Abstract
The Vitamin D3 Receptor (VDR) is present in all microenvironments of the breast, yet is hypothesized to signal through the epithelium to regulate hormone induced growth and differentiation. However, the influence or contribution of the other microenvironments within the breast that express VDR, like the breast adipose tissue, have yet to be investigated. We hypothesized that the breast adipocytes express the signaling components necessary to participate in vitamin D3 synthesis and signaling via VDR, modulating ductal epithelial cell growth and differentiation. We utilized human primary breast adipocytes and VDR wild type (WT) and knockout (KO) mice to address whether breast adipocytes participate in vitamin D3-induced growth regulation of the ductal epithelium. We report in this study that breast primary adipocytes express VDR, CYP27B1 (1α-hydroxylase, 1α-OHase), the enzyme that generates the biologically active VDR ligand, 1α,25-dihydroxyvitamin D3 (1,25D3), and CYP24 (24- hydroxylase, 24-OHase), a VDR-1,25D3 induced target gene. Furthermore, the breast adipocytes participate in bioactivating 25-hydroxyvitamin D3 (25D3) to the active ligand, 1,25D3, and secreting it to the surrounding microenvironment. In support of this concept, we report that purified mammary ductal epithelial fragments (organoids) from VDR KO mice, co-cultured with WT breast adipocytes, were growth inhibited upon treatment with 25D3 or 1,25D3 compared to vehicle alone. Collectively, these results demonstrate that breast adipocytes bioactivate 25D3 to 1,25D3, signal via VDR within the adipocytes, and release an inhibitory factor that regulates ductal epithelial cell growth, suggesting that breast adipose tissue contributes to vitamin D3-induced growth regulation of ductal epithelium.
Keywords: Vitamin D receptor, mammary gland, 1α-hydroxylase, breast adipose tissue
INTRODUCTION
The mammary gland consists of various microenvironments that contribute and crosstalk to maintain ductal epithelial cell homeostasis via communication through the extracellular matrix [McCave et al., 2010]. The epithelial environment has been investigated very extensively to understand the response of the epithelial cells to various hormones and growth factors. Conversely, the stromal environment, which is made up of various components, including fibroblasts, blood vessels, inflammatory cells, preadipocytes and adipocytes [Wiseman and Werb, 2002], has received less investigation and is only vaguely understood. Adipocytes are highly abundant within the stroma of the mammary gland and undergo extensive remodeling during lactation and involution [Neville et al., 1998]. The adipocytes are also believed to participate as an endocrine store house [Halberg et al., 2008] contributing various adipocyte–derived factors (adipokines) including leptin, adiponectin, hepatocyte growth factor (HGF), collagen VI, interleukin-6 (IL-6), and tumor necrosis factor alpha (TNFα) [Trujillo and Scherer, 2006]. There are numerous reports suggesting that the influence of these adipokines to regulate transformed cell growth would also likely contribute to modulate growth signaling during normal mammary gland development. The stromal microenvironment, in addition to providing growth promoting cytokines, also secretes growth inhibitory factors, like transforming growth factor beta (TGFβ) [Daniel and Robinson, 1992; Daniel et al., 1989], that regulate mammary gland development, and likely others that have yet to be discovered.
Vitamin D3 Receptor (VDR) is expressed in various microenvironments of the mammary gland, including the epithelium and stromal environments [Zinser et al., 2002], and participates to regulate hormone induced growth and differentiation in vitro and in vivo throughout development [Zinser et al., 2002; Zinser and Welsh, 2004]. Furthermore, VDR complexes with the active ligand, 1α,25-dihydroxyvitamin D3 (1,25D3), to induce cell cycle arrest, differentiation, and apoptosis in breast epithelial cells regulating growth in normal and transformed cells [Flanagan et al., 2003; Jensen et al., 2001; Kemmis et al., 2006; Narvaez and Welsh, 2001; Simboli-Campbell et al., 1996; Zinser et al., 2003]. Kemmis et al., 2006 established that human mammary epithelial (HME) cells express Cyp27B1, 25-hydroxyvitamin D3 1α-hydroxylase (1α-OHase), the enzyme necessary to convert inactive 25- hydroxyvitamin D3 (25D3) to the active ligand 1,25D3, thus sensitizing HME cells to 25D3-induced growth inhibition. The ability of breast epithelial cells to synthesize 1,25D3 locally within the breast epithelium to regulate cellular growth and differentiation is a potential mechanism by which elevated serum 25D3 is associated with a decrease risk of developing breast cancer or metastatic progression [Goodwin et al., 2009; Janowsky et al., 1999]. However, as breast epithelial cells become transformed, the efficiency of 25D3 uptake by the cells and the synthesis to 1,25D3 declines, resulting in transformed cells that only remain growth inhibited by the active ligand 1,25D3 [Kemmis and Welsh, 2008; Rowling et al., 2006]. Therefore, in the face of this epithelial cell transformation phenomenon, we hypothesized that the surrounding microenvironment would also contribute to vitamin D3-induced signaling. Consequently, we investigated the expression of VDR and 1α-OHase in primary human and mouse breast adipocytes to define the contribution that breast adipose tissue has on vitamin D3-induced growth inhibition within the mammary gland.
Ductal development within the mammary gland is dependent on many factors, those contributing to ductal extension or ductal outgrowth and those that regulate branching morphogenesis [Bocchinfuso et al., 2000; Brisken et al., 1998; Gallego et al., 2001; Humphreys et al., 1997; Wiesen et al., 1999; Yant et al., 1998]. In addition there are signaling components that are expressed primarily in the epithelial cells [Brisken et al., 1998; Mallepell et al., 2006], those expressed in the stromal compartments [Gallego et al., 2001; Sternlicht et al., 2005; Weber-Hall et al., 1994; Wiesen et al., 1999; Wiseman and Werb, 2002], as well as those components that are expressed in both microenvironments of the breast [Humphreys et al., 1997; Kleinberg, 1998]. The influence of the mammary adipose tissue to contribute to vitamin D3- induced regulation of breast development and mammary gland homeostasis stemmed from the concept that excess circulating 25D3 is stored within adipose tissue [Rosenstreich et al., 1971]. Therefore, the synthesis of 1,25D3 occurring locally within the breast tissue would likely regulate breast epithelial cell growth and differentiation, maintaining glandular homeostasis without disrupting systemic calcium levels.
We report in this study that primary breast adipocytes express the signaling components necessary to participate in vitamin D3 synthesis and signal via VDR to contribute to vitamin D3-induced regulation of epithelial cell growth in response to hormone stimulation. The breast adipocytes bioactivate 25D3 to the active ligand 1,25D3 and secrete it to the surrounding microenvironment. Furthermore, the breast adipocytes participate in autocrine/paracrine signaling, upregulating 24-hydroxylase (24-OHase) expression, a VDR-1,25D3 induced target gene, suggesting that breast adipocytes play an active role in vitamin D3-induced signaling within the breast. We provide additional support for adipocyte contribution to vitamin D3-modulated growth, presenting data that suggests VDR wild type (WT) mouse preadipocytes are also capable of bioactivating 25D3 to 1,25D3, signaling via VDR to modulate gene expression, and secreting a growth inhibitory component sufficient to regulate the growth of VDR knockout (KO) ductal epithelial fragments (organoids) using an ex vivo co-culture model. Thus, we report that the adipose tissue likely plays a more active role in vitamin D3 synthesis and signaling that requires further investigation to better comprehend the complexity of this stromal/epithelial interaction.
MATERIALS AND METHODS
Animal Maintenance
Wild type and VDR KO mice on the C57Bl6 background were weaned onto and continuously maintained on a “rescue” diet containing 2% calcium, 1.25% phosphorous, and 20% lactose with 2.2 IU vitamin D3/g (TD96348, Teklad, Madison, WI). This diet prevents the mineral disturbances and impaired growth associated with VDR ablation [Li et al., 1998]. All procedures were approved by the University of Cincinnati institutional animal care and use committee.
Cells and Cell Culture
Breast preadipocyte cell lines were purchased from Zen-Bio, Inc. Specifically, these preadipocytes were isolated from women having breast reduction procedures that had a body mass index between 20–30 kg/m2. The breast preadipocytes were maintained in preadipocyte medium (PM-1, Zen-Bio) at a sub-confluent density. To produce mature adipocytes, preadipocytes were plated and allowed to reach confluence. Differentiation media (DM-2, Zen-Bio) was added and cells were allowed to undergo differentiation for 7–14 days prior to assays. Differentiated adipocytes were maintained in adipocyte medium (AM-1, Zen-Bio) for an additional 5–7 days. By day 14, a majority of the adipocytes contained large lipid droplets and were considered mature adipocytes.
