Abstract
Although little is known about the etiology of progressive supranuclear palsy (PSP), genetic and epigenetic factors, oxidative injury and inflammation are thought to contribute to its development and/or progression. Evidence for activated glia involvement in PSP has raised the possibility that neuroinflammation may contribute to its pathogenesis. To investigate the correlation between neuroinflammation and PSP, a comparative study was conducted on the patterns of cytokine expression in different regions of the brains of PSP, Alzheimer’s disease (AD) patients and normal controls. Our results show different patterns of cytokine expression in each disease, with the expression of IL-1β transcripts being significantly higher in the substantia nigra of PSP than in AD and controls, while AD brains had significantly higher IL-1β expression in the parietal cortex compared to PSP and controls. In addition, expression of TGFβ was significantly higher in the cortical areas (particularly frontal and parietal lobes) of AD compared to PSP and controls. These results show a disease-specific topographical relationship among the expression of certain cytokines (IL-1β and TGFβ), microglial activation and neurodegenerative changes, suggesting that these cytokines may contribute to the pathologic process. If so, the use of cytokine-inhibitors and/or other anti-inflammatory agents may be able to slow disease progression in PSP.
Keywords: Alzheimer’s disease, brain, cytokines, inflammation, microglia, progressive supranuclear palsy
Progressive supranuclear palsy (PSP) is a neurodegenerative disease of unknown etiology characterized by progressive gait instability, supranuclear gaze abnormalities, levodopa-unresponsive parkinsonism and frontal cognitive disturbances [1,2]. PSP is the most common of the atypical parkinsonian disorders [3], which also include corticobasal degeneration, post-encephalitic parkinsonism and multisystem atrophy. The prevalence of PSP accounts for approximately 5-6% of suspected cases of parkinsonism [3]. Neuropathologically, PSP is considered a “tauopathy”, a group of neurodegenerative diseases that also includes Alzheimer’s disease (AD), and is characterized by the presence of abnormal microtubule associated protein tau (MAPT) aggregates as neurofibrillary tangles in both neurons and glia in specific cortical and subcortical regions [1,2,4]. In contrast to AD, tau pathology in PSP is not accompanied by amyloid deposits. Very little is known about the etiology and pathogenesis of PSP and related tauopathies, although genetic and epigenetic factors, oxidative injury and inflammation are thought to contribute to their development and progression. Evidence for activated glia in PSP raises the possibility that neuroinflammation, indeed, may be involved in its pathogenesis [5].
A substantial amount of data supports the idea that inflammation may play a role in many neurodegenerative diseases, with activated microglia being the common denominator [6,7]. While it is clear that not all activation of microglia results in neuronal cell death, un-regulated or over-active microglia are indeed capable of neurotoxic effects [8]. Activation of microglia may be induced by infectious agents, injury or chronic accumulation of abnormal protein aggregates [9]. The chronic release of inflammatory mediators, such as pro-inflammatory cytokines, reactive oxygen and nitrogen intermediates and arachidonic acid metabolites may promote the abnormal activation of microglia and astroglia, recruitment of inflammatory cells and destruction of normal neurons and synapses [10].
A role for pro-inflammatory cytokines in neuronal death is supported by several lines of evidence, including data showing that inhibition of endogenous pro-inflammatory cytokines, such as IL-1, results in decreased neuronal damage in a variety of animal models of neuronal injury [11]. In contrast, increased expression of pro-inflammatory cytokines, such as IL-1 IL-6 and TNFα, has been associated with neuronal damage in both acute and chronic neurologic diseases, including AD and Parkinson’s disease [11-15].
Establishing a correlation between PSP pathology and cytokine alterations would be important not only in understanding the pathogenesis of the disease, but in considering cytokine-inhibitors or other anti-inflammatory agents as potential therapeutic strategies. Thus, the purpose of this study was to investigate and compare the expression patterns of several cytokines (IL-1β, TNFα, IL-6, TGFβ) in different regions of PSP and AD brains. Inasmuch as PSP and AD primarily affect different areas of the brain, dissimilar patterns of expression were predicted. The study consisted in the analysis of cytokine expression and microglial burdens in tissue samples from six different regions obtained from post-mortem brains of patients with PSP, AD and normal controls.
