Conspectus
Nanoparticles offer diagnostic and therapeutic capabilities impossible with small molecules or micro-scale tools. As molecular biology merges with medical imaging to form the field of molecular imaging, nanoparticle imaging is increasingly common with both therapeutic and diagnostic applications. The term theranostic indicates technology with concurrent and complementary diagnostic and therapeutic capabilities. When performed with sub-micron materials, the field may be termed theranostic nanomedicine. Although nanoparticles have been FDA-approved for clinical use as transport vehicles for nearly 15 years, full translation of their theranostic potential is incomplete. Still, remarkable successes with nanoparticles have been realized in the areas of drug delivery and magnetic resonance imaging. Emerging applications include image-guided resection, optical/photoacoustic imaging in vivo, contrast-enhanced ultrasound, and thermoablative therapy.
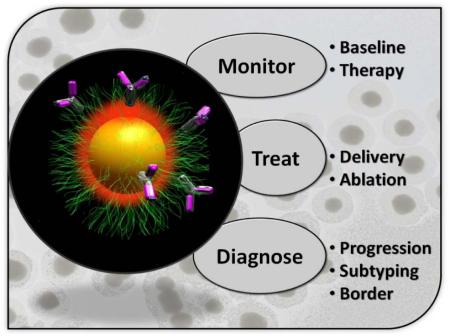
Diagnosis with nanoparticles in molecular imaging involves correlating signal to a phenotype. The disease’s size, stage, and biochemical signature can be gleaned from the location and intensity of nanoparticle signal emanating from a living subject. Therapy with NP uses the image for resection or delivery of small molecule or RNA thererapeutic. Ablation of the affected area is also possible via heat or radioactivity.
The ideal theranostic NP: (1) selectively and rapidly accumulates in diseased tissue, (2) reports biochemical and morphological characteristics of the area, (3) delivers a non-invasive therapeutic, and (4) is safe and biodegrades with non-toxic byproducts. Above is a schematic of such a system which contains a central imaging core (yellow) surrounded by small molecule therapeutics (red). The system targets via ligands such as IgG (pink) and is protected from immune scavengers by a cloak of protective polymer (green). While no nanoparticle has achieved all of the above features, many NPs do fulfill one or more. While the most clinically translatable nanoparticles have been used in the field of magnetic resonance imaging, other types are quickly becoming more biocompatible by overcoming toxicity and biodistribution concerns. The document details diagnostic imaging and therapeutic uses of nanoparticles. We propose five main types of nanoparticles with concurrent diagnostic and thereapeutic uses and offer examples of each.
Keywords: Theranostic, Nanoparticle, Molecular Imaging
Introduction
Molecular imaging (MI) monitors and measures biological processes in living subjects via spectral data. Traditional modalities such as X-Ray, computed tomography (CT), and magnetic resonance imaging (MRI) produce an image of anatomy. MI modalities such as positron emission tomography (PET), single photon emission CT (SPECT), optical techniques, and contrast-enhanced CT or MRI produce an image with details on function. Molecular imaging monitors and measures biological processes similar to a biopsy, but is done non-invasively, in real time, and with potential for sequential, longitudinal monitoring. Applications include early detection of disease, staging of disease, evaluating the response to therapy, and studying biological processes in living subjects.1, 2
Nanoparticles (NPs) are an emerging instrument in the toolkit of MI because of their intense and stable output, large payload delivery, multimodal signaling capacity, strong target binding via multiple ligands, and tunable biodistribution profiles (Table 1). These synthetic materials (with dimensions from one to hundreds of nanometers) have a long history in drug delivery and are increasingly popular in imaging because of the unique way in which they interact with light, sound, and electromagnetic fields.3,4 Nanoparticles are particularly intriguing when used for combination applications that are both therapeutic and diagnostic. Such systems have recently been described as theranostic.5 Theranostic nanomedicine is an emerging field that uses nanometer-scale materials to glean diagnostic insight for well-informed treatment. The fundamental advantage of theranostic nanomedicine is the use of patient-specific test results to tailor a treatment regimen producing improved outcomes, reduced costs and fewer side effects.
Table 1. NP Applications to Diagnosis and Therapy.
Mechanisms of diagnosis via imaging (pink) and treatment of disease (blue) are aided by nanoparticles. NPs are also critical to next-generation in vitro diagnostics (IVD; brown).