Human mammary epithelial (HME) cells were maintained in Medium 171 supplemented with Mammary Epithelial Growth Supplement (MEGS) containing 0.4% bovine pituitary extract, 5mg/L bovine insulin, 0.5mg/L hydrocortisone, and 3μg/L human epidermal growth factor (Invitrogen). MCF-7 breast cancer cells were maintained in Improved MEM media containing 5% FBS. All cells were cultured at 37°C and 5% CO2 in a humidified incubator and passaged every 3–4 days except for the preadipocytes which were passaged once every other week for a maximum of seven passages.
Quantification of Lipid Content
Lipid content was determined using the Oil Red O assay. Preadipocytes were plated and allowed to grow until confluent at which time half the plate was placed into differentiation media (DM-2) as described above. The adipocytes were treated with vehicle (ethanol) or various concentrations of 25D3 or 1,25D3. Media with treatment was changed every 3–4 days. After day 7 and day 14 of the various media changes and treatments, the plates were harvested and lipid content was assessed by Oil Red O staining. Preadipocytes or mature adipocytes were washed in PBS three times, fixed with 10% formalin for 30 minutes and then stained in 10% Oil Red O for 20 minutes. Oil Red O stain was visualized and imaged in addition to being eluted from the plate with 100% isopropanol to quantitate the absorbance at 500nm.
Mammary Gland Microenvironment Isolation and Co-culture
For protein and RNA isolation from individual microenvironments of the mammary gland, female mice were sacrificed at 3–4 weeks of age and inguinal mammary glands were harvested. Total mammary gland, containing ductal epithelium and mammary fat, was isolated from the nipple region up to the lymph node within inguinal mammary glands. To ensure that the mammary fat pad microenvironment isolation did not contain ductal epithelium, fat pad on the medial side of the lymph node, clear of ductal epithelium at 4 weeks of age was harvested for protein and RNA assessment. To harvest purified ductal epithelium, female mice were sacrificed at 8–10 weeks of age and total mammary glands were collected. The glands were placed into a digestion media and purified as described [Rudolph et al., 2009]. Briefly, mammary glands from VDR WT or KO mice were minced into 0.5 cm sized chunks of mammary tissue and incubated at 37°C in 0.22μm filtered Digestion Media (25mL of digestion media/8 mammary glands) consisting of DMEM/F12 media (1:1) containing collagenase A (0.5 mg/ml) (Sigma), hyaluronidase (55 μg/ml) (Sigma), insulin (5 μg/ml), nystatin (60 units/ml), pen/strep (100 units/ml) and gentamycin (50 μg/ml) while shaking at 150–200 rpm. Digestion was terminated when the mammary gland had been digested into small ductal epithelial fragments (organoids) (usually within 1–2 hrs). After centrifugation (400g, 4°C, 5mins), pellets containing fibroblast cells, red blood cells, and epithelial organoids were resuspended in PBS containing 5% FBS and washed 5 times using pulse spins (800–900 rpm) to eliminate red blood cells and fibroblasts which remain in the supernatant media of the wash. Final pellets, containing ductal organoids were placed into protein lysis buffer or TRI Reagent (Molecular Research Center, Inc.) to isolate protein or RNA from the purified epithelial clusters.
To harvest purified ductal organoids for co-culture assays, female mice (2 WT and KO) were sacrificed at 8–10 weeks of age and total mammary glands were harvested making sure to extract the lymph nodes in inguinal glands prior to digestion. After digestion, centrifugation, and purification rinses, the final pellets were then mixed with Cellmatrix Type 1A collagen for ex vivo culture and co-culture assays. Cellmatrix Type 1A collagen was prepared according to manufacture instructions (Waco Chemicals USA). Ductal organoid pellets were resuspended in Cellmatrix Type 1A collagen and plated in 24-well plates (300μl/well). The plated collagen matrix was incubated at 37° for 30 minutes prior to the addition of organoid growth media to allow the collagen to solidify. Organoid growth media consisted of HME media prepared as described above and supplemented to produce media containing the following components 20mM HEPES, 5 μg/ml linoleic acid, 1 mg/ml BSA (fraction V), and 10 μg/ml Insulin. Organoids were cultured overnight prior to cell treatments. Treatments in duplicate wells consisted of vehicle (ethanol, Et), 30nM 17β-estradiol (E), 60nM Progesterone (P), 10 ng/ml epidermal growth factor (EGF), 250nM 25D3, and 100nM 1,25D3 as individual treatments and combination treatments in order to define the impact of 25D3 and 1,25D3 to regulate hormone and growth factor induced organoid growth. The combination treatments consisted of an E/25D3, P/25D3, E/P/25D3, E/1,25D3, P/1,25D3, E/P/1,25D3 and EGF/1,25D3 cultured for 72hrs and retreated for an additional 48–72hrs.
Co-culture assays were conducted by resuspending preadipocytes in Cellmatrix Type 1A collagen and plating the preadipocyte collagen as a basement matrix. The preadipocytes were allowed to grow until reaching 50–60% confluence within the collagen. The preadipocytes were then pretreated with the respective treatments listed above for 48hrs prior to the addition of the organoid (WT or KO) collagen matrix that would rest on top of the preadipocyte matrix. Prior to adding the second collagen layer, the treatment media was removed to ensure that the organoid collagen layer adheres to the preadipocyte collagen layer below. Co-cultured cells were treated 24hrs after organoid plating and treated for a similar duration as described above.
Morphology and Immunofluorescence
For immunodetection of cell specific markers, cells grown on chamber slides (5 × 103 cells/well) were formalin fixed, permeabilized in ice cold methanol, pre-blocked with 1%BSA/PBS and incubated with a rat monoclonal VDR antibody (clone 9A7, Thermo Scientific) or a sheep 25-hydroxyvitamin D3- 1α-hydroxylase (PC290, The Binding Site) antibody for 1hr at 37°C. After washing in PBS, cells were incubated with anti-rat conjugated ALEXA-488 or anti-sheep secondary antibodies. The 1α-OHase was visualized using the ABC tertiary complex (Vector Laboratories) and incubation with DAB. After washing in PBS, cover slips were applied with an anti-fade reagent containing DAPI or counterstained with Harris modified Hematoxylin (Fisher Scientific) and images were captured.
Western Blotting
Human breast preadipocytes were plated in 100mm dishes (5×105 cells/dish) and maintained as preadipocytes or induced to differentiate into mature adipocytes. Differentiation of preadipocytes was accomplished using differentiation media from Zen Bio, Inc. for at least 10 days prior to harvest. Cell monolayers of preadipocytes or mature adipocytes were harvested by scraping in 250μl of 1x Laemmli buffer containing protease and phosphatase inhibitors (1mM dithiothreitol, 10mM benzamidine, 1mM sodium orthovanadate, 25 μg/ml leupeptin, 25 μg/ml aprotinin, 25 μg/ml pepstatin, and 1mM phenylmethylsulfonyl fluoride). Total protein was analyzed by the Micro BCA assay (Pierce). Cell lysates (100μg protein/lane) were separated by SDS-PAGE, transferred to nitrocellulose and immunoblotted with primary antibodies directed against the VDR (clone 9A7γ, Thermo Scientific), 1α-OHase (The Binding Site), Perilipin (Cell Signaling), Cytokeratin 18 (Epitomics), and α-tubulin (Sigma) for 1h room temperature or overnight at 4° C. Specific binding was detected by horseradish peroxidase-conjugated secondary antibodies diluted in PBS/5% skim milk/0.1% Tween 20 and autoradiography with enhanced chemiluminescence (Pierce).
Measurement of 1α-OHase activity
Cells were seeded in six-well culture plates and treated with 1mL of 100nM 25D3 or vehicle (ethanol) in serum free media the following day. Serum free supernatant media was collected after 24h incubation for analysis of 1α-hydroxylated metabolites with the 1,25D3 enzyme immunoassay (EIA) (Immunodiagnostic Systems, Inc.). Assays were conducted according to manufacturer directions for analysis of supernatant 1,25D3. Controls included media alone supplemented with 25D3 to control for cross-reactivity with 25D3 in the EIA. The level of 1,25D3 synthesized and secreted into the supernatant media was measured and expressed as picogram (pg) 1,25D3 produced/ml/106 cells.
