Abstract
The coumarins imperatorin and osthole are known to exert anticonvulsant activity. We have therefore analyzed the modulation of GABA-induced chloride currents (IGABA) by a selection of 18 coumarin derivatives on recombinant α1β2γ2S GABAA receptors expressed in Xenopus laevis oocytes by means of the two-microelectrode voltage clamp technique. Osthole (EC50=14±1 μM) and oxypeucedanin (EC50=25±8 μM) displayed the highest efficiency with IGABA potentiation of 116±4% and 547±56%, respectively. IGABA enhancement by osthole and oxypeucedanin was not inhibited by flumazenil (1 μM) indicating an interaction with a binding site distinct from the benzodiazepine binding site. In general, prenyl residues are essential for the positive modulatory activity, while longer side chains or bulkier residues (e.g. geranyl residues) diminish IGABA modulation. Generation of a binary classification tree revealed the importance of polarisability, which is sufficient to distinguish actives from inactives. A 4-point pharmacophore model based on oxypeucedanin – comprising three hydrophobic and one aromatic feature – identified 6 out of 7 actives as hits. In summary, (oxy-)prenylated coumarin derivatives from natural origin represent new GABAA receptor modulators.
Keywords: GABAA receptor, (Furano)-coumarin, Osthole, Oxypeucedanin, Voltage-clamp technique, Pharmacophore model
1. Introduction
The gamma-aminobutyric acid type A receptor (GABAA) is a ligand gated ion channel mediating fast inhibition of neuronal signal transmission (Mody and Pearce, 2004). Binding of GABA to GABAA receptors induces hyperpolarization of the neuronal membrane due to an increased chloride influx and thus decreases or inhibits ongoing neurotransmission. The GABAA receptors are heteropentameric proteins, which can assembly from 19 different subunits: α1–6, β1–3, γ1–3, δ, π, ε, θ, and ρ1–3 and potentially generate a large variety of receptor subtypes (Simon et al., 2004). From theoretically over 150.000 possible GABAA receptors only a few seem to occur in vivo in the mammalian central nervous system (Olsen and Sieghart, 2009). The most abundant receptor subtype consists of 2 α1, 2 β2 and 1 γ2S/L subunit (McKernan and Whiting, 1996; Sieghart and Sperk, 2002). While binding of GABA opens GABAA receptor channels, there is also evidence for binding sites interacting with benzodiazepines, general anesthetics, barbiturates and many other therapeutically important drugs (Korpi et al., 2002; Sieghart, 1995; Sieghart and Enna, 2006). In addition to drugs that are in clinical use a variety of structurally diverse natural products have been shown to elicit positive modulatory effects on GABAA receptors, e.g. borneol (Granger et al., 2005), thymol (Priestley et al., 2003), valerenic acid (Khom et al., 2007; Trauner et al., 2008), piperin (Zaugg et al., 2010), flavonoids (Fernandez et al., 2008; Huen et al., 2003), polyacetylenes (Baur et al., 2005), and various others (Johnston et al., 2006).
Compared to other natural compound classes like flavonoids or monoterpenes, the action of coumarins on GABAA receptors is largely unknown. However, coumarins often occur in plants that are used as sedatives or spasmolytical agents in traditional medicinal systems worldwide (Murray et al., 1982; O’Kennedy and Thorne, 1997). Furthermore, in vivo antiepileptic activity of coumarins was reported by Luszczki and co-workers (Luszczki et al., 2007a,b, 2009a,b).
Evidence for interaction of coumarins with GABAA receptors comes also from binding studies suggesting that phellopterin and imperatorin interact with the benzodiazepine binding site of the GABAA receptor (Bergendorff et al., 1997; Dekermendjian et al., 1996).
In the present study we examine the effects of 18 (furano-) coumarins on chloride currents (IGABA) through recombinant α1β2γ2S GABAA receptors expressed in Xenopus laevis oocytes and provide first insights into the structural requirement for a positive modulatory effect.
2. Material and methods
2.1. Chemicals and substances
γ-Amino butyric acid (GABA), reagents for ND96 solution, diazepam and flumazenil were purchased from Sigma (Vienna, Austria). Bergamottin, bergapten, bergaptol, coumarin, isobergapten, isopimpinellin, scopoletin and umbelliferone were purchased from Extrasynthese (Lyon, France). Auraptene and isoimperatorin were purchased from LGC Standards (Wesel, Germany). Oxypeucedanin was purchased from Phytolab (Vestenbergsreuth, Germany). Phellopterin was purchased from Sequoia Research Products Ltd. (Pangbourne, UK). Pimpinellin was purchased from Herboreal Ltd. (Edinburgh, UK). Ostruthin (purity≥98%) and ostruthol (purity≥98%) were isolated from Peucedanum ostruthium L. (Koch) by Vogl et al. (2011) and imperatorin and osthole were isolated from Cnidium monnieri L. as follows: a petroleum ether extract of Cnidium monnieri fruits was first subjected to semi-preparative HPLC using a RP-18 column (Nucleosil 100, Machery-Nagel) and a gradient elution consisting of water (solvent A) and acetonitril (solvent B) with a concentration of B of 35% B for 15 min, followed by an increase of B to 80% in 5 min and a steady concentration of B for 7 min followed by a decrease to 35% B in 3 min. Flow rate was set at 27.6 mL/min. Fraction 16, which according to literature contains imperatorin and osthole, was subjected to normal phase column chromatography on silica gel (60 × 0.5 cm i.d.) using n-hexane:EtOAc (95:5) as mobile phase (flow rate 10 mL/h, fraction volume: 5 mL). Fractions were screened by TLC on silica gel coated aluminum plates KG60 F254 (Merck, Germany) using n-hexane:EtOAc (90:10) as mobile phase. Fractions 25–36 (blue fluorescent zone in the TLC screening) and fractions 46–61 (brown fluorescent spot in the TLC screening) were unified to yield two cumulative fractions. Their structure was elucidated by 1- and 2-D 1H and 13 C-NMR as imperatorin (purity: ≥98%) and osthole (purity: ≥97%), respectively. Purity was determined using HPLC by comparing UV spectra and retention time to reference substances which were purchased from Sigma (Vienna, Austria).