MATERIALS AND METHODS
Brain tissue samples
Post-mortem tissue samples were obtained from brains donated to the CurePSP Society Brain Bank, which operates under a specific IRB protocol approved by the Mayo Clinic. The legal next-of-kin or person with power of attorney signed a consent form allowing the use of donated brain tissue for research purposes. One half of each brain was preserved frozen and used for the isolation of RNA, while the other half was formalin-fixed and used for immunohistochemical studies. The relative abundance of cytokine transcripts was studied in the subthalamic nucleus (ST), caudate nucleus (CN), substantia nigra (SN), as well as parietal (PC), frontal (FC) and occipital (OPC) cortices. Tissue samples were dissected from the freshly-thawed, unfixed post-mortem brains of each of 5 PSP, 5 AD and 4 normal controls without neurodegenerative disease under the direct supervision of an experienced neuropathologist (D.W.D.). The ST was identified as a lentiform structure rostral to the SN and ventral to the thalamus. Immunohistochemical studies for assessment of the glial burden were performed on formalin-fixed, paraffin-embedded tissue from the contralateral regions of the same brains. The diagnoses of PSP and AD were based on published criteria [4,16]. The control group included post-mortem brains from three aged-matched normal individuals and one with Agyrophillic grains disease/age-associated calcification. The PSP, AD and control brains were matched for age and RNA Integrity number (RIN), a measure of the quality of isolated RNA [17]. RINs were obtained from frozen brain tissue following microcapillary electrophoretic RNA separation and analysis using an Agilent 2100 Bioanalyzer and Total Eukaryotic RNA nanoscale chips (Agilent Technologies, Santa Clara, CA). The characteristics of these groups are shown in Table 1.
TABLE 1. Summary of characteristics of post-mortem brains groups.
| Group/Pathology | Braak Score | Age (years) | Sex | RIN |
|---|---|---|---|---|
| PSP | 0.9 ±0.9 | 71.4±4.0 | 5M | 5.9±1.3 |
| AD | 5.8±0.4 | 72.4±3.3 | 3M/2F | 5.9±1.0 |
| Normal | 1.9±1.4 | 77.8±3.9 | 2M/2F | 4.5±2.1 |
Numbers represent average and S.D. for each group. PSP: Progressive Supranuclear Palsy; AD: Alzheimer’s Disease; RIN: RNA integrity number; M: male; F: female.
Total RNA Extraction
Approximately 0.2 × 0.2 cm tissue samples from each region were dissected. Samples were homogenized in 1 ml TRI reagent (Ambion, Austin, TX) using a Polytron tissue homogenizer (Kinematica, Switzerland) and total RNA isolated from the homogenates following the manufacturer’s recommendations. The quality and quantity of the RNA was assessed spectrophotometrically. The OD260/OD280 ratios were typically in the 1.8-2.1 range. RNA quality and absence of significant RNA degradation was confirmed by formaldehyde gel electrophoresis and staining with propidium iodide.