Real Time Quantitative PCR
Total RNA was isolated from 90–150mg of total mammary gland or only the mammary fat pad using TRI Reagent. Independent mammary gland RNA preps from three mice of each genotype were made from each of the microenvironments. Ductal epithelial organoids were isolated as described above. Cells were grown to sub-confluence in six-well plates and treated with vehicle (ethanol), 100nM 25D3, or 100nM 1,25D3 for 48h. After concentration and purity of the RNA was determined using a spectrophotometer, total RNA was reverse transcribed using a High Capacity RT kit (Applied Biosystems). Three independent 1.0μg cDNA stocks were generated from each RNA sample, and each was independently analyzed in duplicate (60ng of cDNA/well) using FastStart Universal SYBR green master mix (Roche Diagnostics) and specific primer sets (human VDR forward (For)- 5′-GGCCCAACT-CCAGACACACT -3′, reverse (Rev) – 5′-GGGTCACAGAAGGGTCATCTGA-3′; human 24-OHase For 5′-CAAACCGTGGAAGGCCTATC-3′, Rev- 5′-AGTCTTCCCCTTCCAGGATCAG- 3′. Human 1α-OHase was run using a Taqman probe design with the forward primer sequence of 5′-AGTTGCTATTGGCGGGAGTG-3′, Rev 5′-GTGCCGGGAGAGCTCATACA-3′, and the probe- 5′-ACACGGTGTCCAACACGCTCTCTTGG- 3′; Mouse VDR For 5′-GAAGCGC-AAGGCCCTGTT-3′, Rev 5′-CGCTGCACCTCCTCATCTGT-3′; Mouse 1α-OHase For 5′-GCGGGCTATGCTGGAACTC-3′, Rev 5′-GCACCGCGCCTATACTTTCT-3′; Mouse 24-OHase For 5′-CTGGCCTGGGACACCATTT-3′, Rev 5′-CTCCGTGACAGCAGCGTACA-3′). Gene expression levels were normalized against 18S RNA, and reported as normalized gene equivalence. For data presentation, duplicate values from each run were averaged, and triplicate values were then averaged to generate one value for each animal or cell type. The final data is expressed as the mean ± standard error of three independent tissue isolations or independent cell harvests.
Statistical Evaluation
Data are presented as mean ± standard error, with the number of analyses for each mean indicated. Data were analyzed by Student’s t test, and means were considered significantly different if a p value less than 0.05 was obtained. All statistical evaluations were performed with Instat software (GraphPad Software, Inc., San Diego California USA, www.graphpad.com).
RESULTS
Expression and Localization of Vitamin D3 Signaling components in Human Breast Adipocytes
We hypothesized that breast adipocytes would contribute to vitamin D3-induced growth regulation of mammary epithelial cells by storing, bioactivating and signaling via VDR to modulate cellular growth and differentiation. To begin to address this hypothesis, we first wanted to assure that human primary breast adipocytes, pre and differentiated (mature), expressed the vitamin D3 signaling components necessary to participate in vitamin D3 bioactivation and signaling in response to vitamin D3 exposure. We purchased human breast preadipocytes from Zen-Bio, Inc. to assess the expression of VDR and 1α-OHase, the enzyme needed to convert inactive 25D3 to the biologically active ligand, 1,25D3. Total RNA isolated from preadipocytes and mature adipocytes were assessed for 1α-OHase and VDR expression, normalized to 18S ribosomal RNA and compared to normal human mammary epithelial cells (HME) and MCF-7 cells, a human breast cancer cell line (Figure 1). 1α-OHase expression was present in both pre and mature breast adipocytes similar to MCF-7 cells, yet significantly lower than HME cell expression. In contrast, VDR expression remained similar in pre and mature adipocytes and equivalent to HME expression levels. The protein expression for 1α-OHase and VDR was similar in pre and mature breast adipocytes (Figure 1B) but slightly less than the epithelial cell expression, suggesting a possible change in the stability of the VDR signaling components in each microenvironment of the breast. Ultimately, the expression of VDR and 1α-OHase are present in pre and mature adipocytes.
Figure 1. Expression of Vitamin D3 signaling components in human primary breast adipocytes.
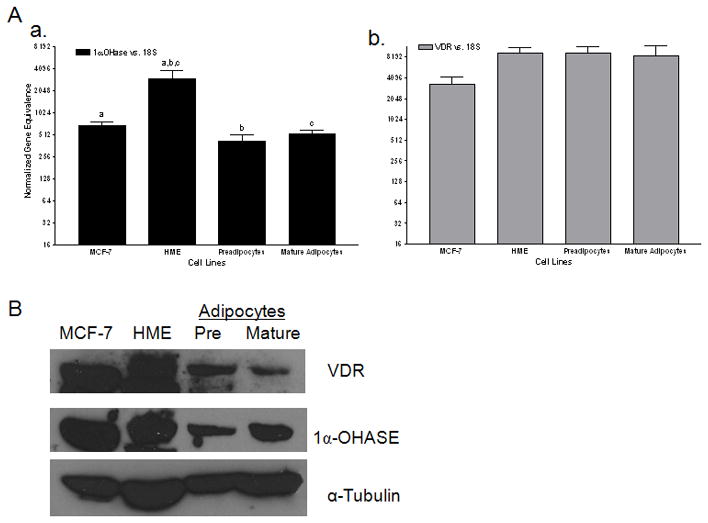
A) Real time PCR for 1α-hydroxylase (1α-OHase) (a) and vitamin D3 receptor (VDR) (b) in MCF-7 human breast cancer cells, non-transformed human mammary epithelial cells (HME), and adipocytes (pre and mature adipocytes). Data are expressed relative to 18S RNA (normalized gene equivalence) and represent mean s.e.m. of triplicate runs. Similar letters above the bars designate significance in the expression of 1α-OHase between cell lines- p<0.05. B) Western blot of VDR and 1α-OHase in MCF-7, HME and adipocytes compared to tubulin, which was used as a loading control.
To assess the localization of VDR and 1α-OHase within adipocytes, we plated preadipocytes on glass chamber slides. Preadipocyte cells, upon reaching 70–80% confluence were formalin fixed and stained for 1α-OHase or VDR. Preadipocyte 1α-OHase expression was shown as brown cytoplasmic staining (Figure 2d) against a blue hematoxylin nuclear counterstain compared to the control which lacked the primary antibody that showed only the blue nuclear counter stain (Figure 2a). Preadipocyte VDR expression was localized to the nucleus, visualized with an Alexa-488 conjugated secondary antibody (Figure 2e) compared with no primary antibody (Figure 2b) and the DAPI staining to show nuclear localization for the control and VDR stained cells (Figure 2c and 2f). We conclude from these experiments that pre and mature adipocytes express the necessary components, which localize to the expected environments within the preadipocytes to participate in vitamin D3 synthesis and signaling.
Figure 2. Localization of VDR and 1α-OHase in human primary breast adipocytes.
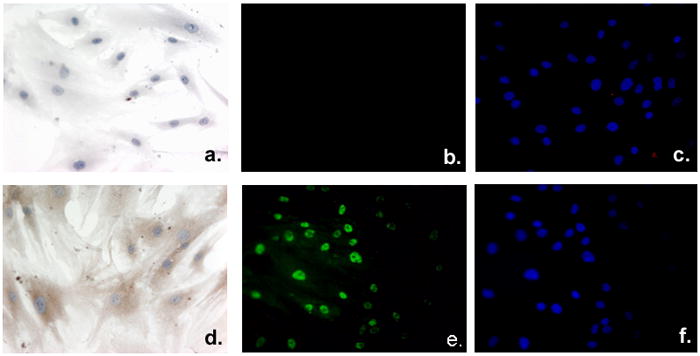
Adipocytes were plated and grown on chamber slides, formalin fixed, and either processed as unstained control cells (a and b) or stained to detect 1α-OHase (d), VDR (e), or the nuclear compartment using DAPI incorporation (c and f). Primary adipocytes were incubated with 1α-OHase (d) or without (a) primary antibody directed against 1α-OHase protein to assess the expression and localization within primary adipocytes. 1α-OHase expression is shown as brown cytoplasmic staining (d) against the blue hematoxylin counterstain (a and d). Immunofluorescent detection of VDR (e) shows nuclear staining within the adipocytes compared to the no primary antibody control (b). Nuclear incorporation of DAPI staining (c and f) of control cells (b) or VDR stained cells (f) emphasizes the nuclear localization of VDR expression.