2.2. Voltage clamp and fast solution exchange on Xenopus oocytes
Preperation of stage V–VI oocytes from Xenopus laevis (NASCO, USA) and injection of cRNA were done as previously described (Khom et al., 2006). Female frogs were anesthetised 15 min prior to surgery using 0.2% solution of MS-222 (Sigma, Vienna, Austria) and parts of the ovaries were removed. Remaining follicle membranes were enzymatically digested with 2 mg/mL collagenase Type 1 A (Sigma, Vienna, Austria). Synthesis of capped off run-off poly (A+) cRNA transcripts was performed from linearized cDNA templates (pCMV vector). cRNAs were diluted with DEPC-treated water and stored at −80 °C. Injection of 10–50 nL of the different cRNA solutions was carried out on the day of isolation. To ensure the expression of the γ-subunit, cRNAs of α1, β2, and γ2S were injected in a ratio of 1:1:10 (Baburin et al., 2008; Boileau et al., 2003). Successful expression of the γ-subunit was determined by application of diazepam (300 nM). Injected oocytes were stored at 18 °C in penicillin and streptomycin supplemented ND96 solution, containing 96 mM NaCl, 2 mM KCl, 1 mM MgCl2*6H2O, 1.8 mM CaCl2 and 5 mM HEPES (pH 7.4) in double distilled water.
Chloride currents through GABAA receptors were measured by means of the two-microelectrode voltage clamp method making use of a TURBO TEC 03X amplifier (npi electronic, Tamm, Germany) at a holding potential of −70 mV as previously described (Baburin et al., 2006). Current measurements were recorded with pCLAMP 10 data acquisition software (Molecular Devices, Sunnyvale, CA, USA). ND96 was used as bath solution. Microelectrodes (Harvard Apparatus, Kent, UK) with resistances between 1 and 3 MΩ were pulled by means of a microelectrode puller (Narashige, Tokyo, Japan) and filled with 2 M KCl.
GABA and compounds were applied to oocytes by means of the ScreeningTool (npi electronic, Tamm, Germany) fast perfusion system as described by Baburin et al. (2006). Stock solutions of the tested compounds (100 mM) were prepared in DMSO and stored at −20 °C. GABA and test solutions were prepared freshly every day. The DMSO concentration of 1% in both, the control and test solutions, did not affect GABA-induced chloride current (IGABA). In the DMSO-stock solutions (10 mM) and the aqueous test solutions used no precipitates or turbidity was observed and thus the compounds were regarded as fully dissolved.
IGABA modulation was measured at a GABA concentration eliciting between 5 and 10% of the maximal current amplitude (EC5–10), corresponding to 3–10 μM GABA. The EC5–10 was established at the beginning of each experiment. In the presence of compound concentrations higher than 30 μM wash out periods were extended to up to 10 min to exclude effects of receptor desensitization on current amplitudes.
2.3. Data analysis
Compound induced changes in chloride current amplitudes were calculated as I(GABA + compound)/IGABA − 1, where I(GABA+compound) is the current response in the presence of a given compound and IGABA is the control GABA current.
Concentration–response curves were generated and the data were fitted by nonlinear regression analysis using Origin Software (OriginLab Corporation, Northampton, MA, US). Data were fitted to the equation 1/(1 + (EC50/[compound]nH), where EC50 is the concentration of the compound that increases the amplitude of the GABA-evoked current by 50% of the compound-induced maximum response, and nH is the Hill coefficient. Responses were graphed as mean±S.E.M. from at least three oocytes out of ≥2 different batches. Statistical significance (*) was calculated using t-test and one-way ANOVA with a confidence interval of P<0.05.
2.4. Molecular modeling
Molecules were built using the builder module in MOE 2009.10 and energy minimized using standard conditions (MMFF94x force field, adjust H and LP, gradient=0.01, calculate forcefield partial charges). A database was built and a small set of physicochemical parameters was calculated. These comprise logP (logP(o/w)), topological polar surface area (TPSA), polarisability (apol), molar refractivity (mr), number of rotable bonds (b_1rotN), as well as number of H-bond donors and –acceptors. These descriptors allow a general description of the physicochemical properties of the molecules and have been successfully applied in classification analyses (Demel et al., 2010). The data set was split into two classes, active/inactive, with a threshold of 10% potentiation. This resulted in 7 active and 11 inactive compounds. Chemical structures, class labels as well as selected physicochemical descriptors are given in Table 1.
Table 1.
Class labels and selected physicochemical descriptors of the (furano-)coumarins including mean potentiation of IGABA by selected coumarin derivatives (100 μM). 7 compounds with an IGABA potentiation above 20% were classified as active (1) while the other 11 components were regarded as inactive (0).