| Technique | Limitation | NP Solution | NPType(s) |
|---|---|---|---|
| CT | Sensitivity | Contrast | Gold, Silver, Iodine NPs |
| Optical | Signal Penetration Poor multiplexing |
Intense Signal Fingerprint Spectra |
Quantum Dots Raman nanoparticles |
| MRI | Anatomic Technique | Contrast | Iron Oxide, Gd3+/Silica |
| PET/SPECT | Spatial Resolution | Multimodal | Radiolabeled NP |
| Ultrasound | Anatomic Technique | Contrast | Silica, Manobubble |
| Resection | Tumor Location | Border Delineation | MRI and/or Fluorescent |
| Radiation Therapy | Site-specific Delivery | Radio-NPs | Sir-Spheres (microparticles) |
| Chemotherapy | Delivery/Stability /Toxicity | Drug carriers | Liposome, Micelles |
| Ablation | Low Efficiency | NP Enhancer | Nanoshell, Nanorod, CuS NPs |
| RIMAi | Delivery | siRNA carrier | Liposome, Polymeric NPs |
| In Vitroi Diagnostics | Detection Limit Multiplexing |
Intense Signal Spectral Resolution |
Quantum Dots Raman/Magnetic NPs |
While the definition of thernostics continues to evolve, we suggest the following: A diagnostic improves the knowledge of a disease state. Diagnostics may be performed in vivo or ex vivo and offer information about a disease’s metabolic/biochemical state, genotype, size, location(s), morphology, chemical composition, rate of change, etc. A therapeutic improves the outcome of a disease state. Small molecules, proteins, RNA interference, gamma ablation, and surgical explanation are all examples of therapeutics. In the following account, we recap the state-of-the-art in theranostic NPs and define such systems as synthetic, nanometer-scale materials that simultaneously improve the knowledge and outcome of aberrant biology. Although we focus on cancer biology and in vivo applications, theranostic nanoparticles also have applications in diabetes, and regenerative medicine.6 Complementary to imaging, theranostic in vitro diagnostic (IVD) approaches also utilize nanoparticles and use an ex vivo test result to guide treatment. Helpful discussions of IVD theranostics exist elsewhere.7
Nanoparticles
Limitations of some MI techniques include poor multiplexing capabilities, poor spatial resolution, low sensitivity, and poor signal penetration through tissues.2 For these reasons, MI researchers have increasingly turned to NPs because of their large payloads, high signal intensity/stability, avidity, and the capacity for multiple, simultaneous applications due to their unique size and the high surface area to volume ratio.8 NPs are bigger than proteins, yet smaller than cells, and thus behave differently in vivo than other therapies and imaging agents. While both therapeutic nanostructures and imaging NPs have a long history, they have only recently begun to coalesce into the theranostic NPs detailed below (Figure 1). There are currently more than 35 FDA-approved NPs, with a larger number in pre-clinical studies for both imaging and therapy.3, 9-13 Most FDA-approved NPs are used as mechanisms of drug delivery with the exception of MRI contrast agents.
Figure 1. Evolution of Theranostic NPs.
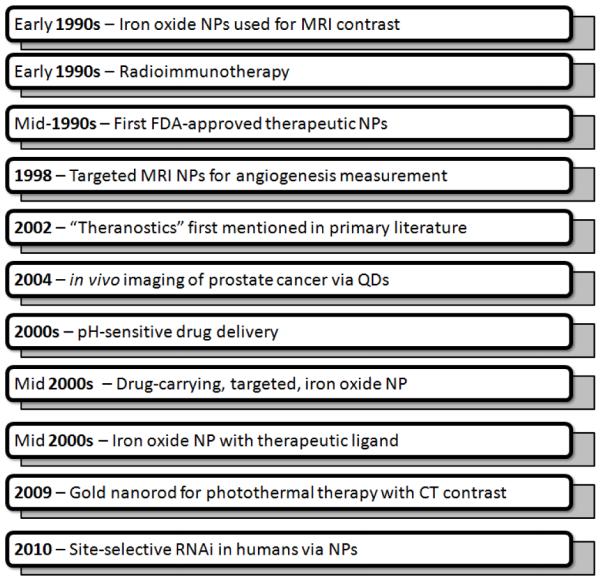
Initial NPs were active in either a therapeutic (delivery) or a diagnostic (imaging) mode. As homing capabilities and multimodal approaches advanced, systems capable of simultaneous therapy and molecular imaging (theranostic) were realized.
Diagnostic Capabilities
The diagnostic role of theranostic NPs reports the presence (location(s)) of disease, the status of disease (sub-type), or a disease’s response to treatment (Figure 2). Through either passive or active targeting, increased binding at the site of interest is achieved. The location and intensity of NP signal after systemic (intra-venous) injection correlates to cell surface receptors or molecular phenotype and measures the tumor’s size, border, stage, etc. NP signaling utilizes radionuclides, fluorophores, or the NP itself may have an intrinsic property for contrast (e.g. iron oxide for MRI.) NPs are often coated with ligands that target angiogenesis markers. For example, the RGD peptide binds to αvβ3 integrin and vascular epidermal growth factor binds to the VEGF receptor (VEGF-R). Both identify angiogeneic tumor. Once bound, the NP can help guide resection or monitor response to therapy. Alternatively, passive targeting can be used for anatomic imaging rather than MI.
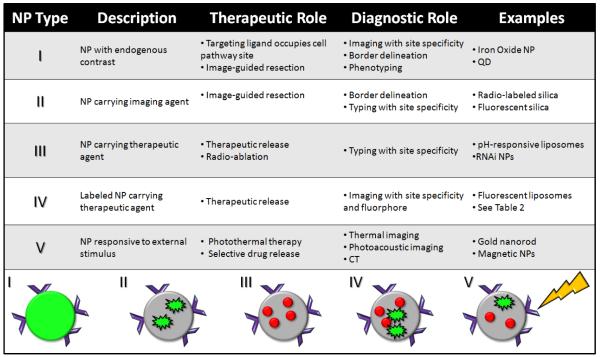
Figure 2. Types of NPs.
The therapeutic (indicated by red circles) and diagnostic (imaging agent indicated by green flash) roles of nanoparticles (grey scaffold) combine in different fashions. Type I and II are self-contained NPs, while types III and IV involve release of an agent. These NPs may target disease area non-specifically or specifically via homing ligands (indicated by purple wedges). Type V NPs may include various elements of the following four types, however they are only activated in the presence of an external stimulus.