Primary adipocytes synthesize Vitamin D3 and Signal via VDR
We hypothesized that human pre and mature breast adipocytes bioactivate 25D3 to 1,25D3 and signal through VDR to modulate signaling within the adipocytes. To begin to specifically address whether human preadipocytes or mature adipocytes synthesize Vitamin D3, we utilized an enzyme immunoassay specific for 1,25D3 to assess supernatant media secreted from the cultured adipocytes. Preadipocytes or mature adipocytes were treated for 24h using serum-free media containing either vehicle (ethanol (Et)) or 25D3 (100nM) treatment. Supernatant media was harvested and assayed for 1,25D3 secretion normalized by the number of cells cultured per well. We compared the synthesis of 25D3 in adipocytes to MCF-7, human breast cancer cells, known to be inefficient at bioactivating 25D3 [Rowling et al., 2006] and normal human mammary epithelial cells (HME), known to be very efficient at bioactivating 25D3 to 1,25D3 [Kemmis et al., 2006]. In vehicle treated cells, the level of 1,25D3 synthesized and excreted into the supernatant media from the cells was minimal except for the mature adipocytes which had a slightly elevated basal level of 1,25D3 synthesis (Figure 3A). In the pre and mature breast adipocytes treated with 25D3, the level of 1,25D3 secreted into the supernatant media increased significantly over the vehicle control, producing at least a 15 fold increase in 1,25D3 detected in the supernatant, similar to the HME cells and in contrast to the MCF-7 cells which only showed a slight increase (<5 fold) in bioactivated and secreted 1,25D3 after 25D3 treatment. Thus, pre and mature breast adipocytes are efficient in the uptake and synthesis of 25D3, producing and secreting active vitamin D3 ligand to the surrounding microenvironment.
Figure 3. Vitamin D3 synthesis and signaling within human primary breast adipocytes.
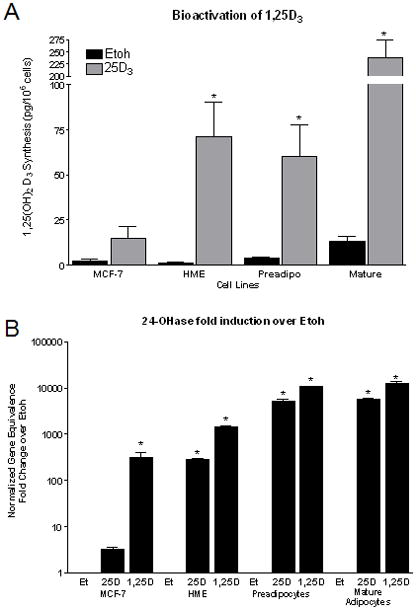
A) Breast cancer cells (MCF-7, negative control), non-transformed human mammary epithelial cells (HME, positive control), preadipocytes and mature adipocytes were treated with vehicle (Etoh) or 25D3 for 24h to assess the ability of breast adipocytes to bioactivate 25D3 to the active form, 1,25D3. Culture media was immunoextracted using a 1,25D3 Enzyme ImmunoAssay. Data are expressed as pg/ml/106 cells. B) Bioactivation and signaling through the VDR to regulate 24-OHase, a vitamin D3 target gene. 24-OHase gene induction by 25D3 and 1,25D3 in primary preadipocytes and mature adipocytes compared to HME cells (positive control) and MCF-7 breast cancer cells (negative control). 24-OHase mRNA expression was normalized to 18S from cultured cells treated for 48h with ethanol (Et), 25D3, or 1,25D3. Vehicle treated cells provided the baseline level of 24-OHase expression and 1,25D3 provided the upper level of 24-OHase expression in each cell line. *- p <0.05 compared to vehicle control in each cell line.
To determine whether the preadipocytes and mature adipocytes not only synthesize but also signal via VDR to regulate 1,25D3/VDR modulated genes, we plated cells and treated with either vehicle, 25D3 (100nM), or 1,25D3 (100nM) for 48h and harvested total RNA to asses gene expression changes in 24-OHase. 24-hydroxylase contains vitamin D3 response elements in its promoter and is very responsive to 1,25D3/VDR- induced gene upregulation. Therefore, we treated human MCF-7 breast cancer cells, which have been shown to be inefficient at bioactivating 25D3 to 1,25D3, thus serving as a negative control.for 24-OHase upregulation with 25D3 treatment. In contrast, HME cells serve as a positive control regarding 25D3 bioactivation and signaling via VDR to upregulate 24-OHase gene expression. As shown in Figure 3B, treatment with the active ligand, 1,25D3, represents full 24-OHase gene induction in all four cell types (500–10000 fold upregulation) and vehicle (ethanol) represents the baseline level of 24- OHase gene expression in each cell line. Pre and mature adipocytes experience a significant fold induction of 24-OHase mRNA expression in response to 25D3 (>5000 fold induction), similar to that seen in the HME cells and absent in the MCF-7 breast cancer cell line (~3 fold induction). Collectively, these results indicate that inactive Vitamin D3 is being bioactivated to the active ligand and is signaling via the VDR within the adipocytes to participate in feedback gene regulation of 24-OHase expression.
Effect of Vitamin D3 on Human Adipocyte Differentiation
It has been reported that vitamin D3 induces a block in 3T3-L1 preadipocyte differentiation that is associated with an inhibition of peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer binding protein alpha (C/EBPα) expression [Blumberg et al., 2006; Kong and Li, 2006]. Using primary human breast preadipocytes, we wanted to assess not only the ability of 1,25D3 to inhibit human preadipocyte differentiation, but also the ability of 25D3 to be synthesized and utilized by the preadipocytes to inhibit differentiation. Human adipocyte differentiation was first evaluated with Oil Red O staining 14 days after the differentiation media was added. As shown in Figure 4A, human breast preadipocytes maintained in the preadipocyte media remained undifferentiated, lacking any red/orange lipid droplet staining (Figure 4A, a), whereas preadipocytes cultured in the presence of differentiation media for 14 days displayed lipid droplets that appear red/orange in a halo shape around the nuclei of differentiated (mature) adipocytes (Figure 4A, b). To assess the impact of 25D3 and 1,25D3 to inhibit the differentiation of human breast preadipocytes, we plated preadipocytes in 24-well plates and allowed them to grow to confluence. Upon reaching confluence, half the plate remained cultured in preadipocyte media and half of the plate was placed in differentiation media in the presence of vehicle (ethanol), 25D3 (100nM and 500nM), or 1,25D3 (100nM) treatments. Representative plates were harvested at day 7 and day 14 with treatment media consisting of either preadipocyte media or differentiation media being changed every 3–4 days. By 14 days, the extent of preadipocyte differentiation (Oil Red O staining) was more apparent than at day 7. To quantitate the level of Oil Red O staining at each time point and the impact that vitamin D3 treatments have on adipocyte differentiation, Oil Red O stain was visualized and eluted from the plate with 100% isopropanol to quantitate the absorbance reading in each well (Figure 4B). Similar to previous work in 3T3-L1 cells [Blumberg et al., 2006; Kong and Li, 2006], 1,25D3 also inhibits early stages of human breast preadipocyte differentiation (day 7), but by day 14 the impact of 1,25D3 has subsided and differentiation occurs equally well in vehicle and vitamin D3 treated wells. Interestingly, 25D3 works equally as well to inhibit preadipocyte differentiation at day 7, indicating that the preadipocytes are synthesizing (bioactivating) 25D3 to the active ligand, 1,25D3 and signaling via VDR to regulate adipocyte differentiation similar to the direct 1,25D3 treatment. Although treatment with 25D3 or 1,25D3 showed a trend suggesting a reduction in adipocyte differentiation after 14 days, it was only at day 7 that we detected a significant reduction in adipocyte differentiation when exposed to either 25D3 or 1,25D3 treatments. Thus, vitamin D3 may inhibit the initiation of preadipocyte differentiation, but it does not completely block the differentiation process in human primary preadipocytes.
Figure 4. Differentiation of human primary breast preadipocytes in the presence and absence of vitamin D3.