| Compound | Potentiation (%) | Class | apol | a_acc | a_don | b_1rotN | logP(o/w) | mr | TPSA |
|---|---|---|---|---|---|---|---|---|---|
| Coumarin | 1,5 | 0 | 21,44 | 1 | 0 | 0 | 2,18 | 4,14 | 26,30 |
| Umbelliferone | −4,4 | 0 | 22,25 | 2 | 1 | 0 | 1,90 | 4,26 | 46,53 |
| Scopoletin | −1,8 | 0 | 26,14 | 3 | 1 | 1 | 1,90 | 4,90 | 55,76 |
| Osthole | 124,5 | 1 | 39,47 | 2 | 0 | 3 | 3,20 | 7,05 | 35,53 |
| Auraptene | −0,1 | 0 | 50,52 | 2 | 0 | 6 | 3,85 | 8,85 | 35,53 |
| Ostruthin | 2,6 | 0 | 50,52 | 2 | 1 | 5 | 3,88 | 8,81 | 46,53 |
| Bergaptol | −19,9 | 0 | 26,56 | 2 | 1 | 0 | 2,17 | 5,16 | 59,67 |
| Isopimpinellin | 6,3 | 0 | 33,56 | 3 | 0 | 2 | 2,35 | 6,31 | 57,90 |
| Bergapten | −10,5 | 0 | 29,66 | 2 | 0 | 1 | 2,43 | 5,68 | 48,67 |
| Isoimperatorin | 33,8 | 1 | 40,70 | 2 | 0 | 3 | 3,25 | 7,49 | 48,67 |
| Imperatorin | 54,1 | 1 | 40,70 | 2 | 0 | 3 | 3,25 | 7,49 | 48,67 |
| Phellopterin | 56,5 | 1 | 44,60 | 3 | 0 | 4 | 3,16 | 8,13 | 57,90 |
| Heraclenin | 32,9 | 1 | 41,51 | 3 | 0 | 3 | 2,99 | 7,45 | 61,20 |
| Oxypeucedanin | 550 | 1 | 41,51 | 3 | 0 | 3 | 2,99 | 7,45 | 61,20 |
| Bergamottin | −7,2 | 0 | 54,84 | 2 | 0 | 6 | 4,11 | 9,77 | 48,67 |
| Ostruthol | −6,6 | 0 | 57,24 | 4 | 1 | 6 | 3,06 | 10,15 | 95,20 |
| Isobergapten | 4,6 | 0 | 29,66 | 2 | 0 | 1 | 2,43 | 5,68 | 48,67 |
| Pimpinellin | 65,4 | 1 | 33,56 | 3 | 0 | 2 | 2,10 | 6,31 | 57,90 |
2.4.1. Binary classification tree
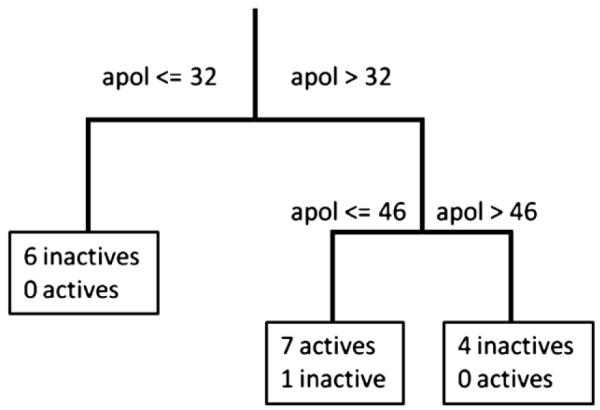
A binary classification tree was built using the QuaSAR-Classify tool in MOE 2009.10 and the physicochemical descriptors outlined above. The tree was constructed applying standard conditions (number of test samples: 18; number of cross-validation subsets: 2; cross-validation subset size: 9; random subset selection: on; minimum node size for splitting: 10; the maximum growth depth: 10; classes equally important). Quality of the models was assessed by identifying the number of true positives (TP), true negatives (TN), false positives (FP) and false negatives (FN) obtained in leave one out cross validation runs. The overall prediction accuracy (A), the sensitivity (SE) which represents the accuracy on actives, and the specificity (SP), which illustrates the accuracy on inactives, were calculated as follows: A=(TP+TN)/(TP+TN+FP+FN), SE=TP/(TP+FN) and SP=TN/(TN+FP).
2.4.2. Creating a pharmacophore model
3D structures were built interactively using MOE 2009.10. The number of conformers generated using the ‘best’ feature of the program for each substrate was limited within the program to a maximum of 255 with an energy range of 15.00 kcal/mol beyond the calculated potential energy minimum. A 4-point pharmacophore model using the most efficient modulator, oxypeucedanin, as template (Fig. 7A), was created using the pharmacophore modeling tools implemented in MOE. The final model features 3 hydrophobic regions at the prenyl residue and in position 4 of the carbon skeleton of oxypeucedanin, a position directly opposite to the attachment site of the prenyl residue, as well as one aromatic feature.
Fig. 7.
Binary decision tree based on polarisability (apol).
3. Results
3.1. Potentiation of IGABA by osthole and oxypeucedanin
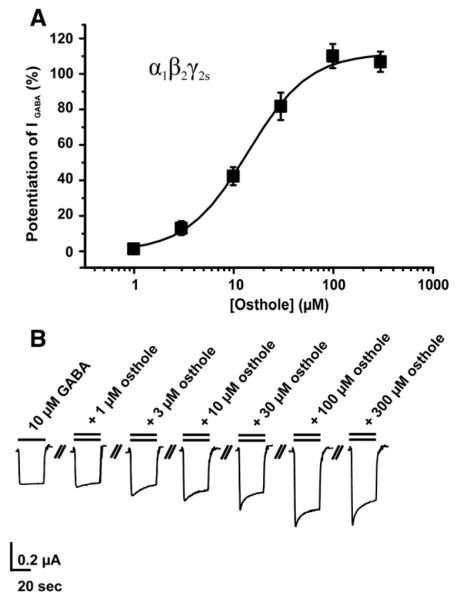
Recombinant α1β2γ2S receptors were expressed in Xenopus laevis oocytes and GABA-induced chloride current (IGABA) modulation by 18 coumarin derivates (Figs. 1 and 2) was investigated by means of two-microelectrode voltage clamp and a fast perfusion technique (see Materials and methods). The 18 tested compounds consist of 6 simple coumarins, 10 linear furanocoumarins and 2 angular furanocoumarins (Fig. 1). Oxypeucedanin and osthole at 100 μM enhanced IGABA most efficiently by 550±71% (n=5) and 124±11% (n=5), respectively (Fig. 2, Table 1). The concentration-dependent potentiation of IGABA by osthole (maximum enhancement by 116±4%) is illustrated in Fig. 3. The EC50 value was determined as 14±1 μM with a Hill coefficient (nH) of 1.4±0.2 (n=4).
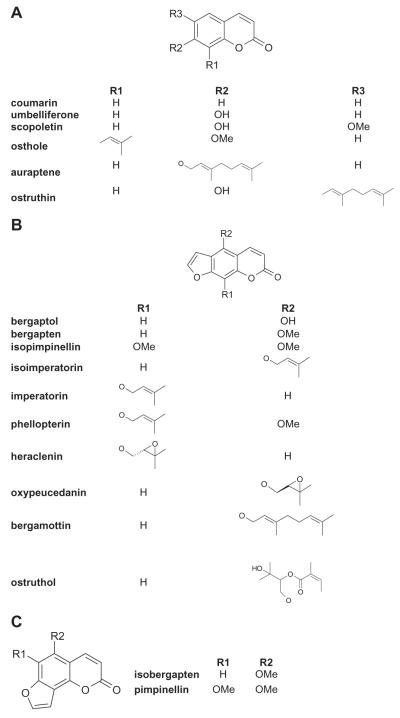
Fig. 1.