Diagnostic NPs include superparamagnetic iron oxide (SPIO) and ultra-small SPIO (USPIO) for MRI contrast and targeted SPIO NPs, which allow MI via MRI.14 Gold NPs are used for CT and radiograph contrast and MI via ligands.15 Silica nanoparticles have applications in MRI as gadolinium containers or to protect inner imaging cores.16 Optical reporters include quantum dots (QDs), fluorophore-doped silica NPs, and fluorophore-doped polymeric NPs.17 Carbon nanotubes and gold nanorods produce photoacoustic contrast.18 Surface-enhanced Raman scattering (SERS) NPs are used for multiplexed approaches.19 Some NPs are multimodal—that is, they report signal through more than one method (e.g., fluorescence and MRI) and are useful when the two modalities have complementary spatial resolution, sensitivity, or depth penetration.20
Therapeutic Capabilities
The therapeutic role of the NP can take several forms as well (Figure 2). It may be a material delivered or released to the diseased area. Traditionally, this has been small molecule chemotherapeutics such as doxorubicin, paclitaxel, etc. Next-generation systems deliver or miRNA for RNA interference with gene expression.13, 21-23 Mirkin’s group has demonstrated that gold NPs not only facilitate cellular delivery of oligonucelotides, but also stabilize them from nucleases.24 The release may be the ablative effect of radionuclides loaded into NPs for destruction of the tumor, causing DNA damage and retarding cell growth.25 Finally, the release may be in the form of heat or vibrational energy that disrupts the structure of the cells and shrinks the tumor volume. Second, the therapeutic role may be to guide surgery for tumor resection. NPs that contain an imaging agent can thus be both diagnostic (determine tumor type, location, and borders) and therapeutic (use that image to guide tumor removal). Intra-operative imaging is visualization of diseased areas exposed during surgery and is especially important as the location of the tumor may change after pre-surgical imaging and during resection. Finally, the therapeutic role may be in disrupting a cellular or metabolic pathway. This approach utilizes a ligand to target the NP and disrupt cell regulation. An example of this is Herceptin-labeled NPs, which occupy the Her-2 cell surface receptors.26
In delivery applications, the NP carriers stabilizes the payload (up to 105 copies per nanoparticle)27, allowing a metered release of drug, reducing toxicity and side effects. Hydrophobic drugs are protected by the NP interior.28 Most therapeutic drug-carrying NPs are in the form of liposomes or lipid-based complexes, as well as polymeric micelles or biodegradable polymer/drug composites.29 The most common substrate is a blend of poly(lactic-co-glycolic acid) (PLGA) and polyethylene glycol (PEG), (PLGA-PEG). Metallic NPs used in tandem with infrared heating are thermoablative NPs; nanoshells and nanorods are the most common examples.30 Sir-spheres™ are the trademarked name of Yttrium-90 loaded nanoparticles used to treat liver cancers. These particles are injected into the hepatic artery and accumulate in the tumor where they ablate the tumor in vivo.25
Nanoparticle Types
In Figure 2, five common approaches to designing theranostic NPs are detailed. All have one or more of the above therapeutic and diagnostic roles. While there are obviously many variations on this theme and this list is not exclusive, many NPs do fall into one of these categories. Type I and II NPs are used in tandem with surgery for resection/removal of the tumor/lesion. They either have an intrinsic (Type I) signaling nature such as MRI or are loaded with a reporter (Type II) like a fluorophore. The diagnostic role is due to the site-specific accumulation of the NP, caused by the NP’s size/shape, sensitivity to local environment, or targeting ligand. Thus, imaging is used to radically treat the tumor and the location of the NP causes both the diagnosis (tumor location/border/type) and therapy (surgical resection).
Type I NPs include dextran-stabilized iron oxide (MRI), gold nanoshells (photoacoustic imaging), nanobubbles (US), and PEG-coated quantum dots.31 Type II NPs include dye-loaded silica, radiolabeled silica, and multimodal NPs. Resection using type I or II NPs is one of the best applications of multimodal NPs because the advantages of each modality can be used at different points in the procedure. The mapping of a tumor’s sentinel lymph nodes is a key application of these NPs. Limitations to type I and II NPs include non-specific accumulation, inadequate circulation times/biodistribution, poor biodegradation profiles, and toxicity.32
Type III and IV NPs are less invasive (no surgical intervention) and more sophisticated as they carry an agent to the site of interest. Site-specific accumulation allows molecular imaging of the tumor or specific delivery of therapeutic. Drug-carrying liposomes, micelles, and other NPs are type III. Examples include Doxil which is PEGylated, liposomal doxorubicin and Abraxane which is a NP carrier of paclitaxel.9 More than one type of therapeutic can be loaded per NP. The diagnostic mechanism of type III NPs is their specific accumulation in diseased area, which is an in vivo evaluation of tissue state. Type IV NPs have the additional diagnostic capacity for imaging via a loaded or intrinsic reporter with simultaneous release of a treatment mechanism to the site of interest. One challenge is balancing the limited NP exterior or volume, especially for surface-functionalized NPs. A high amount of therapeutics may reduce the number of bound ligands and vice versa. Still, simultaneous imaging and delivery are illustrated in Table 2 and demonstrate the broad range of deliverable products and targeting ligands.33,34
Table 2. Examples of Type III-V Thernostic NPs.
Both active and passively targeted NPs are currently in clinical and pre-clinical work. For clinically approved NPs see Zweck.3 (EGFR = epidermal growth factor receptor; PSMA = prostate specific membrane antigen; MSKCC = Memorial Sloan Kettering Cancer Center).