Reverse-transcription and Real-time PCR
Approximately 1 μg of total RNA per sample was used to prepare cDNA using a Reverse transcription kit according to the manufacturer’s recommendations (Applied Biosystems, Foster City, CA). The relative abundance of cytokine transcripts was then assessed by real time polymerase chain reaction (PCR) amplification using specific primers and SYBR green I reagents for detection in an Applied Biosystems 7300 Real Time PCR System. Reactions in which the enzyme or cDNA were omitted were be used as negative controls. The relative gene expression of the different cytokines was analyzed using the 2−ΔΔCt method by normalizing to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene in all the experiments. With exception of the cerebellum, uniform levels of GAPDH expression has been reported in different areas of the brain, thus making this a convenient housekeeping gene for normalization [18]. Melting curve analysis of each reaction was used to discard primer-dimer artifacts or contamination and to ensure reaction specificity. Primers used in these studies were:
GAPDH: (122-141): 5′-GAGTCAACGGATTTGGTCGT-3′
(335-316): 5′-TGGAAGATGGTGATGGGATT-3′
IL-1β: (1009-1028): 5′-TCTACACCAATGCCCAACTG-3′
(1213-1194): 5′-AGCGAATGACAGAGGGTTTC-3′
IL-6: (241-262): 5′-CACACAGACAGCCACTCACCTC-3′
(375-356): 5′-CTGCCAGTGCCTCTTTGCTG-3′
TNFα: (251-269): 5′-AGGCGGTGCTTGTTCCTCA-3′
(417-394): 5′-GTTCGAGAAGATGATCTGACTGCC-3′
TGFβ1: (1407-1427): 5′-CAACAATTCCTGGCGATACCT-3′
(1542-1522): 5′-GCTAAGGCGAAAGCCCTCAAT-3′
Microglial burden
The microglial burden was investigated in formalin-fixed, paraffin-embedded tissue samples using the method described by Ishizawa and Dickson [5], based on immunohistochemical staining of activated microglia with a monoclonal anti-human HLA-DR (LN3; ICN Biomedicals, Costa Mesa, CA) followed by image analysis. The captured images were processed with a Sigma Scan Pro image analysis software (Jandel Scientific, San Rafael, CA) by conversion to gray-scale images and detection of the immunolabel by its pixel intensity. The area occupied by the labeling was measured and a ratio of immunoreactive (LN3) to total pixels was calculated to estimate the burden. Microglial burden is expressed as the LN3 %-area.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Data distribution was analyzed using the D’Agostino and Pearson omnibus normality test. Comparisons between groups were performed using one-way ANOVA followed by Bonferroni’s post-hoc test. Correlation analyses were performed by the Pearson’s method. Values of p<0.05 were considered statistically significant.
RESULTS
Comparison of the relative abundance of cytokine transcripts
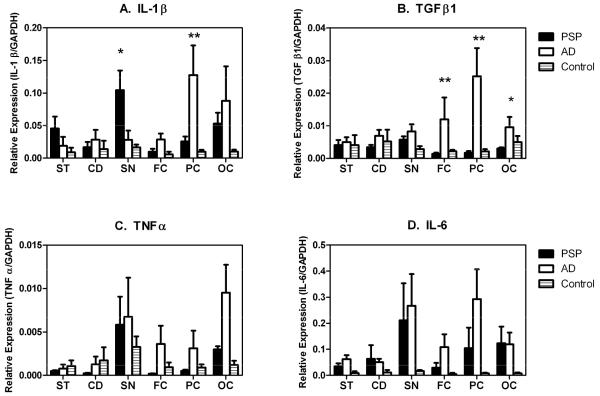
Transcripts for all four cytokines investigated were consistently detected in all brain groups. The relative abundance of cytokine transcripts was IL-6>IL-1β>TGFβ>TNFα in all brain groups when expressed as the ratio of the cytokine transcripts relative to that of the house-keeping gene control, GAPDH (Figure 1A-D). Comparison of the results showed that IL-1β expression levels were higher in the ST and SN of PSP brains, when compared to those of AD and normal controls. In contrast, they were higher in the cortical regions of AD brains, particularly the FC and PC, compared to PSP or normal controls (Figure 1A). There were significant differences (p<0.05) between PSP and AD patients for the SN (higher in PSP) and PC (higher in AD). While the expression levels of TGFβ transcripts in the ST and SN of PSP were not different from AD, their expression in the cortical areas (FC, PC and OC) was statistically higher for AD patients compared to PSP patients and controls (Figure 1B).
Figure 1.