Structure of the selected compounds divided in the three groups: (A) simple coumarins, (B) linear furanocoumarins and (C) angular furanocoumarins.
Fig. 2.
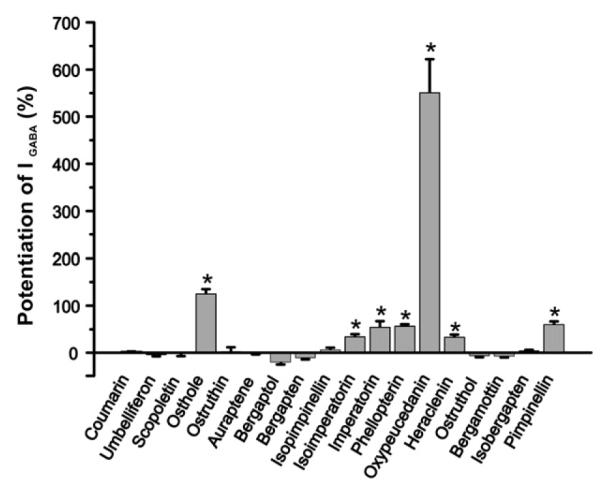
Potentiation of IGABA by different coumarins and furanocoumarins (100 μM) from a selection of 18 coumarin derivatives in oocytes expressing α1β2γ2S GABAA receptors. The compounds are ordered in following groups: simple coumarins, linear coumarins and angular coumarins. Within these groups the columns are arranged according to the length of the sidechain – small (−OH, −OMe), medium (−OC5, −C5) and large (−OC10, −C10). Statistical significance (t-test, P<0.05) is indicated with (*).
Fig. 3.
(A) Concentration–response curve for IGABA enhancement by osthole (EC50=14±1 μM, nH=1.4±0.3, n=4) in oocytes expressing α1β2γ2S GABAA receptors. (B) Representative currents through α1β2γ2S GABAA receptors in the presence of GABA (10 μM, single bar, control) and currents recorded during co-application of GABA (10 μM) and 1, 3, 10, 30, 100 and 300 μM of osthole (double bar).
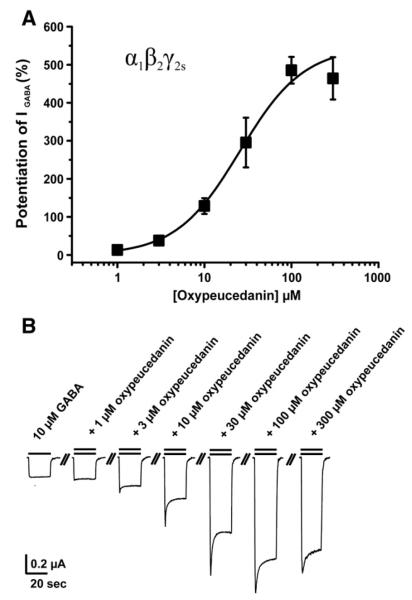
The concentration–response curve for oxypeucedanin (EC50 of 26±8 μM, nH=1.2±0.1, n=4) is shown in Fig. 4. Neither of the tested compounds activated the GABAA receptor in the absence of GABA in concentrations up to 300 μM suggesting an allosteric modulation. The observed enhancement of IGABA was always reversible.
Fig. 4.
(A) Concentration–response curve for IGABA enhancement by oxypeucedanin (OPD, EC50=26±8 μM, nH=1.2±0.1, n=4) in oocytes expressing α1β2γ2S GABAA receptors. (B) Representative currents through α1β2γ2S GABAA receptors in the presence of GABA (10 μM, single bar, control) and currents recorded during co-application of GABA (10 μM) and 1, 3, 10, 30, 100 and 300 μM of oxypeucedanin (double bar).
3.2. Osthole and oxypeucedanin modulate GABAA receptors not via the benzodiazepine binding site
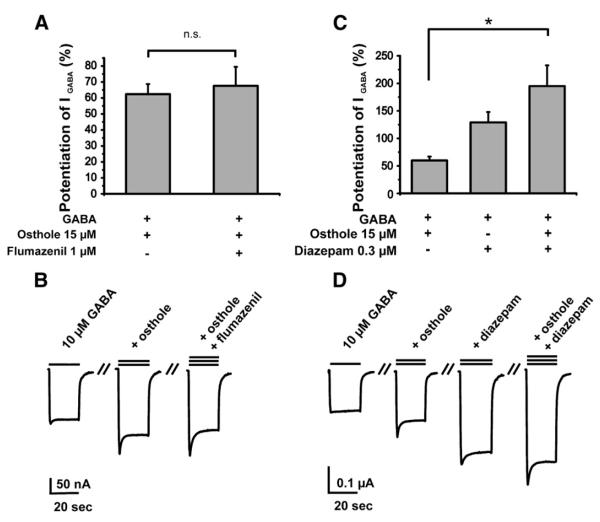
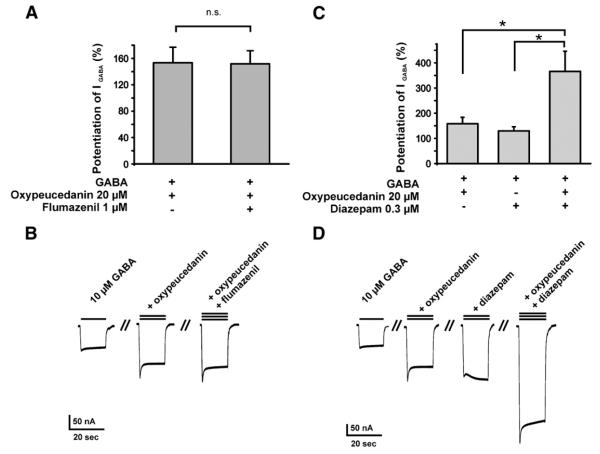
To determine if osthole or oxypeucedanin modulate GABAA receptors by interaction with the benzodiazepine binding site, IGABA modulation by these two compounds was studied in the presence and absence of flumazenil (1 μM) or diazepam (300 nM). Co-application of flumazenil (1 μM) did neither inhibit osthole- (15 μM) nor oxypeucedanin-induced (20 μM) potentiation of IGABA (62±6%, n=4 vs. 68±12%, n=4, Fig. 5A; and 152±20%, n=5 vs. 153±24%, n=5, Fig. 6A). When osthole (15 μM) and diazepam (300 nM) were co-applied an additive increase in the IGABA amplitude (195±38%, n=5) was observed compared to IGABA modulation by diazepam (129±19, n=4) and osthole (60±7%, n=5, Fig. 5C). Similar observations were made for oxypeucedanin (Fig. 6C, 300 nM diazepam: 130±16%, n=3, 20 μM oxypeucedanin: 158±26%, n=5 vs. oxypeucedanin and diazepam co-applied: 366±80%, n=3).