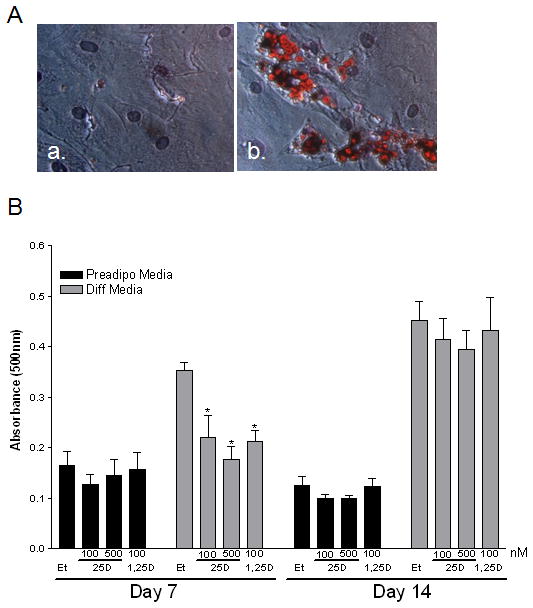
A) Representative image (320x) of preadipocytes (a) and mature (b) adipocytes stained with Oil red O after 14 days of culture in preadipocyte media (a) or differentiation media (b). Oil red O was used to assess intracellular triglyceride and measure lipid content within the cells. The lipid droplets appear red/orange in a halo shape around the nuclei of mature adipocytes. B) Effect of vitamin D3 to influence preadipocyte differentiation in the presence of preadipocyte media or differentiation media. Cells were exposed to vehicle or varying concentrations of vitamin D3 for 7 (a) or 14 (b) days. Assays were terminated on Day 7 or 14 and stained with Oil red O, dried, eluted from the plate with 100% isopropanol to quantitate the absorbance at 500nm in response to vitamin D3 in the preadipocyte or differentiation media. *- p <0.05 compared to vehicle control at each time point.
Mammary Microenvironment isolation and Expression of vitamin D3 signaling components
After investigating the expression of essential vitamin D3 signaling components within human pre and mature adipocytes and determining that adipocytes not only bioactivate vitamin D3, but also signal via VDR to regulate gene expression, we decided to utilize wild type (WT) and VDR knockout (KO) mice to investigate the microenvironments of the mammary gland for VDR and 1α-OHase expression. As shown in Figure 5, we isolated total mammary gland, ductal epithelial organoids (isolated mammary ductal epithelial structures, cleared of surrounding adipose tissue), and mammary adipose tissue (cleared of ductal epithelium) to investigate the expression of 1α-OHase (Figure 5A, a) and VDR (Figure 5A, b) using kidney as a positive control. Mouse mammary epithelial cells (organoids) have elevated 1α-OHase and VDR expression compared to the mammary adipose tissue similar to what was shown in the human primary cell lines, yet both microenvironments of the mammary gland have a detectable level of mRNA and protein expression (Figure 5A, B). As shown in Figure 5B, VDR protein expression is present in all mammary gland microenvironments, except from VDR KO organoids as anticipated. 1α-OHase is also expressed in each mammary gland microenvironment as well as total mammary gland. To assess the quality of our ex vivo isolation and purification procedure, we show protein expression of perilipin, an adipocyte marker, and cytokeratin 18, a glandular epithelial marker, to indicate the purity of our mammary gland microenvironment isolation procedures. These data suggest that there is no contamination of epithelium in our adipose fraction or adipose tissue remaining in our epithelial organoid fraction. Furthermore, in support of our human adipocyte and epithelial cell line expression data, VDR WT ex vivo isolated cells also express VDR and 1α-OHase in both microenvironment fractions of the mammary gland.
Figure 5. Expression of Vitamin D3 signaling components in individual microenvironments of the mammary gland.
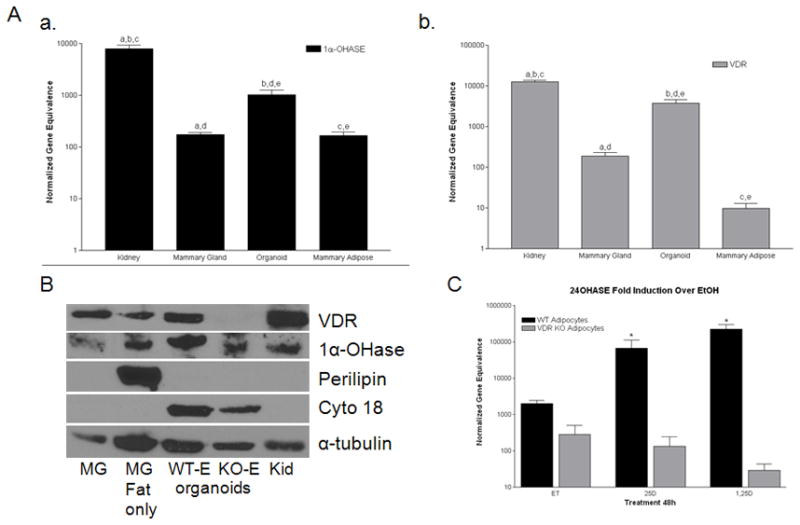
A) Real time PCR 1α-OHase (a) and vitamin D3 receptor (VDR) (b) in total mammary gland (Mammary Gland), purified epithelial organoids (Organoids), and cleared mammary fat pad (Mammary Adipose). Data are expressed relative to 18S RNA (normalized gene equivalence) and represent mean s.e.m. of triplicate runs. Similar letters above the bars designate comparison and significance in the expression of 1α-OHase or VDR within total mammary glands or the individual microenvironments of the mammary gland compared to a traditional target tissue for 1α-OHase and VDR expression - p<0.05. B) Western blot of VDR,1α-OHase, Perilipin, Cytokeratin 18 (cyto 18), and α-tubulin in total mammary gland (MG), cleared mammary fat pad (MG fat only), purified epithelial organoids from wild type (WT-E) and VDR knockout (KO-E) mice, and kidney (Kid). C. Bioactivation and signaling through the VDR to regulate 24-OHase in VDR WT and KO primary preadipocytes. 24- OHase induction by 25D3 and 1,25D3 in VDR WT primary mammary preadipocytes compared to VDR KO that lack a functional VDR. 24-OHase mRNA expression normalized to 18S from cultured cells treated for 48h with ethanol (Et), 25D3, or 1,25D3. *- p <0.05 compared to vehicle control at each genotype.
To investigate the ability of mouse adipocytes to regulate 24-OHase gene expression in the presence of vitamin D3 treatments, we plated VDR WT and KO primary mouse preadipocytes in 6-well plates and allowed them to grow to confluence. We treated with vehicle (ethanol), 25D3 (100nM), and 1,25D3 (100nM) similar to what was performed in the human adipocytes. After 48h, total RNA was isolated and real time PCR was performed to assess 24-OHase gene expression modifications in VDR WT and KO preadipocytes. As shown in Figure 5C, VDR WT adipocytes show a significant fold upregulation with 25D3 (~100 fold) and 1,25D3 (~150 fold) treatments compared to VDR KO adipocytes, that show no upregulation with vitamin D3 treatments. The VDR KO adipocytes serve as a negative control since they lack a functional VDR to participate in the binding of 1,25D3 and regulation via the vitamin D3 response elements within the 24-OHase promoter. Thus, our microenvironment isolations are pure and the primary adipose cells isolated from WT mice maintain the ability to bioactivate vitamin D3 and signal via VDR to modulate vitamin D3 target genes, suggesting a contribution to vitamin D3 signaling within the breast adipose tissue.