| NP Type | NP Size (nm) |
Therapeutic Agent |
Diagnostic Agent |
Disease State |
Target | Reference | |
|---|---|---|---|---|---|---|---|
| Pre-clinical | Iron Oxide | 10 | Anti-EGFRIgG | Iron Oxide | Brain CA | EGFR | Maoetal.31 |
| Silica | 100–200 | Paclitaxel | Iron Oxide | Many | FolicAcid | Zinketal.34 | |
| Liposome | 100–200 | Paclitaxel | pH-responsive membrane |
Ovarian CA/ ManyTypes |
EPR | Langeret al.53 | |
| Gold Nanorod | 10x40 | Heat | Thermal/CT | Many | EPR | Bhatiaet al.35 | |
| QD | 30 | Doxorubicin | QD | Prostate CA | PSMA | Farokhzadet al.45 | |
| Clinical Trials | Cyclodextrin | 70 | RNAi | Transferrin | Melanoma | Transferrin Receptor |
CalandoPharma, (NCT00689065) |
| Gold Nanoshell ,(Aurolase) |
150 | Nanoshell (Photothermal) |
Nanoshell (MR and optical) |
Head and NeckCA |
EPR | NanoSpectra (NCT00848042) |
|
| Silica | 3-10 | cRGD | Melanoma | avB3lntegrin | MSKCC (NCT01266096) |
||
| Iron Oxide | 120–180 | Injected Cell | Iron Oxide (Endorem) |
Healthy Volunteers |
None | Univ. of Edinburgh (NCT00972946) |
|
| Gold | 27 | TumorNecrosis Factor |
Gold NP Size | SolidTumors | EPR | NCI/Cytimmune (NCT00356980) |
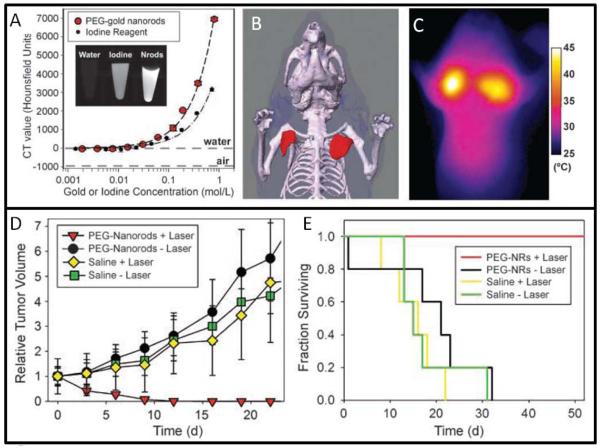
Type V NPs are responsive to external stimulus; NPs with magnetic or thermoablative capacities are of this class. In thermoablative therapy, nanoshells and nanorods are effective because they have absorption peaks in the near infra-red (IR; IR light passes more easily through tissue) and are effective at converting light into heat energy. Bhatia and co-workers report nanorods accumulation in tumor followed by laser treatment with heating to nearly 70° C in vivo (Figure 3.)35
Figure 3. Gold Nanorods.
Nanorods have both imaging and therapeutic capabilities as illustrated in a murine model of breast cancer. (A) Gold absorbs X-Rays during CT more strongly than iodine. (B) Nanorods can be used to create a CT map of the tumor. When irradiated with NIR light, the nanorods increase in temperature, which can be mapped via thermal imaging (C). The heating causes tumor death and shrinkage of the tumor (D) resulting in increased survival of treated animals (E). Adapted and reprinted by permission from the American Association for Cancer Research: Reference 35.
Targeting Ligands
Theranostic NPs demonstrate specific accumulation at the site of interest, which can occur through either active or passive targeting. Passive targeting takes advantage of the enhanced extravasation, permeation, and retention (EPR) effect in which NPs escape from a tumor’s leaky vasculature and accumulate non-specifically in the lesion. Passive targeting is dependent on the size, shape, and charge of the NP. A key challenge is reducing non-specific binding. NPs larger than 100 nm, with surface charges (zeta potential ± > −20 mV), or with solid cores are often rapidly (< 15 minutes) cleared from circulation by the liver and spleen before accumulating at the tumor. Drug-containing liposomes are a classic example of NPs (Type III) that target non-specifically.9
Alternatively, actively targeted NPs anneal a recognition element to the NP to bind to cell surface markers for even greater accumulation in the diseased tissue. While passive targeting can increase accumulation, only NPs with ligands that specifically bind to a receptor indicative of a metabolic process are true MI NPs. Ligand types include antibodies, small peptides or molecules, lectins, aptamers, engineered proteins, and protein fragments. Commonly targeted tissue biomarkers include vascular markers and markers of angiogenesis. Folate receptor is over-expressed in many cancer types and folate-labeled NPs are common.36 Some NPs are therapeutic by using the monoclonal antibody to occupy a cell surface marker involved in signal transduction preventing tumor growth. In general, expression levels 2-10 times higher at target than the non-targeted area is sufficient.37
A key advantage of using NPs versus molecular scaffolds is the multiple copies of ligand that can be loaded onto the NP. Small NPs like QDs may have tens of ligands, while larger liposomal NPs may have thousands. This multivalent mode of attachment between the NP and disease area is known as avidity. In addition to the number of ligands per NP, the many different types of ligands attached to a NP is very advantageous. Because cancer cells often rapidly develop resistance to one type of ligand, combination approaches that target/inhibit through more than one mechanism can be especially useful.38
Magnetic NPs
Particles enhancing MRI signal are one of the most established Type I NPs and iron oxide (stabilized with dextran, PEG, oleic or pluoronic acid) are routine and clinically approved under many brand names including Resovist, Feridex, Ferumoxtran-10, or Gastromark. The main benefits of these NPs are the depth insensitive nature of MRI detection, low toxicity, and hours of circulation time. SPIO and USPIO decrease T1 and T2 relaxation times in a dose-dependent manner. They are used as contrast for lymphography and angiography, bone marrow contrast, or as a perfusion agent of the brain and kidney. Iron oxide particles can be targeted through the addition of a therapeutic ligand such as Herceptin (tratuszamab.)26 There are approximately 20 current clinical trials of SPIO or USPIO.39 Approved clinical applications of SPIO include imaging of liver metastasis.