Fig. 5.
Effect of osthole on IGABA in the presence of flumazenil and diazepam in oocytes expressing α1β2γ2S GABAA receptors. Statistical significance (one-way ANOVA, P<0.05) is indicated with (*), n.s.= not significant (P>0.05). (A) Stimulation of IGABA by osthole in the presence of flumazenil (1 μM).The left bar shows the positive allosteric modulation of the GABA (EC5–10)-induced chloride currents by 15 μM osthole. The right bar illustrates that flumazenil (1 μM) does not antagonize the osthole-induced enhancement of IGABA. (B) Representative currents through α1β2γ2S receptors in the absence and presence of the indicated concentrations of osthole or osthole and flumazenil, respectively. The leftmost current represents the GABA control current (10 μM, single bar). (C) Additive effects of osthole and diazepam on IGABA. The left bar illustrates the enhancement of IGABA by 15 μM osthole, the middle bar by 300 nM diazepam, and the right bar illustrates the enhancement of IGABA by co-application of osthole (15 μM) and diazepam. (300 nM). (D) Representative currents through α1β2γ2S receptors in the absence and presence of the indicated concentrations of osthole, diazepam, or osthole and diazepam, respectively. The leftmost current represents the GABA control current (10 μM, single bar).
Fig. 6.
Effect of oxypeucedanin on IGABA in the presence of flumazenil and diazepam in oocytes expressing α1β2γ2S GABAA receptors. Statistical significance (one-way ANOVA, P<0.05) is indicated with (*), n.s. = not significant (P>0.05). (A) Stimulation of IGABA by oxypeucedanin in the presence of flumazenil (FLZ, 1 μM). The left bar shows the positive allosteric modulation of the GABA (EC5–10)-induced chloride currents by 20 μM oxypeucedanin. The right bar illustrates that flumazenil (1 μM) does not antagonize the oxypeucedanin-induced enhancement of IGABA. (B) Representative currents through α1β2γ2S receptors in the absence and presence of the indicated concentrations of oxypeucedanin, or oxypeucedanin and flumazenil, respectively. The leftmost current represents the GABA control current (10 μM, single bar). (C) Additive effects of oxypeucedanin and diazepam on IGABA The enhancement of IGABA by oxypeucedanin (20 μM, left bar) or 300 nM diazepam (middle bar) is increased in an additive manner when oxypeucedanin and diazepam are co-applied (right bar). (D) Representative currents through α1β2γ2S receptors in the absence and presence of the indicated concentrations of oxypeucedanin, diazepam, or oxypeucedanin and diazepam, respectively. The leftmost current represents the GABA control current (10 μM, single bar).
3.3. Isopentenyl residues are a structural requirement for allosteric modulation of GABAA receptors
Insights into the structural requirements for GABAA receptor modulation by coumarins were obtained by comparing the action of 18 different coumarin derivatives (Fig. 2, Table 1). From the 6 simple coumarins only the prenylated osthole (100 μM) significantly potentiated IGABA by 124±12%. All other coumarins, which contained small hydroxyl or methoxyl groups (coumarin, umbelliferone, scopoletin) as well as the components with bulkier (geranyl/geranyloxy/other) residues (ostruthin, auraptene), did not enhance IGABA when co-applied with GABA at 100 μM (Fig. 2, Table 1). From the linear furanocoumarins (100 μM), only compounds with oxyprenylresidues modulated IGABA. The epoxy-group containing oxypeucedanin (100 μM) induced the strongest potentiation (550±71%, n=5, Fig. 2, Table 1). The same concentration of heraclenin induced much less IGABA stimulation (33±6%, n=3). Other tested furanocoumarins with an oxyisopentenyl residue – isoimperatorin, imperatorin, and phellopterin (all at 100 μM) – potentiated IGABA by 34±6% (n=4), 54±13% (n=4) and 57±4% (n=3), respectively. None of the other components displayed IGABA enhancement above ≥+10%, e.g. the furanocoumarins with only methyoxy or hydroxyl groups e.g. bergaptol (−20±5%, n=4), bergapten (−11±4%, n=4) and isopimpinellin (6±5%, n=4). The same is valid for the furanocoumarins with bulkier residues such as the oxygeranylated bergamottin (−7±3%, n=4) and the ester compound ostruthol (−7±3%, n=3, Fig. 2, Table 1). These data correlate with the results of the simple coumarin group (see Fig. 1 for classification). Interestingly, while the angular furanocoumarin isobergapten showed no activity, its two times methoxylated derivative pimpinellin enhanced IGABA by 65±5% (n=4).
3.4. Molecular modeling
First insights into the structural features necessary for significant IGABA potentiation were further complemented by preliminary computational studies. As more than half of the compounds are inactive, classification algorithms rather than classical QSAR analysis were chosen. Binary classification tree analysis as implemented in the software package MOE revealed a highly significant model (total accuracy=88.9%) based solely on polarisability (apol) as descriptor. Compounds with apol values <31,6 or >47,6 are assigned as inactive, whereas those exhibiting values within this range show GABA-modulating potency.
Furthermore, a pharmacophore model was constructed using oxypeucedanin as template. The model consists of three hydrophobic regions and one aromatic feature resulting in 4 false positive and 1 false negative annotations. All 4 false positives (ostruthin, auraptene, bergamottin, and ostruthol) are compounds with either a geranyl or oxy-geranylresidue. To exclude these compounds, several volume exclusion domains were placed along the templates, but no decrease in the number of false positives was achieved. The inclusion of hydrogen acceptor regions (Acc) in the pharmacophore model always led to a reduction of the number of true positives. Further addition of Hyd or AtomQ features in the prenyl residue or the ring system had the same effect. The final model (18 compounds) produced 6 true positives, 7 true negatives, 1 false negative (phellopterin) and 4 false positives, resulting in an overall accuracy of 72%, a sensitivity of 86% and a specificity of 64%.