Mammary preadipocytes contribute to regulate mammary organoid hormone induced growth
To begin to address the significance of adipose tissue contribution to vitamin D3 signaling within the mammary gland, we utilized an ex vivo co-culture model that included mammary ductal epithelial organoids from WT and VDR KO mice as well as human preadipocytes cultured in a collagen matrix. Human breast preadipocytes were plated in the collagen matrix and allowed to culture until they reached 50–60% confluence at which time they received a 48h pretreatment of the following treatments prior to the addition of the organoid cultures from VDR WT and KO mice. The treatments consisted of vehicle (Ethanol), 17β-estradiol (E, 30nM), progesterone (P, 60nM), epidermal growth factor (EGF, 10ng/ml), 25D3 (250nM), 1,25D3 (100nM) or combinations of stimulating hormone/growth factor and either 25D3 or 1,25D3. Organoids were then harvested from 8–10wk old VDR WT or KO mice and purified as described in the Materials and Methods. Purified organoids were resuspended in collagen matrix and plated on top of the existing pretreated preadipocyte cell containing collagen matrix being cultured in a controlled hormonal milieu. After an overnight incubation, the co-cultures were then treated with the previously mentioned treatments and allowed to culture for 72hrs at which time the co-cultures were retreated and allowed to grow an additional 48–72 hrs. These experiments provided the platform to assess adipocyte signaling in response to inactive and active forms of vitamin D3 as well as investigating vitamin D3-induced release of growth inhibitory components from the co-cultured adipocytes. In Figure 6, the organoids pictured at day 0 (6A and 6H), shortly after plating and containing no secondary branch points from the organoid body, and at day 5, showing multiple branch cites from the organoid body, are representative images of WT (6B, E) and KO (6I, L) organoids cultured alone (B, I) or co-cultured with WT adipocytes (E, L) in the absence (ethanol – vehicle control, E, B, I, L) or presence of vitamin D3 (25D3 (F, C, J, M) or 1,25D3 (G, D, K, N)). Note the reduction in WT organoid size and ductal branching when cultured in the presence of 1,25D3 (6D) compared with the ethanol control (6B). There was also a slight reduction in organoid growth when treated with 25D3 (6C), suggesting bioactivation of vitamin D3 via the mammary ductal epithelial cells. However, there was no change in VDR KO organoid size or ductal branching when cultured in the presence of 25D3 (6J) or 1,25D3 (6K) compared with the ethanol control group (6I).
Figure 6. Preadipocytes contribute to vitamin D3-induced growth inhibition of mammary organoids.
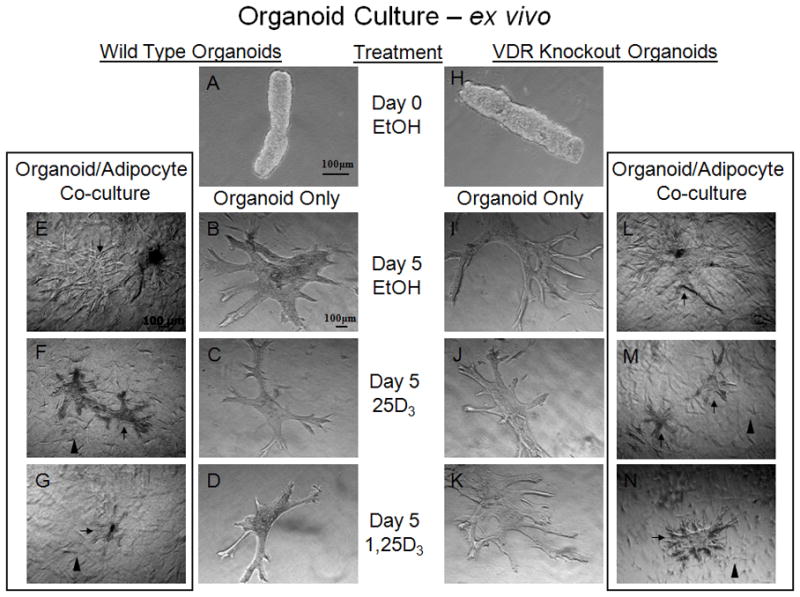
VDR WT (A–G) and KO (H–N) organoids (isolated mammary ductal structures, cleared of surrounding adipose) cultured in Type I Collagen in the presence of vehicle (ethanol) (Day 0 (A & H) or Day 5 (B & I)), 25D3 (100nM) (C & J), 1,25D3 (100nM) (D & K). WT organoids (C) show a slight reduction in branching with 25D3 (100nM) treatment, suggesting bioactivation of vitamin D3 via the mammary ductal epithelial cells compared to KO organoids (J) that show no reduction in size of branching compared with the ethanol control groups (B & I, respectfully). Further reduction in WT organoid (D) size and branching was seen with 1,25D3 treatment compared to KO organoid (K) and ethanol control groups (B & I). VDR wild type adipocytes (arrowheads, visible in F,G,M,N) cultured with VDR WT (E–G) or KO (L–N) organoids (arrows) embedded in Type I Collagen in the presence of vehicle (ethanol) (Day 5 (E & L)), 25D3 (100nM) (F & M), 1,25D3 (100nM) (G & N). Note the reduction in WT and KO organoid size and ductal branching when cultured with wild type adipocytes in the presence of 25D3 (100nM) (F & M) compared with the ethanol control groups (E & L) and without adipocyte co-culture (C & J), suggesting bioactivation via wild type adipocytes. There was also a similar reduction in WT and KO organoid growth when treated with 1,25D3 (G & N), suggesting VDR signaling and growth inhibitory secretion via WT adipocytes that results in growth inhibition of VDR KO organoids. n=3–5 organoids/genotype/treatment and n>5 branches measured/organoid.
In contrast, the organoid/WT Adipocyte co-culture provides different results. The WT organoid growth was again inhibited by vitamin D3 (25D3 and 1,25D3), similar to the organoid only culture, but showing a slight additive reduction in growth induced by WT adipocytes that are likely participating in the bioactivation and signaling via the VDR to induce growth inhibition in the developing organoids. To further support this concept of adipocyte contribution, the VDR KO organoids show a greatly reduced growth response when treated with either 25D3 (6J vs. M) or 1,25D3 (6K vs. N), suggesting VDR WT adipocytes induce a reduction in organoid size and ductal branching when cultured in the presence of vitamin D3 (6M and N) compared with treated VDR KO organoid only cultures (6J and K) as well as the ethanol control groups (6I and L), suggesting VDR signaling via WT adipocytes. Since there was also a reduction in organoid growth when treated with 25D3 (6M), this would suggest that bioactivation and signaling via the WT adipocytes results in a growth inhibitory signal that regulates VDR KO ductal epithelial cells. We quantitated these ex vivo co-culture results (Figure 7) as well as those in the presence of estrogen, progesterone and epidermal growth factor using Axiovision software to assess ductal length, the number of ductal branches and the area of each organoid. In the presence of WT adipocytes, 25D3 and 1,25D3 were able to inhibit hormone stimulated branching and ductal extension in the WT organoids (Figure 7A) and also in the VDR KO organoids (Figure 7B) comparing either vehicle versus hormone or growth factor stimulation in the absence or presence of 25D3 or 1,25D3. We also made statistical comparisons using the baseline hormone or growth factor stimulation alone versus being stimulated in the presence of 25D3 or 1,25D3 to determine the ability of vitamin D3 to inhibit hormone/growth factor stimulated growth. Vitamin D3 not only was able to inhibit hormone or growth factor stimulated growth in the WT organoids (Figure 7A), but was also able to inhibit VDR KO organoid stimulated growth when cultured in the presence of WT adipocytes (Figure 7B). Thus, we conclude that mammary adipocytes take an active role in vitamin D3 synthesis and signaling, contributing to hormone and growth factor induced growth regulation of the mammary ductal epithelial cells.
Figure 7. Preadipocytes treated with vitamin D3 modulate hormone induced organoid growth.
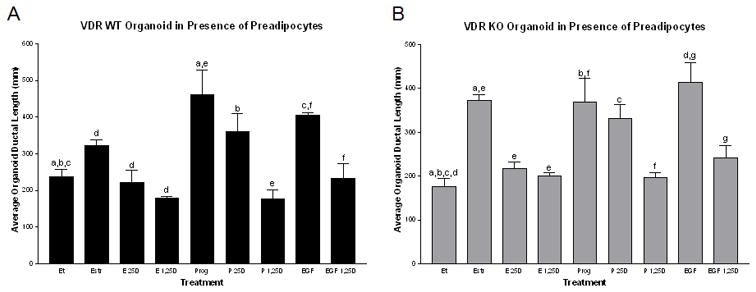
Ability of wild type adipocytes to modulate the growth of VDR WT (A) and KO (B) organoids in the presence of hormone or growth factor stimulation. Organoids cultured along with wild type adipocytes embedded in Type I Collagen in the presence of vehicle (Et), estrogen (E, 30nM), progesterone (P, 60nM) or epidermal growth factor (EGF, 10ng/ml) in the absence or presence of 25D3 (250nM) or 1,25D3 (100nM). 25D3 and 1,25D3 induce organoid growth inhibition even in the presence of hormone or growth factor stimulation in both WT and KO organoids when co-cultured with wild type breast adipocytes. n=3–5 organoids/genotype/treatment and n>5 branches measured/organoid. Similar letters above the bars designate significance between treatments comparing either vehicle (Et) versus hormone or growth factor stimulation in the presence or absence of 25D3 or 1,25D or comparing the baseline hormone or growth factor stimulation alone versus having that stimulation in the presence of 25D3 or 1,25D3 -p<0.05.