Relative expression of transcripts for IL-1β (A), TGFβ1 (B), TNFα (C) and IL-6 (D) in the subthalamic nucleus (ST), caudate nucleus (CD), substantia nigra (SN), frontal cortex (FC), parietal cortex (PC) and occipital cortex (OC) of post-mortem brains from PSP, AD patients and controls. Transcript expression was measured by real time PCR after reverse transcription of total RNA extracted from the dissected tissues. The relative abundance of each transcript is expressed as its ratio to that of the house-keeping gene, GAPDH. Results represent the mean and standard error (n=5 [PSP and AD]; n= 4 [controls]). Statistically-significant differences, * (p < 0.05) and ** (p<0.01), over the corresponding groups are indicated.
The expression pattern of IL-6 and TNFα generally mirrored that of TGFβ, being higher in the cortical areas of AD compared to PSP (Figures 1C and 1D). Although the expression of these two cytokine transcripts in the SN appeared to be higher than in the ST and CN, there were no appreciable differences between PSP and AD. The average levels of expression for the control samples were relatively lower, suggesting a relative overall higher expression in the brains affected by PSP and AD.
Microglial burden
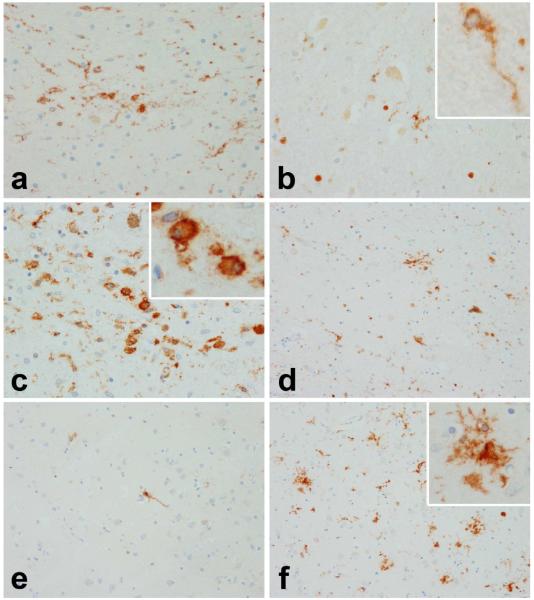
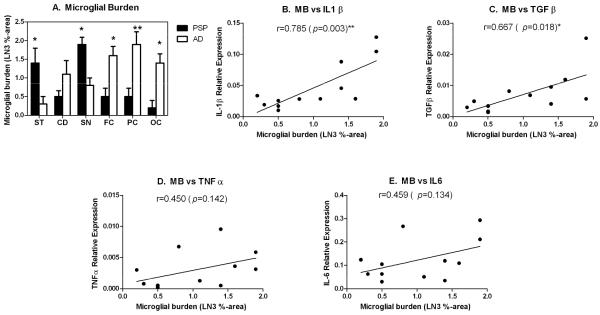
To investigate and compare the degree of microglial activation in the same brain areas analyzed in the cytokine expression studies, the microglial burden was determined in the contralateral regions of the same brains. Two clearly distinct patterns were observed when comparing PSP to AD brains. Figure 2a-f shows micrographs depicting representative staining patterns in the ST (a,b), SN (c,d) and mid-FC (e,f) of PSP (a,c,e) and AD brains (b,d,f). In PSP, activated microglia are present in those regions with neurodegeneration (ST, SN), while resting-type microglia (i.e., ramified) are present in an unaffected area (FC). In contrast, in AD sparse ramified microglia are present in areas relatively unaffected (ST, SN) while affected areas (FC) have activated microglia. Insets in Figures 2b and 2c show ramified and a phagocytic (ameboid) microglial cells, respectively. Inset in Figure 2f shows activated microglia associated with a senile plaque. Figure 3A presents the quantification of the microglial burden in the different brain regions. Whereas PSP had higher microglial burdens in the SN (average score of 1.9) and ST (average score: 1.4) compared to other brain regions, AD had the highest microglial burden scores in cortical areas, particularly the PC and FC (average score: 1.9).
Figure 2.