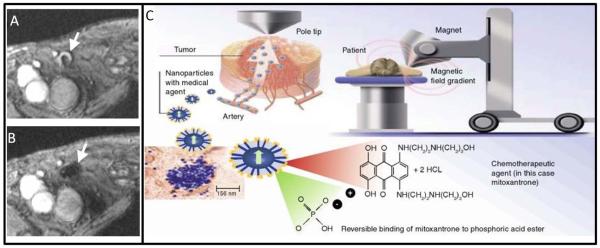
Theranostic applications of SPIO include labeling cells with iron oxide NPs to be tracked in vivo with potential applications in stem cell monitoring (Figure 4).40 Figdor and coworkers have shown that cells labeled with magnetic nanoparticles can be monitored and tracked in human patients.41 Loading of SPIO with NPs was done by co-culturing immature cells with 200 mg/ml SPIO. Patients were imaged with gradient echo transversal MRI before both before and after injection of 7.5 × 106 cells in 200 uL into a lymph node. Sequential imaging showed migration of cells to sentinel lymph nodes. The limit of detection was 1.5 × 105 cells.
Figure 4. Tracking Injected Cells via Magnetic NPs.
Human lymph nodes before (A) and after (B) intranodal injection of iron oxide-labeled cells. Cells could be tracked for 2 days after injection as they traveled through lymphatic system. (C) Magnetic NPs can also be loaded with small molecule therapeutics and immobilized at the disease site via external magnetic field to increase dose. Adapted from reference 40 and 42.
Magnetic NPs have also been used to deliver drugs to a diseased area.42 After systemic injection, a high field magnet is positioned over the tumor to increase NP accumulation by immobilizing circulating particles for cargo release. Plank’s group has used SPIO to deliver gene therapy to Wister rat gut.43 Labhasetwar’s group has shown loading and delivery of paclitaxel and doxorubicin with iron oxide NPs and concurrent MRI.44
Multimodal MRI NPs often use optical methods. MRI is a depth insensitive technique and can be used for deep tissue imaging, yet suffers from poor sensitivity and cannot be used in real time. Fluorescence imaging is highly sensitive (fM or pM detection limits), yet has poor depth penetration. Thus, NPs dually functionalized employ the MRI modality for tumor staging and localization and the fluorescent modality intra-operatively to find tumor margins and insure complete removal of the lesion These particles have been validated in a green fluorescent protein (GFP)-tagged 9L rat gliosarcoma model.20 Twenty-four hours after 15 mg/kg i.v. injection Cy5.5 fluorophore-labeled cross-linked iron oxide imaging both MRI and fluorescence imaging clearly showed tumor margins.
Quantum Dots
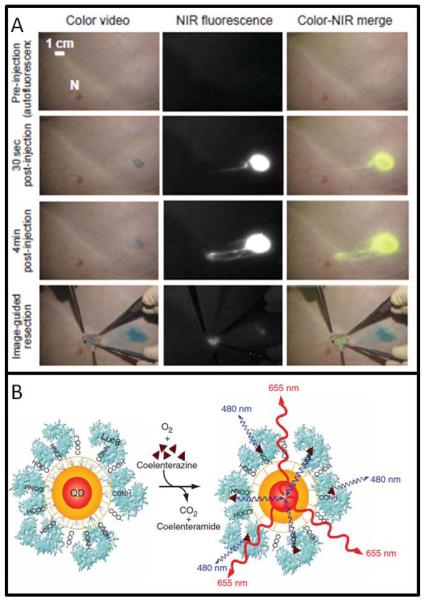
QD NPs have broad applications both in vivo and ex vivo.45, 46 Their theranostic applications as Type II NPs have been to identify disease via targeting ligands. Gambhir and Cai used PEGylated QDs with the RGD peptide to image αvβ3 integrins of a U87 glioma model in vivo and observed tumor to background ratios of 4.42 ± 1.88 at six hours versus 0.84 ± 0.21 for untargeted QDs.47 Bhatia has illustrated homing of QDs in cell culture via the nucelolin-binding F3 peptide. These same QDs were also coated with siRNA to inhibit GFP. The authors demonstrated delivery of this siRNA to the nucleus via 50 nM QD incubation and subsequent inhibition by 80% of GFP gene expression.33 This work has yet to be translated in vivo. Bawendi and colleagues have used QDs in porcine models (Figure 5A). 200 pmol of near IR QDs imaged the sentinel lymph nodes in swine in less than five minutes.48, 49
Figure 5. Quantum Dots.
QDs deployed to theranostic applications include the mapping of sentinel lymph nodes (A). 400 pmol of QDs were injected 1 cm deep into living swine tissue. In less than 5 minutes, the QDs have begun to accumulate in the nearest lymph node for image-guided resection. Surgery is continued until all fluorescence (tumor) is removed. (B) Self-illuminating QDs containing a bioluminescent protein and a QD core use coelenterazine substrate to generate signal. Reproduced courtesy of references 49 and 52.
QD toxicity is the primary barrier to clinical use and two different approaches are under development to reduce this concern. Encapsulation of the CdSe core with a silica coat prevents leaching of the heavy metal and decreased liver uptake from 57.2 to 16.2 %ID/g and splenic uptake from 46.1 to 3.7 %ID/g.50 This decreases leaching of the heavy metal core, but increases the size of the NP such that renal clearance is modulated. The second approach removes heavy metals, i.e. Cd, for ZnSe or ZnS QDs.51 Self-illuminating QDs combine a bioluminescent protein with QD core remove the need for an excitation source and improve signal to noise because of the low background (Fig. 5B).52
Activatable TNPs
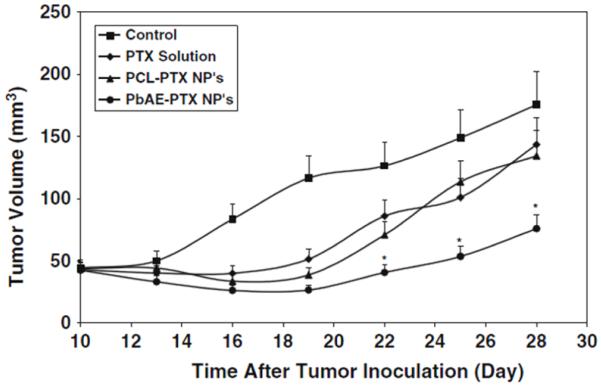
Activatable or “smart” NPs respond to a change in local environment to instigate the therapeutic/diagnostic mechanism. The key advantage is site-selective therapy for a reduction in side effects; challenges include effective delivery and leaky carriers. The most common triggers for activatable NPs are pH, proteases, and light. First, hypoxia in the tumor microenvironment results in lactic acid production and hence acidic conditions. The diagnostic modality of these NPs is selectivity for tumor environment to release the therapy payload. Liposomes between 100 and 200 nm were constructed from pH-sensitive poly(ethylene oxide)-modified poly(beta-amino ester) carrying paclitaxel and treat a murine model of ovarian cancer (Figure 6.)53 One hour after injection there was a 3.0-fold greater accumulation of drug in tumor treated with smart liposome versus non-pH-responsive carrier.