DISCUSSION
The importance of vitamin D3 signaling in the prevention of breast cancer has been suggested as a contributor in the maintenance of breast health [Goodwin et al., 2009; Janowsky et al., 1999]. Our studies begin to address a contributing mechanism behind maintaining long term breast health, involving not only vitamin D3 synthesis and signaling through the breast epithelial cells, but also the breast stromal compartment including the adipose tissue. To our knowledge, this is the first direct evidence showing that human primary breast pre and mature adipocytes express the necessary vitamin D3 signaling components to participate in the synthesis and signaling via VDR within the breast adipose tissue. Furthermore, we established that human primary breast adipocytes bioactivate inactive 25D3 to the active ligand, 1,25D3, and secrete it to surrounding cells in a paracrine manner influencing co-cultured VDR WT and KO ductal organoid outgrowth. The adipocytes also signal via VDR to regulate gene expression in response to 25D3 and 1,25D3, similar to what has been shown to occur in human mammary epithelial cells [Kemmis et al., 2006]. Therefore, maintaining optimal circulating vitamin D3 levels, allowing for excess circulating 25D3 to be stored in the adipose tissue, will likely induce vitamin D3 signaling locally within the breast without disturbing systemic calcium homeostasis and participating in local growth regulation. The most significant suggestion of this work is that local VDR signaling within the breast will be modulated by both microenvironments of the breast in order to maintain breast health.
The presence of VDR and 1α-OHase expression within mouse mammary adipose tissue has been previously reported using immunohistochemical methods during pubertal mammary gland development and pregnancy [Zinser et al., 2002; Zinser and Welsh, 2004], but the contribution of the adipose tissue to participate in VDR signaling had not yet been defined. Studies using epididymal fat tissue and 3T3-L1 cells suggested that adipocytes express 1α-OHase and that 3T3-L1 cells do have the ability to participate in the bioactivation of 25D3 to 1,25D3 [Li et al., 2008], yet the expression of VDR and 1α-OHase, the activity of 1α-OHase, and the impact of VDR signaling within human breast adipocytes remained unknown. Thus, we report in this study that pre and mature human breast adipocytes express VDR and 1α-OHase mRNA and protein at levels similar to human mammary epithelial cells. Furthermore, the localization of VDR and 1α-OHase in primary adipocyte cultures are consistent with previously reported immunohistochemical data reported in vivo using mouse models of breast development [Zinser et al., 2002; Zinser and Welsh, 2004]. Thus, we report that the human breast adipocytes do express the necessary signaling components associated with vitamin D3 synthesis and required for VDR-induced signaling to regulate growth and differentiation.
The bioactivation of 25D3 to 1,25D3 within human mammary epithelial cells and the ability of the HME cells to be growth inhibited by 25D3 [Kemmis et al., 2006] was an essential finding related to epidemiological data associating elevated circulating 25D3 and lower breast cancer risk [Knight et al., 2007]. Furthermore, the influence of mammary cell transformation to inhibit the uptake of 25D3 [Rowling et al., 2006] and the decrease in the efficiency by which 25D3 is bioactivated after transformation [Kemmis and Welsh, 2008] suggests that adequate vitamin D3-induced prevention would benefit by having assistance from the surrounding microenvironment of the breast to produce the active ligand, 1,25D3. Thus, we have shown that the breast adipocytes express 1α-OHase and have presented in comparison to HME cells that breast pre and mature adipocytes bioactivate 25D3 to 1,25D3 efficiently. Furthermore, the breast adipocytes also signal through VDR to modulate vitamin D3 target gene expression within the adipocytes, indicating that the breast adipocyte likely contribute to vitamin D3-induced gene signaling, potentially modulating cellular growth and differentiation within the breast.
The role of vitamin D3 participating or regulating adipocyte differentiation has also been assessed by various groups, including work done over 20 years ago which indicated that treatment of 3T3-L1 pre- adipocytes in culture with 1,25D3 influenced differentiation and adipocyte metabolism [Ishida et al., 1988]. More recently, it was found that 1,25D3 inhibited thiazolidinedione-induced 3T3-L1 preadipocyte differentiation and was associated with inhibition of PPARγ2 protein expression [Hida et al., 1998], which normally occurs during the first 48h after the initiation of preadipocyte differentiation. Studies conducted in the last five years have continued to describe the mechanism behind vitamin D3-induced regulation of adipocyte differentiation, including the dependence of VDR in mediating the effects of 1,25D3 on PPARγ expression and the induction of VDR expression throughout the differentiation process [Kong and Li, 2006]. Therefore, in our work, we wanted to confirm the role of Vitamin D3 signaling during the differentiation of human primary breast preadipocytes. We showed that preadipocyte differentiation is delayed but not prevented in the presence of 1,25D3, resulting in a decrease in Oil Red O staining early during the differentiation process, but having similar lipid content after two weeks of adipocyte differentiation. Thus, our work in human breast adipocytes supports work done previously in 3T3-L1 cells where 1,25D3 participates to inhibit the early stages of preadipocyte differentiation [Blumberg et al., 2006; Kong and Li, 2006]. However, we also established that preadipocytes treated with 25D3 induced an inhibition of early preadipocyte differentiation similar to 1,25D3, which suggests that 25D3 is being synthesized within the preadipocytes to 1,25D3 and signaling through the VDR to regulate differentiation at a similar rate as the active ligand directly.
The contribution of mammary adipose tissue to regulate normal mammary gland development and maintenance of ductal epithelial morphology is well supported, suggesting that the influence of stromal/epithelial interactions are essential to maintain normal mammary gland development [Couldrey et al., 2002; Landskroner-Eiger et al., 2010]. Thus, knowing that the adipose tissue participates in such an essential way, influencing hormone metabolism [Ahima and Flier, 2000; Siiteri, 1987], growth factor storage and secretion [Hovey et al., 2001; Rahimi et al., 1994; Walden et al., 1998], release of adipokines [Trujillo and Scherer, 2006], and the fact that excess circulating vitamin D3 is stored in adipose tissue [Rosenstreich et al., 1971], we hypothesized that it was likely that the adipose tissue would contribute to vitamin D3-induced regulation of the mammary epithelium. Using VDR WT and KO mouse models, we isolated mammary tissue and digested it into individual pure microenvironments containing either epithelial ductal organoids or mammary preadipocytes. The purified microenvironments enabled us to confirm that VDR WT mammary adipocytes have the ability to bioactivate 25D3 to 1,25D3 and signal via VDR to upregulate 24-OHase gene expression in response to either 25D3 or 1,25D3 treatment. In contrast, the VDR KO isolated mammary adipocytes, which lack a functional VDR necessary to bind ligand and the vitamin D3 response element within the 24-OHase promoter, lacked 24-OHase gene induction above baseline. This ex vivo data confirms vitamin D3 bioactivation and vitamin D3-induced signaling within mammary adipocytes, supporting the need of a more thorough investigation into the contribution of VDR signaling via the mammary adipose tissue in order to comprehend the significance of the mammary adipose tissue in the prevention of breast disease.
These studies have revealed a novel process by which mammary adipocytes likely participate in vitamin D3-induced prevention of abnormal breast development and the onset of breast disease. We have reported for the first time that VDR WT primary breast preadipocytes co-cultured with VDR WT or KO mammary ductal organoids in the presence of 25D3 or 1,25D3 have the ability to growth inhibit ductal organoid outgrowth. This data suggests that not only are the preadipocytes bioactivating 25D3 to 1,25D3, but the preadipocytes are also signaling through their VDR to induce signaling, triggering a release of a negative growth regulator in a paracrine manner that inhibits the outgrowth of VDR KO organoids. Although the specific inhibiting factor secreted from WT adipocytes in response to 25D3 or 1,25D3 remains unknown, the growth inhibition resulted in fewer ductal branches and stunted ductal outgrowth from the body of the organoid in both VDR WT and KO organoids. The influence of the co-cultured VDR WT preadipocytes to inhibit ductal organoid outgrowth was evident even in the presence of hormone and growth factor stimulated conditions. Thus, VDR signaling within breast pre and mature adipocytes likely participates to regulate ductal epithelial growth and differentiation throughout mammary gland development, particularly at times of elevated hormonal exposure. Future studies will begin to investigate the vitamin D3-regulated inhibitory factor that is released from the breast adipocytes that could be a small or large molecular weight protein, a small molecule inhibitor, or an adipokine secreted from mammary adipocytes to regulate ductal development.
