: 10.1007/s00223-008-9156-z. [DOI] [PubMed] [Google Scholar]
- Kimura H, Akiyama H, Nakamura T, de Crombrugghe B. Tenascin-W inhibits proliferation and differentiation of preosteoblasts during endochondral bone formation. Biochem Biophys Res Commun. 2007;356:935–41. doi: 10.1016/j.bbrc.2007.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozawa O, Hatakeyama D, Yoshida M, Kamiya Y, Kondo C, Matsuno H, Uematsu T. Activation of p44/p42 Mitogen-Activated Protein Kinase Limits Triiodothyronine-Stimulated Alkaline Phosphatase Activity in Osteoblasts. Biochemical and Biophysical Research Communications. 2001;286:1140–1143. doi: 10.1006/bbrc.2001.5515. [DOI] [PubMed] [Google Scholar]
- Kühl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends in Genetics. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Kurihara K, Sato I. Distribution of tenascin-C and -X, and soft X-ray analysis of the mandibular symphysis during mandible formation in the human fetus. Okajimas Folia Anat Jpn. 2004;81:49–55. doi: 10.2535/ofaj.81.49. [DOI] [PubMed] [Google Scholar]
- Lau KH, Kapur S, Kesavan C, Baylink DJ. Up-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in C57BL/6J osteoblasts as opposed to C3H/HeJ osteoblasts in part contributes to the differential anabolic response to fluid shear. J Biol Chem. 2006;281:9576–88. doi: 10.1074/jbc.M509205200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bodine PV, Billiard J. Ror2, a novel modulator of osteogenesis. J Musculoskelet Neuronal Interact. 2007;7:323–4. [PubMed] [Google Scholar]
- Luvalle P, Ma Q, Beier F. The Role of Activating Transcription Factor-2 in Skeletal Growth Control. J Bone Joint Surg Am. 2003;85:133–136. doi: 10.2106/00004623-200300002-00018. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang H-y. Mitogen-activated Protein Kinase p38 Regulates the Wnt/Cyclic GMP/Ca2+ Non-canonical Pathway. Journal of Biological Chemistry. 2007;282:28980–28990. doi: 10.1074/jbc.M702840200. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Ramsey S. Modulation of osteoblast behaviour by tenascin. J Cell Sci. 1996;109 ( Pt 6):1597–604. doi: 10.1242/jcs.109.6.1597. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Thesleff I, Chiquet-Ehrismann R. Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol. 1987;105:2569–79. doi: 10.1083/jcb.105.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsell R, Einhorn TA. The role of endogenous bone morphogenetic proteins in normal skeletal repair. Injury. 2009;40:S4–S7. doi: 10.1016/S0020-1383(09)70003-8. [DOI] [PubMed] [Google Scholar]
- Meloty-Kapella CV, Degen M, Chiquet-Ehrismann R, Tucker RP. Effects of tenascin-W on osteoblasts in vitro. Cell Tissue Res. 2008;334:445–55. doi: 10.1007/s00441-008-0715-4. [DOI] [PubMed] [Google Scholar]
- Mikic B, Wong M, Chiquet M, Hunziker EB. Mechanical modulation of tenascin-C and collagen-XII expression during avian synovial joint formation. Journal of Orthopaedic Research. 2000;18:406–415. doi: 10.1002/jor.1100180312. [DOI] [PubMed] [Google Scholar]
- Mikura A, Okuhara S, Saito M, Ota M, Ueda K, Iseki S. Association of tenascin-W expression with mineralization in mouse calvarial development. Congenit Anom (Kyoto) 2009;49:77–84. doi: 10.1111/j.1741-4520.2009.00227.x. [DOI] [PubMed] [Google Scholar]
- Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Current Opinion in Pharmacology. 10:322–330. doi: 10.1016/j.coph.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Patil C, Zhu X, Rossa C, Kim YJ, Kirkwood KL. p38 MAPK Regulates IL-1β Induced IL-6 Expression Through mRNA Stability in Osteoblasts. Immunological Investigations. 2004;33:213–233. doi: 10.1081/imm-120034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazzaglia L, Conti A, Chiechi A, Novello C, Magagnoli G, Astolfi A, Pession A, Krenacs T, Alberghini M, Picci P, Benassi MS. Differential gene expression in classic giant cell tumours of bone: Tenascin C as biological risk factor for local relapses and metastases. Histopathology. 2010;57:59–72. doi: 10.1111/j.1365-2559.2010.03597.x. [DOI] [PubMed] [Google Scholar]
- Pilbeam CC, Harrison JR, Raisz LG. In: Prostaglandins and bone metabolism. Blilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology; Elsevier: 2002. pp. 979–994. [Google Scholar]
- Robling AG, Castillo AB, Turner CH. Biomechanical and Molecular Regulation of Bone Remodeling. Annual Review of Biomedical Engineering. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- Scherberich A, Tucker RP, Degen M, Brown-Luedi M, Andres AC, Chiquet-Ehrismann R. Tenascin-W is found in malignant mammary tumors, promotes alpha8 integrin-dependent motility and requires p38MAPK activity for BMP-2 and TNF-alpha induced expression in vitro. Oncogene. 2005;24:1525–32. doi: 10.1038/sj.onc.1208342. [DOI] [PubMed] [Google Scholar]
- Scherberich A, Tucker RP, Samandari E, Brown-Luedi M, Martin D, Chiquet-Ehrismann R. Murine tenascin-W: a novel mammalian tenascin expressed in kidney and at sites of bone and smooth muscle development. J Cell Sci. 2004;117:571–81. doi: 10.1242/jcs.00867. [DOI] [PubMed] [Google Scholar]
- Secreto FJ, Hoeppner LH, Westendorf JJ. Wnt signaling during fracture repair. Curr Osteoporos Rep. 2009;7:64–9. doi: 10.1007/s11914-009-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist MR. Bone: Formation by Autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Wan M, Cao X. BMP signaling in skeletal development. Biochemical and Biophysical Research Communications. 2005;328:651–657. doi: 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- Webb CM, Zaman G, Mosley JR, Tucker RP, Lanyon LE, Mackie EJ. Expression of tenascin-C in bones responding to mechanical load. J Bone Miner Res. 1997;12:52–8. doi: 10.1359/jbmr.1997.12.1.52. [DOI] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Wozney JM. The bone morphogenetic protein family and osteogenesis. Mol Reprod Dev. 1992;32:160–7. doi: 10.1002/mrd.1080320212. [DOI] [PubMed] [Google Scholar]
- Wu CC, Li YS, Haga JH, Wang N, Lian IY, Su FC, Usami S, Chien S. Roles of MAP kinases in the regulation of bone matrix gene expressions in human osteoblasts by oscillatory fluid flow. J Cell Biochem. 2006;98:632–41. doi: 10.1002/jcb.20697. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Dang QN, Kennedy SP, Osathanondh R, Kelly RA, Lee RT. Induction of Tenascin-C in Cardiac Myocytes by Mechanical Deformation. Journal of Biological Chemistry. 1999;274:21840–21846. doi: 10.1074/jbc.274.31.21840. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ishihara A, Hirokawa Y, Kusakabe M, Sakakura T. Tenascin in breast cancer development -- is epithelial tenascin a marker for poor prognosis? Cancer Letters. 1995;90:65–73. doi: 10.1016/0304-3835(94)03679-d. [DOI] [PubMed] [Google Scholar]
- You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276:13365–71. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- Zhao G-Q. Consequences of knocking out BMP signaling in the mouse. genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]