Micrographs of representative LN3-staining (HLA-DR) used for assessment of microglial burden in the subthalamic nucleus (a,b), substantia nigra (c,d) and mid-frontal cortex (e,f) of PSP (a, c & e) and AD brains (b, d & f). Insets in Figures 2b and 2c show ramified and a phagocytic (ameboid) microglial cells, respectively. Inset in Figure 2f shows activated microglia associated with a senile plaque. HLA-DR staining was performed by immunohistochemistry on paraffin-embeded tissue from the contralateral areas of the same brains used for cytokine analyses.
Figure 3.
A. Microglial burden in the subthalamic nucleus (SN), caudate nucleus (CD), substantia nigra (SN), frontal cortex (FC), parietal cortex (PC) and occipital cortex (OC) of post-mortem brains from PSP and AD patients. Microglial burden was measured after staining for HLA-DR expression by immunohistochemistry with the LN3 antibody followed by image analysis and expressed as the percent of the total area occupied by the LN3 label (LN3 %-area). Scores represent the mean and standard error (n=5). B-E. Linear regression analysis of the correlation between cytokine transcript expression and the microglial burden (MB). Expression of transcripts for IL-1β (B), TGFβ1 (C), TNFα (D) and IL-6 (E) in all six brain areas examined were plotted against the microglial burden scores. Pearson correlation coefficients (r) are indicated in each graph. Statistically significant correlations, * (p < 0.05) and ** (p<0.01), are indicated.
Correlation between cytokine expression and microglial burden
The degree of correlation between the cytokine expression and the microglial burden scores was investigated in all six brain regions (Figure 3B-E). The most significant correlation with microglial burden was with IL-1β, which had a positive correlation (r=0.785; p=0.003). The microglial burden also showed a positive correlation with the expression of TGFβ (r=0.667; p=0.018). The expression of TNFα and IL-6 did not show significant correlations with microglial burden (r= 0.450, p=0.142; and r=0.459, p= 0.134, respectively).
DISCUSSION
Results of this study indicate that expression of IL-1β as well as the microglial burden in brain tissue are increased in the ST and SN of PSP, and FC and PC of AD brains, the areas primarily affected in each disease. Moreover, the expression of TGFβ and two pro-inflammatory cytokines, IL-6 and TNFα, appeared to be increased in cortical regions of AD. These results point to a correlation between the local alterations in the expression of cytokines, particularly IL-1β, and the areas preferentially affected in each disease, suggesting that these cytokines might play a role in the neurodegenerative process.
In a previous publication comparing microglial activation in PSP and corticobasal degeneration, Ishizawa and Dickson demonstrated that there was a high degree of correlation between the microglial activation, tau burden and the areas of degeneration in each disease, suggesting that microglial activation may play a role in their pathogenesis [5]. In PSP, microglial burden was highest in the basal ganglia and cerebellar output and input systems. The two neuroanatomic nuclei with the highest levels of microglial burden were the SN and the ST, which were precisely the areas that showed the highest levels of expression for IL-1β (and difference with AD) in our studies.
In addition to IL-1β, the other pro-inflammatory cytokines, TNFα and IL-6, also tended to be expressed at relatively higher levels in the SN, particularly when compared to normal controls. Their expression, however, was not significantly different when compared to the same areas of AD. The higher expression of pro-inflammatory cytokines in these areas is consistent with models proposing a role for inflammation in the CNS as a key event contributing to neuronal cell death. Although microglia normally respond to neuronal damage and remove damaged cells, conditions leading to chronic microglial activation and the sustained release of pro-inflammatory cytokines in addition to other potentially toxic factors, may promote neuronal cell death and contribute to the neurodegenerative process [8-10]. Whether pro-inflammatory cytokines, particularly IL-1β, play a causal role or are simply secondary to neuronal damage in PSP, remains to be determined.
In regard to a potential causal role, a model has been proposed in which the production of IL-1 by activated microglia is part of a feedback cascade whereby IL-1-mediated MAPK-p38 overexpression leads to tau hyperphosphorylation, decreased synaptophysin expression and neurofibrillary pathology [19. Thus, the involvement of IL-1β in neurodegenerative diseases is not only supported by its expression pattern but by its activity. In addition, evidence that certain polymorphisms that increase expression of IL-1α and IL-1β genes increase the risk of AD, further supports a potential role for these cytokines in the pathogenesis of neurodegenerative disorders [20].