4. Discussion
Coumarins are a class of secondary metabolites commonly found in various plant families. Despite the well known anticoagulant action of the class of 3-substitued 4-hydroxycoumarins (Gebauer, 2007; Hirsh et al., 2001; Sadler, 2004), the pharmacological properties of many natural coumarin derivatives are insufficiently characterized (Yarnell and Abascal, 2009). Previous studies with (furano)coumarins revealed photosensitizing (Abouelzahab et al., 1987; Eisenbrand, 2007), antimicrobial (Tsassi et al., 2010; Widelski et al., 2009), antioxidant (Kostova, 2006; Piao et al., 2004) and cytotoxic activity (Kostova, 2005; Thanh et al., 2004; Yang et al., 2003). There is also evidence for neuroprotective (Epifano et al., 2008) and antiepileptic effects (Luszczki et al., 2007a,b, 2009a,b) induced by coumarins.
Effects of coumarins and furanocoumarins on GABAA receptors were first suggested by Bergendorff et al. (1997) and Dekermendjian et al. (1996) who observed [3H]diazepam displacement in the presence of furanocoumarins, especially phellopterin. Direct evidence for potential effects of a furanocoumarin related substance on the GABAA receptor comes from recent studies, which described a positive allosteric modulation of IGABA by a novel plant derived dihydroisocoumarin (Li et al., 2010) and coumarins from Angelica pubescens L. (Zaugg et al., 2011).
We have therefore systematically analyzed 18 structurally diverse coumarin derivatives for IGABA enhancement. A comparison of their activity on GABAA receptors enabled first insights into their structure–activity relationship. From the tested 18 structurally diverse coumarins, imperatorin, isoimperatorin, phellopterin, osthole, oxypeucedanin, heraclenin, and pimpinellin potentiated IGABA by more than 20% when applied at 100 μM (Fig. 2). All 7 components, except the angular furanocoumarin pimpinellin, bear either an oxyprenyl or a prenyl residue, while the position of the side chain varies. This indicates that the C5 side chain represents a structural requirement for IGABA modulation. While osthole, the second most active compound, is a simple coumarin, the most efficient substance – oxypeucedanin – represents a furanocoumarin with an epoxylated oxyprenylresidue. The stabilizing effect of the two geminal methyl groups rules out an unspecific effect caused by the chemical reactivity of the epoxide moiety. Interestingly, the regioisomeric heraclenin showed a more than 10-fold loss of activity (31%). Furthermore, both regioisomers of the respective furanocoumarin analogue (isoimperatorin and imperatorin) were almost equally active, which indicates that the different activities of oxypeucedanin and heraclenin could be due to the different configuration of the chiral center rather than to the different position of the side chain. Extending the prenyl side chain of isoimperatorin by one additional isopentenyl moiety (bergamottin) or attaching a large and sterically complex group (ostruthol) completely abolished biological activity. Finally it’s worth mentioning that in the group of angular furanocoumarins one additional methoxy group leads to a remarkable increase in IGABA potentiation (pimpinellin vs isobergapten). This also accounts for the configurational isomers pimpinellin and isopimpinellin, where the compound with the angular scaffold (pimpinellin) is more active than the respective linear analogue (isopimpinellin).
In order to gain deeper insights into the molecular features relevant for high biological activity, we also performed preliminary computational studies utilizing both a decision tree algorithm and pharmacophore modeling.
Abinary classification tree based on a small set of physicochemical descriptors was able to classify 17 out of 18 compounds correctly, using only polarisability (apol) as descriptor (Fig. 7). The only compound miss-classified in this model was isopimpinellin (FP). However, one needs to consider that the descriptor used (apol) cannot distinguish between the configurational isomeres isopimpinellin (inactive) and pimpinellin (active), giving for both compounds a value of 33.56. In conclusion, this model might be useful for predicting the activity of structurally analogous derivatives.
Finally, a pharmacophore model was constructed which could aid in the understanding of the main pharmacophoric features necessary for IGABA enhancement by coumarins. Using oxypeucedanin as template, 6 out of 7 actives were correctly annotated as active. Having additionally 4 false positives, a total accuracy of 72% with a sensitivity of 86% and a specificity of 64% was achieved. The high sensitivity indicates that the model would be a versatile tool for in silico screening attempts in order to identify potentially actives out of a coumarine-based compound library. The pharmacophore used consisted of three hydrophobic regions placed at the prenyl residue and opposite the carbon atom linking the basic skeleton with the side chain, and one aromatic feature (Fig. 8). Interestingly, introduction of other typical features like hydrogen-bond acceptor zones or an additional aromatic domain could not improve the model but rather resulted in assignment of active compounds as false negatives.
Fig. 8.
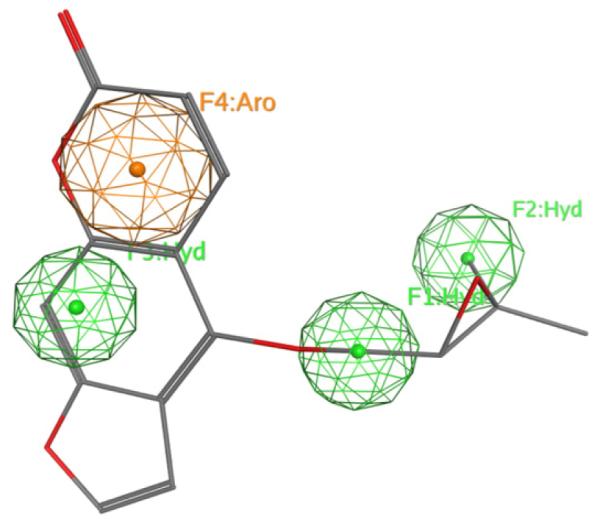
4-point pharmacophore model for coumarins. Superposition of oxypeucedanin with the pharmacophore query. Green color indicates hydrophobic regions and orange represents the aromatic feature.