Figure 6. pH-sentivive NPs.
NPs that selectively release paclitaxel in the presence of acidic tumor environment (PbAE-PTX) show statistically (*p<0.05) greater reduction in tumor volume than control NPs or free paclitaxel. Reproduced courtesy reference 53.
A second class uses the proteases up-regulated by tumor for a cleavage event. The family of matrix metalloproteases (MMP) are commonly used. Bhatia and coworkers demonstrated that upon cleavage of protective PEG chains by protease, SPIO NPs aggregate at the site of interest.54 A final class of NPs uses light for activation. One example incorporates the chlorine meso-tetraphenylporpholactol into PLGA nanoparticles, which is quenched in NP formulation, but regains fluorescence in the presence of cellular lipids, producing singlet oxygen for photodynamic therapy. These NPs were injected in vivo and irradiated with 650 nm light at 191 mW/cm2, causing a significant reduction in tumor volumes.55
Challenges
Some fundamental challenges hamper NP deployment to the clinic. The first among these are delivery obstacles, especially uptake by the reticuloendothelial system (RES) in which NPs are rapidly shuttled out of the circulation to the liver, spleen, and bone marrow. Coating the NP with polyethylene glycol can reduce recognition of the NP by the RES and increase circulation times 2-10 times.56 Nanoparticles are often limited to vascular applications because their size prevents easy extravasation. NPs below 100 nm are often used to increase extravasation (for solid particles)55, although extravasation of 400 nm liposomes is reported.57 NP toxicity concerns often arise because of this RES accumulation. Aggregation can lead to a loss of function or NP entrapment in the liver, lungs or elsewhere due to capillary occlusion.58 Toxicity can also result from the composition of the NP itself, e.g. Cd in QDs.
The dose of NP required for a therapeutic effect may be markedly higher than that required for a diagnostic effect. For example, a drug may need to be present at milligrams/kg of body weight where a radioactive tracer agent needs much less than 1 μg/kg. A final problem specific to theranostic NPs is circulation time. Imaging requires the area of interest to have higher signal than surrounding tissue for contrast. Thus, most imaging agents are designed to clear from the blood quickly (e.g, in a few minutes to hours). A therapeutic strategy needs to have NPs with longer circulation times for adequate exposure to the tumor and for drug release. A final challenge is oral bioavailability. Current NP design requires I.V. injection; transitioning to a less invasive approach is key.
Theranostic Nanoparticles in the Clinic
Although most NPs are used in pre-clinical models, some have entered human use (Table 2). The first class are liposomes or micelles for drug delivery. While their diagnostic content is limited to tumor accumulation, nearly 20 are currently commercially available and offer reduced systemic cytotoxicy and drug stability.3 For example, Abraxane as a NP formulation of paclitaxel demonstrated significantly higher response rates versus free paclitaxel (33% v 19%, respectively; P = 0.001), longer time to progression (23.0 v 16.9 weeks.) The side effect of neutropenia was lower for Abraxane versus paclitaxel (P<0.001) despite a 49% higher paclitaxel dose.59 Ongoing work creates liposomes with active targeting and longer circulation times.60
Gold nanoshells under the brand name Aurolase evolved from work by Halas and West. Work in canine models used 1.25 × 109 nanoshells/gram body weight. Patients with neck and throat cancers are injected with 120 nm gold nanoshells. These circulate and accumulate in the tumor by EPR. After immobilization of the NPs, laser irradiation at 808 nm causes a temperature increase of ~ 20 degrees which has been shown to ablate tumor in a wide variety of small animal studies.30, 61 Advantages to photoablative treatment include the ability to customize the treatment with the location and duration of the light pulse. One limitation is that deeper tissue may not receive the same thermal dose as superficial tissue and that location of the tumor is needed prior to the initiation of treatment.
Recent work by Davis and colleagues described a cyclodextrin NP stabilized with PEG and adamantane and targeted to melanoma cells via human transferrin protein.13 When injected into human melanoma patients, site-selective tumor accumulation was monitored by a Cy3 tag on the siRNA and ribonucleotide reductase-M2 subunit (RRM2) mRNA decreased by 40-70% and protein transcript decreased by ~ 30% versus pre-dose tissue.21 Finally, work in the Gambhir and Contag labs has shown that SERS NPs can label markers of colon cancer via either affibodies or small peptides.19 Work is underway to deploy this imaging modality to molecular imaging guided colonosocopy.