In the case of TGFβ1, our studies detected significantly increased expression in cortical areas of AD, but not PSP. These results are consistent with reports that the expression of the TGFβ cytokine family is induced by Aβ amyloid and increased in areas of plaque deposition [21]. The roles of TGFβ1 and other members of the TGFβ family (TGFβ2, TGFβ3) in the pathology of AD and other neurodegenerative diseases are not clear, since they have been reported to have both protective and harmful effects [22-24]. TGFβ1 is normally considered an anti-inflammatory cytokine, and thus its increased expression near amyloid plaques may be beneficial, probably representing an attempt to counteract inflammatory reactions, including inhibition of the production of pro-inflammatory cytokines [7]. In this regard, TGFβ1 has been reported to repress activation and resultant death of microglia through inhibition of phosphatidyl inositol 3-kinase activity [23]. On the other hand, there is also substantial evidence that expression of TGFβ1 may also promote amyloidogenesis [22,24]. For example, co-expression of amyloid precursor protein and TGFβ1 in astrocytes resulted in accelerated deposition of amyloid-β peptide in transgenic mice [22]. Although the role of TGFβ family members in AD is supported by many reports, its potential role in PSP remains unclear. Even though our studies did not detect significantly increased expression of TGFβ1 in PSP brains compared to controls, the finding of increased expression of TGF-β receptor-I (TGFβ RI) in reactive glia of both AD and PSP [25] suggests that some members of this cytokine family may play a role in PSP.
Inasmuch as transcripts for both pro-inflammatory cytokines and TGFβ were detected in our studies, the potential modulatory effects of latter on the actual expression of cytokines such as IL-1β and TNFα need to be considered. In this regard, TGFβ has been reported to regulate the production of TNFα and IL-1β at multiple levels, in some cases inhibiting translation of their transcripts [26-28]. Thus, it is possible that expression of TNFα and IL-1β at the protein level might be tempered by the activity of TGFβ.
A key issue and potential limitation to analyses of expression of RNA transcripts in post-mortem brains, such as the present study, is the quality of tissue/RNA used. Inasmuch as post-mortem interval has been demonstrated to be a poor indicator of brain RNA integrity [29], we attempted to match our samples for RIN, which more closely match the tissue quality when it was actually used. RINs integrate effects on RNA due to freeze-thaw, storage, in addition to postmortem delay and effects of agonal state. Another limitation to our study was the relatively low number of individuals analyzed and the statistical implications of a low sample size. Our study succeeded in establishing that expression of IL-1β was significantly increased in those brain areas affected by neurodegeneration in PSP brains. However, differences in the clinical manifestations of PSP may be related to variability in the degree of tau pathology in different brain regions [2], thus studies with larger numbers of brains and exploring additional regions are necessary. Moreover, the potential involvement of TGFβ1 and other cytokines such as TNFα and IL-6 (which did not reach statistical significance in our study) needs to be analyzed in using a larger sample size. In this regard, cytokine expression at the protein level along with identification of the cells responsible for their synthesis need also be considered to better define the role that cytokines play in the pathogenesiss of PSP.
In conclusion, our study shows a topographical relationship between the expression of certain cytokines (IL-1β and TGFβ) and brain areas affected by neurodegeneration in PSP and AD, suggesting that they may be involved in the pathologic process. If confirmed to play a causal role, this kind of evidence would favor the idea that anti-inflammatory treatments may be beneficial. Although in the case of AD the evidence from anti-inflammatory clinical trials remains controversial [30], no such trials have been conducted in PSP patients.
Research Highlights.