However, the feasibility of this 4-point pharmacophore for virtual screening should be taken with caution, since these rather unspecific hydrophobic zones will probably lead to a high number of false positives when used for screening. Nonetheless, given that geranylated coumarins are not active in vitro, screening of a coumarin database which has upfront been cleaned from such compounds might lead to interesting new hits, especially when combined with the binary classification tree.
Interestingly, neither osthole nor oxypeucedanin induce a current in the absence of GABA, which distinguishes the action of osthole and oxypeucedanin from other modulators like etomidate, or the barbiturates. Furthermore, our data clearly show that the two components do not interact with the binding sites of the benzodiazepines (Figs. 5 and 6). Future studies employing point-mutated receptors will clarify the exact binding site of constituents like osthole and oxypeucedanin on the GABAA receptor.
The effect of the tested coumarins occurs at very high concentrations which makes a therapeutic application unlikely, although all compounds tested met the Lipinski Rule of Five, indicating a low risk of insufficient bioavailability. However, it is currently not known if such a high concentrations can enter the brain. When referring to their anti-convulsant activity in vivo, the high concentrations needed for IGABA by such compounds as imperatorin suggest that coumarins exert their effects not exclusively via the GABAA receptor, but may additionally interact with other receptors supporting an anticonvulsive (Luszczki et al., 2007a,b, 2009a,b).
Acknowledgments
This work was supported by the University of Vienna (Initiative Group “Molecular Drug Targets”) and P 19614-B11 (S.H.). Special thanks to Prof. Ernst Urban for NMR and Dr. Sophia Khom for helpful suggestions on methodology and manuscript.
References
- Abouelzahab MM, Metwally MA, Dawidar AM, Abdelmogib M, Abumustafa EA. Photochemical-reactions of the natural furocoumarin, imperatorin. Bull. Chem. Soc. Jpn. 1987;60:4433–4435. [Google Scholar]
- Baburin I, Beyl S, Hering S. Automated fast perfusion of Xenopus oocytes for drug screening. Pflugers Arch. Eur. J. Phy. 2006;453:117–123. doi: 10.1007/s00424-006-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baburin I, Khom S, Timin E, Hohaus A, Sieghart W, Hering S. Estimating the efficiency of benzodiazepines on GABA(A) receptors comprising gamma1 or gamma2 subunits. Br. J. Pharmacol. 2008;155:424–433. doi: 10.1038/bjp.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Simmen U, Senn M, Sequin U, Sigel E. Novel plant substances acting as beta subunit isoform-selective positive allosteric modulators of GABA(A) receptors. Mol. Pharmacol. 2005;68:787–792. doi: 10.1124/mol.105.011882. [DOI] [PubMed] [Google Scholar]
- Bergendorff O, Dekermendjian K, Nielsen M, Shan R, Witt R, Ai J, Sterner O. Furanocoumarins with affinity to brain benzodiazepine receptors in vitro. Phytochemistry. 1997;44:1121–1124. doi: 10.1016/s0031-9422(96)00703-0. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA. Effects of [gamma]2S subunit incorporation on GABA(A) receptor macroscopic kinetics. Neuropharmacology. 2003;44:1003–1012. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Dekermendjian K, Ai J, Nielsen M, Sterner O, Shan R, Witt M-R. Characterization of the furanocoumarin phellopterin as a rat brain benzodiazepine receptor partial agonist in vitro. Neurosci. Lett. 1996;219:151–154. doi: 10.1016/s0304-3940(96)13183-9. [DOI] [PubMed] [Google Scholar]
- Demel MA, Kraemer O, Ettmayer P, Haaksma E, Ecker GF. Ensemble rule-based classification of substrates of the human ABC-transporter ABCB1 using simple physicochemical descriptors. Mol. Inf. 2010;29:233–242. doi: 10.1002/minf.200900079. [DOI] [PubMed] [Google Scholar]
- Eisenbrand G. Toxicological assessment of furocoumarins in foodstuffs. Mol. Nutr. Food Res. 2007;51:367–373. doi: 10.1002/mnfr.200600270. [DOI] [PubMed] [Google Scholar]
- Epifano F, Molinaro G, Genovese S, Ngomba RT, Nicoletti F, Curini M. Neuroprotective effect of prenyloxycoumarins from edible vegetables. Neurosci. Lett. 2008;443:57–60. doi: 10.1016/j.neulet.2008.07.062. [DOI] [PubMed] [Google Scholar]
- Fernandez SP, Mewett KN, Hanrahan JR, Chebib M, Johnston GAR. Flavan-3-ol derivatives are positive modulators of GABA(A) receptors with higher efficacy for the alpha2 subtype and anxiolytic action in mice. Neuropharmacology. 2008;55:900–907. doi: 10.1016/j.neuropharm.2008.06.069. [DOI] [PubMed] [Google Scholar]
- Gebauer M. Synthesis and structure–activity relationships of novel warfarin derivatives. Bioorg. Med. Chem. 2007;15:2414–2420. doi: 10.1016/j.bmc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Granger RE, Campbell EL, Johnston GAR. (+)- and (−)-borneol: efficacious positive modulators of GABA action at human recombinant [alpha]1[beta]2[gamma]2 L GABA(A) receptors. Biochem. Pharmacol. 2005;69:1101–1111. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119:8S–21S. doi: 10.1378/chest.119.1_suppl.8s. [DOI] [PubMed] [Google Scholar]
- Huen MS, Hui KM, Leung JW, Sigel E, Baur R, Wong JT, Xue H. Naturally occurring 2′-hydroxyl-substituted flavonoids as high-affinity benzodiazepine site ligands. Biochem. Pharmacol. 2003;66:2397–2407. doi: 10.1016/j.bcp.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Johnston GAR, Hanrahan JR, Chebib M, Duke RK, Mewett KN, Enna SJ. Modulation of Ionotropic GABA Receptors by Natural Products of Plant Origin. Advances in Pharmacology. 2006:285–316. doi: 10.1016/s1054-3589(06)54012-8. Elsevier, San Diego, CA. [DOI] [PubMed] [Google Scholar]
- Khom S, Baburin I, Timin EN, Hohaus A, Sieghart W, Hering S. Pharmacological properties of GABA(A) receptors containing gamma1 subunits. Mol. Pharmacol. 2006;69:640–649. doi: 10.1124/mol.105.017236. [DOI] [PubMed] [Google Scholar]