Theranostic nanomedicine beyond imaging includes IVD in which an in vivo treatment decision is based on an ex vivo test result. The Mirkin group has developed a bio-barcode assay with excellent sensitivity via gold NPs.62 Others utilize QDs and magnetic particles. Here, “companion diagnostics” stratify a disease, e.g. breast cancer, into subtypes via measurement of a biomarker, e.g. circulating Her-2.7 In general, IVD tests have a shorter time course to gain approval for use in humans than in vivo imaging/diagnostics.7
Future Directions
Observing the progression of theranostic NPs in the past decades shows a trend toward less invasive approaches. We suggest NP invasiveness may be staged in the following categories. The current design of NPs can be divided into the following categories of invasiveness:
Stage A NP – The NP is injected for diagnostic imaging with surgery for therapy
Stage B NP – NP is injected and gives information for necessary oral therapy
Stage C NP – NP is injected and identifies disease state and selectively treats affected area
Stage D NP – NP is constantly circulating and activates theranostics when disease begins
Stage D NPs have yet to be reported. These systems could be considered synthetic cells and are often describe as “nanobots” in the popular scientific press.
Work with theranostic NPs will continue to solve the above challenges. Alternatives to metallic or liposome NPs will allow for tailored circulation times unique to application. Time-sensitive coatings that shed over time could be used for custom circulation profiles. Especially intriguing are the use of bacteria, viruses, or other naturally occurring NP scaffolds.63 These materials are similar to naturally occurring systems and thus may penetrate the cell membrane much more efficiently than synthetic nanoparticles. Biodegradable nanoparticles are an important nanoparticle type and overcome many of the toxicity and accumulation concerns of other NPs.64
Acknowledgements
This work is funded in part by the National Cancer Institute CCNE U54 CA119367 (S.S.G.) and In Vivo Cancer Molecular Imaging Centers ICMIC P50 CA114747 (S.S.G.). J.V.J is grateful for fellowship support from the Stanford Molecular Imaging Scholars Program SMIS R25-T CA118681.
Biography

Jesse V. Jokerst was born in Missouri and earned his Ph.D. in Analytical Chemistry under the supervision of John T. McDevitt (currently Rice University) at The University of Texas at Austin in 2009. His Ph.D. work used microfluidics and nanoscience to create tools for saliva-based diagnostics and HIV monitoring. He is currently a NIH R25T postdoctoral scholar in the labs of Sanjiv Sam Gambhir in the Stanford University School of Medicine. Jokerst’s current research interests are Raman imaging, cell tracking via ultrasound, multimodal nanoparticles, and nano-characterization.

Sanjiv Sam Gambhir, M.D., Ph.D., is the Chair of Radiology and Virginia & D.K. Ludwig Professor of Radiology, Bioengineering (by courtesy), and Materials Science & Engineering (by courtesy) at Stanford University. He also heads up the new Canary Center at Stanford for Cancer Early Detection. He received his M.D./Ph.D. from the UCLA Medical Scientist Training Program. He has over 375 publications in the field and over 30 patents pending or granted. He is also co-editor of the leading textbook in Nuclear Medicine and a recent comprehensive book on Molecular Imaging.
An internationally recognized researcher in molecular imaging with over $75 million of National Institutes of Health funding as the Principal Investigator, Dr. Gambhir’s lab has focused on interrogating fundamental molecular events in living subjects. He has developed and clinically translated several multimodality molecular imaging strategies including imaging of gene and cell therapies. He has also developed strategies for monitoring fundamental cellular events such as protein-protein interactions and protein phosphorylation in living subjects. Much of his work has bridged scientific disciplines including applied physics, chemistry, cell/molecular biology, life sciences, engineering, and biomathematics. He holds several FDA eIND/IND’s and has clinically translated PET imaging agents.
Dr. Gambhir serves as an advisor to several major imaging and pharmaceutical companies and has also co-founded several imaging startups. He serves on numerous academic advisory boards for universities around the world and is also a member of the Board of Scientific Advisors of the National Cancer Institute. He was also elected as one of the youngest members of the Institute of Medicine (IOM) of the U.S. National Academies in 2008.
References
- 1.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17(5):545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R, et al. Molecular Imaging: Principles and Practice. People’s Medical Publishing House; Shelton, CT: 2010. [Google Scholar]
- 3.Wagner V, et al. The emerging nanomedicine landscape. Nat. Biotechnol. 2006;24(10):1211–1218. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 4.Kim BY, et al. Nanomedicine. N. Eng. J. Med. 2010;363(25):2434–43. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 5.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine. 2008;3(2):137–140. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, et al. Nanoparticles in medicine: therapeutic applications and developments. Clinical Pharmacology & Therapeutics. 2007;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulos N, et al. The role of companion diagnostics in the development and use of mutation-targeted cancer therapies. Nat. Biotechnol. 2006;24(8):985–995. doi: 10.1038/nbt1234. [DOI] [PubMed] [Google Scholar]
- 8.Jain PK, et al. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41(12):1578–86. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien ME, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–9. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 10.Scranton R, Cincotta A. Bromocriptine--unique formulation of a dopamine agonist for the treatment of type 2 diabetes. Expert Opin Pharmacother. 11(2):269–79. doi: 10.1517/14656560903501544. [DOI] [PubMed] [Google Scholar]
- 11.Lim WT, et al. Phase I pharmacokinetic study of a weekly liposomal paclitaxel formulation (Genexol-PM) in patients with solid tumors. Ann Oncol. 2009;21(2):382–8. doi: 10.1093/annonc/mdp315. [DOI] [PubMed] [Google Scholar]
- 12.Winter PM, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108(18):2270–4. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 13.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artemov D, et al. MR molecular imaging of the Her 2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magnetic Resonance in Medicine. 2003;49(3):403–408. doi: 10.1002/mrm.10406. [DOI] [PubMed] [Google Scholar]
- 15.Geso M. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2007;80(949):64–5. doi: 10.1259/bjr/28250432. author reply 65. [DOI] [PubMed] [Google Scholar]
- 16.Mulvaney SP, et al. Glass-Coated, Analyte-Tagged Nanoparticles: A New Tagging System Based on Detection with Surface-Enhanced Raman Scattering. Langmuir. 2003;19(11):4784–4790. [Google Scholar]