We studied expression of cytokine mRNAs in brains of PSP vs. Alzheimer’s disease
Higher expression of IL-1β in substantia nigra of brains from PSP patients
Higher expression of IL-1β in parietal cortex of brains from Alzheimer’s patients
Higher expression of TGFβ in cortical areas of Alzheimer’s patients
Correlated IL-1β expression, microglial activation and neurodegeneration
ACKNOWLEDGEMENTS
We are indebted to the CurePSP brain bank for making the study samples available. Funded in part by American Heart Association grant 0350352N to R.F.B. Dr. Dickson is supported by NIH grants P50NS072187 and P50AG16475 and Dr. Litvan NIH grant R01 PAS-03-092.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Litvan I. Update on progressive supranuclear palsy. Curr Neurol Neurosci Reports. 2004;4:296–302. doi: 10.1007/s11910-004-0055-z. [DOI] [PubMed] [Google Scholar]
- 2.Williams DR, Lees AJ. Progressive supranuclear plasy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–279. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 3.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771–5. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- 4.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–9. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 5.Ishizawa K, Dickson DW. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol. 2001;60:647–57. doi: 10.1093/jnen/60.6.647. [DOI] [PubMed] [Google Scholar]
- 6.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease – A double-edged sword. Neuron. 2002;35:419–32. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 7.Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–97. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 8.Block ML, Hong JS. Microglia and inflammation-mediated neuro-degeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–39. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 10.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegeneration. 2009;4:47–60. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allan SM, Tyrrell P, Rothwell NJ. Interleukin-1 and neuronal injury. Nature Rev Immunol. 2005;5:629–40. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 12.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 13.Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- 14.Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 15.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 16.Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, et al. Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol. 2008;67:649–56. doi: 10.1097/NEN.0b013e31817d7a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assessing integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–95. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–11. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicoll JAR, Mrak RE, Graham DI, Stewart J, Wilcock G, MacGowan S, et al. Association of interleukin-1 (IL-1) gene polymorphisms with Alzheimer’s disease. Ann Neurol. 2000;47:365–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Grammas P, Ovase R. Cerebrovascular transforming growth factor-β contributes to inflammation in the Alzheimer’s disease brain. Am J Pathol. 2002;160:1583–7. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, et al. Amyloidogenic role of cytokine TGF-β1 in transgenic mice and in Alzheimer’s disease. Nature. 1997;389:603–6. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- 23.Kim WK, Hwang SY, Oh ES, Piao HZ, Kim KW, Han IO. TGF-β1 represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J Immunol. 2004;172:7015–23. doi: 10.4049/jimmunol.172.11.7015. [DOI] [PubMed] [Google Scholar]
- 24.Town T, Laouar Y, Pittenger C, Szekely CA, Tan J, Duman RS, et al. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–7. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippa CF, Flanders KC, Kim ES, Croul S. TGF-beta receptors-I and -II immunoexpression in Alzheimer’s disease: a comparison with aging and progressive supranuclear palsy. Neurobiol Aging. 1998;19:527–33. doi: 10.1016/s0197-4580(98)00089-x. [DOI] [PubMed] [Google Scholar]
- 26.Chantry D, Turner M, Abney E, Feldmann M. Modulation of cytokine production by transforming growth factor-β. J Immunol. 1989;142:4295–300. [PubMed] [Google Scholar]
- 27.Bogdan C, Paik J, Vodovotz Y, Natham C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-β and interleukin-10. J Biol Chem. 1992;267:23301–8. [PubMed] [Google Scholar]
- 28.Wahl SM. Transforming growth factor β: the good, the bad and the ugly. J Exp Med. 1994;180:1587–90. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ervin JF, Heinzen EL, Cronin KD, Goldstein D, Szymanski MH, Burke JR, et al. Postmortem delay has minimal effect on brain RNA integrity. J Neuropathol Exp Neurol. 2007;66:1093–99. doi: 10.1097/nen.0b013e31815c196a. [DOI] [PubMed] [Google Scholar]
- 30.Bennet DA, Whitmer RA. NSAID exposure and risk of Alzheimer’s disease. Is timing everything? Neurology. 2009;72:1884–5. doi: 10.1212/WNL.0b013e3181a81664. [DOI] [PubMed] [Google Scholar]