Collectively, we report our novel finding that breast adipose tissue contributes to vitamin D3- induced growth regulation of breast ductal epithelium by synthesizing vitamin D3 to the active metabolite, secreting 1,25D3 to the surrounding cellular microenvironment, signaling via the VDR to modulate gene expression and releasing growth inhibitory factors that participate in glandular epithelial cell regulation. This data indicates the need to investigate more thoroughly the relevance of VDR signaling within breast adipose tissue to modulate mammary gland development and the influence the mammary adipose tissue has at the onset of breast transformation.
Acknowledgments
Grants Supporting the Paper: National Institutes of Health/Office of Research on Women’s Health K12 and the NICHD (HD051953-01), Susan G. Komen Career Catalyst (KG090377), National Institutes of Health/National Cancer Institute R21 (CA137310-01A1)
The authors are grateful to Mallory McKeehan for her assistance with animal maintenance, genotyping and tissue collection. We further appreciate the thoughtful discussion and critical comments on the manuscript provided by Dr. JoEllen Welsh. This research was supported by grants to G.M.Z. from the National Institutes of Health/Office of Research on Women’s Health (HD051953), National Institutes of Health (CA137310), and Susan G. Komen Breast Cancer Foundation (KG090377).
References
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–32. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem. 2006;281:11205–13. doi: 10.1074/jbc.M510343200. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, Korach KS. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–94. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95:5076–81. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, Green J. Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn. 2002;223:459–68. doi: 10.1002/dvdy.10065. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Robinson SD. Regulation of mammary growth and function by TGF-beta. Mol Reprod Dev. 1992;32:145–51. doi: 10.1002/mrd.1080320210. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB, Van Horn K, Strickland P, Robinson S. TGF-beta 1-induced inhibition of mouse mammary ductal growth: developmental specificity and characterization. Dev Biol. 1989;135:20–30. doi: 10.1016/0012-1606(89)90154-1. [DOI] [PubMed] [Google Scholar]
- Flanagan L, Packman K, Juba B, O’Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol. 2003;84:181–92. doi: 10.1016/s0960-0760(03)00028-1. [DOI] [PubMed] [Google Scholar]
- Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, Kopchick JJ, Oka T, Kelly PA, Hennighausen L. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol. 2001;229:163–75. doi: 10.1006/dbio.2000.9961. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–63. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–68. x–xi. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida Y, Kawada T, Kayahashi S, Ishihara T, Fushiki T. Counteraction of retinoic acid and 1,25-dihydroxyvitamin D3 on up-regulation of adipocyte differentiation with PPARgamma ligand, an antidiabetic thiazolidinedione, in 3T3-L1 cells. Life Sci. 1998;62:PL205–11. doi: 10.1016/s0024-3205(98)00059-9. [DOI] [PubMed] [Google Scholar]
- Hovey RC, Goldhar AS, Baffi J, Vonderhaar BK. Transcriptional regulation of vascular endothelial growth factor expression in epithelial and stromal cells during mouse mammary gland development. Mol Endocrinol. 2001;15:819–31. doi: 10.1210/mend.15.5.0635. [DOI] [PubMed] [Google Scholar]
- Humphreys RC, Lydon J, O’Malley BW, Rosen JM. Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol Endocrinol. 1997;11:801–11. doi: 10.1210/mend.11.6.9891. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Taniguchi H, Baba S. Possible involvement of 1 alpha,25-dihydroxyvitamin D3 in proliferation and differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 1988;151:1122–7. doi: 10.1016/s0006-291x(88)80482-0. [DOI] [PubMed] [Google Scholar]
- Janowsky EC, Lester GE, Weinberg CR, Millikan RC, Schildkraut JM, Garrett PA, Hulka BS. Association between low levels of 1,25-dihydroxyvitamin D and breast cancer risk. Public Health Nutr. 1999;2:283–91. doi: 10.1017/s1368980099000385. [DOI] [PubMed] [Google Scholar]
- Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol. 2001;15:1370–80. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- Kemmis CM, Salvador SM, Smith KM, Welsh J. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J Nutr. 2006;136:887–92. doi: 10.1093/jn/136.4.887. [DOI] [PubMed] [Google Scholar]
- Kemmis CM, Welsh J. Mammary epithelial cell transformation is associated with deregulation of the vitamin D pathway. J Cell Biochem. 2008;105(4):980–988. doi: 10.1002/jcb.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg DL. Role of IGF-I in normal mammary development. Breast Cancer Res Treat. 1998;47:201–8. doi: 10.1023/a:1005998832636. [DOI] [PubMed] [Google Scholar]
- Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:422–9. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290:E916–24. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- Landskroner-Eiger S, Park J, Israel D, Pollard JW, Scherer PE. Morphogenesis of the developing mammary gland: stage-dependent impact of adipocytes. Dev Biol. 2010;344:968–78. doi: 10.1016/j.ydbio.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK, Burgess JR, Teegarden D. 1alpha,25-Dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112:122–6. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–6. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 2006;103:2196–201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCave EJ, Cass CA, Burg KJ, Booth BW. The normal microenvironment directs mammary gland development. J Mammary Gland Biol Neoplasia. 2010;15:291–9. doi: 10.1007/s10911-010-9190-0. [DOI] [PubMed] [Google Scholar]
- Narvaez CJ, Welsh J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem. 2001;276:9101–7. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- Neville MC, Medina D, Monks J, Hovey RC. The mammary fat pad. J Mammary Gland Biol Neoplasia. 1998;3:109–16. doi: 10.1023/a:1018786604818. [DOI] [PubMed] [Google Scholar]
- Rahimi N, Saulnier R, Nakamura T, Park M, Elliott B. Role of hepatocyte growth factor in breast cancer: a novel mitogenic factor secreted by adipocytes. DNA Cell Biol. 1994;13:1189–97. doi: 10.1089/dna.1994.13.1189. [DOI] [PubMed] [Google Scholar]
- Rosenstreich SJ, Rich C, Volwiler W. Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J Clin Invest. 1971;50:679–87. doi: 10.1172/JCI106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowling MJ, Kemmis CM, T’ffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136:2754–9. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph MC, Wellberg EA, Anderson SM. Adipose-depleted mammary epithelial cells and organoids. J Mammary Gland Biol Neoplasia. 2009;14:381–6. doi: 10.1007/s10911-009-9161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45:277–82. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–76. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–33. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–78. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- Walden PD, Ruan W, Feldman M, Kleinberg DL. Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology. 1998;139:659–62. doi: 10.1210/endo.139.2.5718. [DOI] [PubMed] [Google Scholar]
- Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation. 1994;57:205–14. doi: 10.1046/j.1432-0436.1994.5730205.x. [DOI] [PubMed] [Google Scholar]
- Wiesen JF, Young P, Werb Z, Cunha GR. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126:335–44. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–9. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant J, Buluwela L, Niranjan B, Gusterson B, Kamalati T. In vivo effects of hepatocyte growth factor/scatter factor on mouse mammary gland development. Exp Cell Res. 1998;241:476–81. doi: 10.1006/excr.1998.4028. [DOI] [PubMed] [Google Scholar]
- Zinser G, Packman K, Welsh J. Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Development. 2002;129:3067–76. doi: 10.1242/dev.129.13.3067. [DOI] [PubMed] [Google Scholar]
- Zinser GM, McEleney K, Welsh J. Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Mol Cell Endocrinol. 2003;200:67–80. doi: 10.1016/s0303-7207(02)00416-1. [DOI] [PubMed] [Google Scholar]
- Zinser GM, Welsh J. Accelerated mammary gland development during pregnancy and delayed postlactational involution in vitamin D3 receptor null mice. Mol Endocrinol. 2004;18:2208–23. doi: 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]