- Khom S, Baburin I, Timin E, Hohaus A, Trauner G, Kopp B, Hering S. Valerenic acid potentiates and inhibits GABA(A) receptors: molecular mechanism and subunit specificity. Neuropharmacology. 2007;53:178–187. doi: 10.1016/j.neuropharm.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Gründer G, Lüddens H. Drug interactions at GABA(A) receptors. Prog. Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Kostova I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anticancer Agents. 2005;5:29–46. doi: 10.2174/1568011053352550. [DOI] [PubMed] [Google Scholar]
- Kostova I. Synthetic and natural coumarins as antioxidants. Mini-Rev. Med. Chem. 2006;6:365–374. doi: 10.2174/138955706776361457. [DOI] [PubMed] [Google Scholar]
- Li Y, Plitzko I, Zaugg J, Hering S, Hamburger M. HPLC-based activity profiling for GABA(A) receptor modulators: a new dihydroisocoumarin from Haloxylon scoparium. J. Nat. Prod. 2010;73:768–770. doi: 10.1021/np900803w. [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Glowniak K, Czuczwar SJ. Time–course and dose–response relationships of imperatorin in the mouse maximal electroshock seizure threshold model. Neurosci. Res. 2007a;59:18–22. doi: 10.1016/j.neures.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Glowniak K, Czuczwar SJ. Imperatorin enhances the protective activity of conventional antiepileptic drugs against maximal electroshock-induced seizures in mice. Eur. J. Pharmacol. 2007b;574:133–139. doi: 10.1016/j.ejphar.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Andres-Mach M, Cisowski W, Mazol I, Glowniak K, Czuczwar SJ. Osthole suppresses seizures in the mouse maximal electroshock seizure model. Eur. J. Pharmacol. 2009a;607:107–109. doi: 10.1016/j.ejphar.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Wojda E, Andres-Mach M, Cisowski W, Glensk M, Glowniak K, Czuczwar SJ. Anticonvulsant and acute neurotoxic effects of imperatorin, osthole and valproate in the maximal electroshock seizure and chimney tests in mice: a comparative study. Epilepsy Res. 2009b;85:293–299. doi: 10.1016/j.eplepsyres.2009.03.027. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABA(A)-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Murray RDH, Méndez J, Brown SA. The Natural Coumarins-Occurrence, Chemistry and Biochemistry. John Wiley and Sons; New York: 1982. pp. 20–53. [Google Scholar]
- O’Kennedy R, Thorne RD. Coumarins: Biology, Applications and Mode of Action. Wiley; Chichester: 1997. pp. 23–66. [Google Scholar]
- Olsen RW, Sieghart W. GABA(A) receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao XL, Park IH, Baek SH, Kim HY, Park MK, Park JH. Antioxidative activity of furanocoumarins isolated from Angelicae dahuricae. J. Ethnopharmacol. 2004;93:243–246. doi: 10.1016/j.jep.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Priestley CM, Williamson EM, Wafford KA, Sattelle DB. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABA(A) receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003;140:1363–1372. doi: 10.1038/sj.bjp.0705542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler JE. Medicine: K is for koagulation. Nature. 2004;427:493–494. doi: 10.1038/427493a. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA (A) receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Enna SJ. Structure, Pharmacology, and Function of GABA(A) Receptor Subtypes. Advances in Pharmacology. 2006:231–263. doi: 10.1016/s1054-3589(06)54010-4. Elsevier, San Diego, CA. [DOI] [PubMed] [Google Scholar]
- Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABA(A) receptor genes in the human genome. J. Biol. Chem. 2004;279:41422–41435. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- Thanh PN, Jin W, Song G, Bae K, Kang SS. Cytotoxic coumarins from the root of Angelica dahurica. Arch. Pharm. Res. 2004;27:1211–1215. doi: 10.1007/BF02975883. [DOI] [PubMed] [Google Scholar]
- Trauner G, Khom S, Baburin I, Benedek B, Hering S, Kopp B. Modulation of GABAA receptors by valerian extracts is related to the content of valerenic acid. Planta Med. 2008;74:19–24. doi: 10.1055/s-2007-993761. [DOI] [PubMed] [Google Scholar]
- Tsassi VB, Hussain H, Meffo BY, Kouam SF, Dongo E, Schulz B, Greene IR, Krohn K. Antimicrobial coumarins from the stem bark of Afraegle paniculata. Nat. Prod. Commun. 2010;5:559–561. [PubMed] [Google Scholar]
- Vogl S, Zehl M, Picker P, Urban E, Wawrosch C, Reznicek G, Saukel J, Kopp B. Identification and quantification of coumarins in Peucedanum ostruthium (L.) Koch by HPLC-DAD and HPLC-DAD-MS. Food Chem. 2011;59:4371–4377. doi: 10.1021/jf104772x. [DOI] [PubMed] [Google Scholar]
- Widelski J, Popova M, Graikou K, Glowniak K, Chinou I. Coumarins from Angelica lucida L. –antibacterial activities. Molecules. 2009;14:2729–2734. doi: 10.3390/molecules14082729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LL, Wang MC, Chen LG, Wang CC. Cytotoxic activity of coumarins from the fruits of Cnidium monnieri on leukemia cell lines. Planta Med. 2003;69:1091–1095. doi: 10.1055/s-2003-45188. [DOI] [PubMed] [Google Scholar]
- Yarnell E, Abascal K. Plant Coumarins: Myths and Realities. Alternative & Complementary Therapies. 2009;15:24–30. [Google Scholar]
- Zaugg J, Baburin I, Strommer B, Kim HJ, Hering S, Hamburger M. HPLC-based activity profiling: discovery of piperine as a positive GABA(A) receptor modulator targeting a benzodiazepine-independent binding site. J. Nat. Prod. 2010;73:185–191. doi: 10.1021/np900656g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg J, Eickmeier E, Rueda DC, Hering S, Hamburger M. HPLC-based activity profiling of Angelica pubescens roots for new positive GABA(A) receptor modulators in Xenopus oocytes. Fitoterapia. 2011;82:434–440. doi: 10.1016/j.fitote.2010.12.001. [DOI] [PubMed] [Google Scholar]