- 17.Cai W, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6(4):669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 18.De la Zerda A, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat. Nanotechnol. 2008;3(9):557–62. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jokerst JV, et al. Affibody Functionalized Gold-Silica Nanoparticles for Raman Molecular Imaging of the Epidermal Growth Factor Receptor. Small. 2011 doi: 10.1002/smll.201002291. In Press -DOI: smll.201002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kircher MF, et al. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 2003;63(23):8122–5. [PubMed] [Google Scholar]
- 21.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 1067;464(7291):1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malek A, et al. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(-PEG)/siRNA complexes. Toxicol Appl Pharmacol. 2009;236(1):97–108. doi: 10.1016/j.taap.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson GB, et al. Biodegradable nanoparticles with sustained release of functional siRNA in skin. J Pharm Sci. 2010;99(10):4261–6. doi: 10.1002/jps.22147. [DOI] [PubMed] [Google Scholar]
- 24.Seferos DS, et al. Polyvalent DNA Nanoparticle Conjugates Stabilize Nucleic Acids. Nano Lett. 2009;9(1):308–311. doi: 10.1021/nl802958f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray B, et al. Randomised trial of SIR-Spheres (R) plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Annals of Oncology. 2001;12(12):1711. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nature medicine. 2006;13(1):95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 27.Noble C, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66(5):2801. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 28.Kwon GS. Polymeric micelles for delivery of poorly water-soluble compounds. Crit Rev Ther Drug Carrier Syst. 2003;20(5):357–403. doi: 10.1615/critrevtherdrugcarriersyst.v20.i5.20. [DOI] [PubMed] [Google Scholar]
- 29.Davis M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Reviews Drug Discovery. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch L, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100(23):13549. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadjipanayis CG, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70(15):6303–12. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi Hak S, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derfus A, et al. Targeted quantum dot conjugates for siRNA delivery. Bioconjugate Chem. 2007;18(5):1391–1396. doi: 10.1021/bc060367e. [DOI] [PubMed] [Google Scholar]
- 34.Liong M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2(5):889–96. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Maltzahn G, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69(9):3892–900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonvico F, et al. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: synthesis, physicochemical characterization, and in vitro experiments. Bioconjug Chem. 2005;16(5):1181–8. doi: 10.1021/bc050050z. [DOI] [PubMed] [Google Scholar]
- 37.Phelps ME. PET: Physics, Instrumentation, and Scanners. Springer; 2006. [Google Scholar]
- 38.Schiffelers RM, et al. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic acids research. 2004;32(19):e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NIH www.clinicaltrials.gov.
- 40.de Vries IJ, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23(11):1407–13. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 41.de Vries IJM, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 2005;23(11):1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 42.Barakat NS. Magnetically modulated nanosystems: a unique drug-delivery platform. Nanomedicine. 2009;4(7):799–812. doi: 10.2217/nnm.09.66. [DOI] [PubMed] [Google Scholar]
- 43.Scherer F, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene therapy. 2002;9(2):102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 44.Jain TK, et al. Magnetic resonance imaging of multifunctional pluronic stabilized iron-oxide nanoparticles in tumor-bearing mice. Biomaterials. 2009;30(35):6748–56. doi: 10.1016/j.biomaterials.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalet X, et al. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science. 2005;307(5709):538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagalkot V, et al. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007;7(10):3065–70. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 47.Cai W, et al. Peptide-Labeled Near-Infrared Quantum Dots for Imaging Tumor Vasculature in Living Subjects. Nano Lett. 2006;6(4):669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 48.Soltesz E, et al. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. The Annals of thoracic surgery. 2005;79(1):269–277. doi: 10.1016/j.athoracsur.2004.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22(1):93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma N, et al. Facile synthesis, silanization, and biodistribution of biocompatible quantum dots. Small. 1520;6(14):1520–8. doi: 10.1002/smll.200902409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao J, et al. Ultrasmall near-infrared non-cadmium quantum dots for in vivo tumor imaging. Small. 6(2):256–61. doi: 10.1002/smll.200901672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.So M-K, et al. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotechnol. 2006;24(3):339–343. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 53.Devalapally H, et al. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 3. Therapeutic efficacy and safety studies in ovarian cancer xenograft model. Cancer Chemother Pharmacol. 2007;59(4):477–84. doi: 10.1007/s00280-006-0287-5. [DOI] [PubMed] [Google Scholar]
- 54.Harris TJ, et al. Proteolytic actuation of nanoparticle self-assembly. Angew Chem Int Ed Engl. 2006;45(19):3161–5. doi: 10.1002/anie.200600259. [DOI] [PubMed] [Google Scholar]
- 55.McCarthy JR, et al. Polymeric nanoparticle preparation that eradicates tumors. Nano Lett. 2005;5(12):2552–2556. doi: 10.1021/nl0519229. [DOI] [PubMed] [Google Scholar]
- 56.Jokerst JV. Nanoparticle PEGylation for Imaging and Therapy. Nanomedicine. 2011 doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kong G, et al. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60(16):4440. [PubMed] [Google Scholar]
- 58.Knop K, et al. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. 49(36):6288–308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 59.Gradishar WJ, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005;23(31):7794. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 60.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4(3):95–9. doi: 10.1186/bcr432. Epub 2002 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz JA, et al. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res. 2009;69(4):1659. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- 62.Nam JM, et al. Bio-bar-code-based DNA detection with PCR-like sensitivity. J Am Chem Soc. 2004;126(19):5932–5933. doi: 10.1021/ja049384+. [DOI] [PubMed] [Google Scholar]
- 63.Akin D, et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol. 2007;2(7):441–9. doi: 10.1038/nnano.2007.149. [DOI] [PubMed] [Google Scholar]
- 64.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced drug delivery reviews. 2003;55(3):329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]