Abstract
Hereditary Vitamin D Resistant Rickets (HVDRR) is a rare disease caused by mutations in the vitamin D receptor (VDR). The consequence of defective VDR is the inability to absorb calcium normally in the intestine. This leads to a constellation of metabolic abnormalities including hypocalcemia, secondary hyperparathyroidism and hypophosphatemia that cause the development of rickets at an early age in affected children. An interesting additional abnormality is the presence of alopecia in some children depending on the nature of the VDR mutation. The data indicate that VDR mutations that cause defects in DNA binding, RXR heterodimerization or absence of the VDR cause alopecia while mutations that alter VDR affinity for 1,25(OH)2D3 or disrupt coactivator interactions do not cause alopecia. The cumulative findings indicate that hair follicle cycling is dependent on unliganded actions of the VDR. Further research is ongoing to elucidate the role of the VDR in hair growth and differentiation.
The Metabolic Abnormalities in Hereditary Vitamin D Resistant Rickets
Vitamin D, the primary regulator of calcium homeostasis in the body, is particularly important in skeletal development and in bone mineralization (reviewed in Feldman et al., 2007). The active form of vitamin D, 1α, 25-dihydroxyvitamin D3 [1,25(OH)2D3 or calcitriol], functions by binding with high affinity to the vitamin D receptor (VDR) (reviewed in Haussler et al., 2008). The VDR is a member of the steroid-thyroid-retinoid receptor gene superfamily of nuclear transcription factors that regulate the expression of specific target genes in response to hormone binding. Hereditary 1,25-dihydroxyvitamin D-Resistant Rickets (HVDRR), also known as Vitamin D Dependent Rickets type II (VDDR-II), is a rare genetic disease that is due to generalized resistance to 1,25(OH)2D3 action (reviewed in Malloy and Feldman, 2010, Malloy et al., 1999, Malloy et al., 2011). HVDRR is caused by heterogeneous mutations in the VDR gene that cause loss of function of the receptor leading to complete or partial target organ resistance to 1,25(OH)2D3 (reviewed in Malloy and Feldman, 2010, Malloy et al., 1999, Malloy et al., 2011). HVDRR is clinically manifested by a constellation of signs and symptoms caused by a loss of VDR-mediated actions. The main clinical features of HVDRR are severe rickets, hypocalcemia, secondary hyperparathyroidism, hypophosphatemia and elevated alkaline phosphatase (Marx et al., 1978, Rosen et al., 1979). The metabolic pathways are intact and the hypocalcemia, due to impaired intestinal calcium absorption, appropriately leads to secondary hyperparathyroidism and hypophosphatemia. However, the resulting high levels of 1,25(OH)2D3 are unable to reverse the hypocalcemia or suppress the elevated PTH because of defective VDR in intestine and parathyroid glands. The hallmark of the disease is therefore hypocalcemia despite elevated 1,25(OH)2D3 levels indicating resistance to even elevated levels of 1,25(OH)2D3 (reviewed in Malloy and Feldman, 2010, Malloy et al., 1999, Malloy et al., 2011). Some patients also exhibit total or partial alopecia that will be extensively discussed in this review (Malloy and Feldman, 2010, Malloy et al., 1999, Malloy et al., 2011, Marx et al., 1986, Marx et al., 1978, Rosen et al., 1979).
In cases of HVDRR the first signs of rickets generally appear early in life, usually within months of birth. Characteristic bowing of the legs develops as the infant begins to walk. The rickets is usually severe and affected children suffer from bone pain, muscle weakness, and hypotonia. Children are often growth retarded and they frequently develop severe dental caries or exhibit enamel hypoplasia of the teeth (Balsan et al., 1983, Bliziotes et al., 1988, Kudoh et al., 1981, Laufer et al., 1987, Liberman et al., 1980, Rosen et al., 1979, Sockalosky et al., 1980). Some infants have died from pneumonia as a result of poor respiratory movement due to severe rickets of the chest wall (Balsan et al., 1983, Fraher et al., 1986, Liberman et al., 1980). In the most severe cases of hypocalcemia and metabolic disruption, convulsions due to the hypocalcemia have occurred.
Alopecia
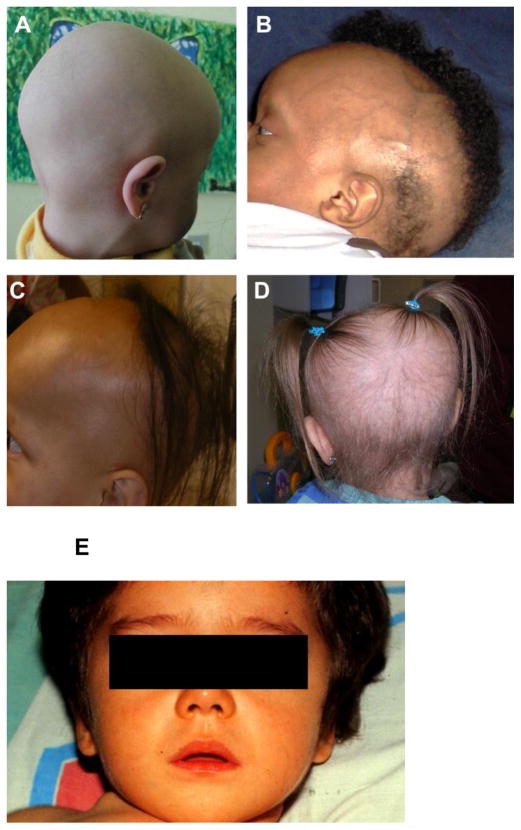
Alopecia (sometimes called atrichia) is a clinical feature that is found in many children with HVDRR and can be quite variable in appearance and extent. As shown in Fig. 1, the pattern ranges from total absence of hair to generalized sparse hair, to patches of total alopecia adjacent to areas of dense hair, or in one case a single tuft of hair. Some patients have sparse body hair and some exhibit total scalp and body alopecia (Hochberg et al., 1984, Hochberg et al., 1985, Marx et al., 1986). Children with extreme alopecia often lack eyebrows and in some cases eyelashes, which may slowly develop as the child grows. Hair loss may be evident at birth or it develops during the first half year of life as some hair the child is born with falls out and is not replaced. In families with a prior history of the disease, the loss of scalp hair in newborns provides initial diagnostic evidence for HVDRR. During childhood, after the hair present at birth sheds, new hair regrowth does not occur (Hochberg et al., 1985). Examination of skin biopsies from patients with HVDRR and alopecia has shown that some hair follicles may be absent and contain follicular remnants and cysts (Bergman et al., 2005, Miller et al., 2001). On the other hand, when hair follicles were examined microscopically other investigators reported that the number of hair follicles were within the normal range but were empty of hair shafts (Hochberg et al., 1985). In some patients papular lesions develop on the face and shoulders, but usually not on the scalp. Both the clinical presentation and the hair histology are similar to the findings in the autosomal recessive disease total alopecia known as ‘Atrichia with Papular Lesions’ (APL) (Ahmad et al., 1998) that is due to mutations in the hairless (hr) gene (Ahmad et al., 1998, Zlotogorski et al., 1998, Zlotogorski et al., 2003, Zlotogorski et al., 2002). These findings have led investigators to conclude that the alopecia in HVDRR patients is a phenocopy of APL (Bergman et al., 2005, Miller et al., 2001). Evidence that the VDR directly interacts with HR has led to the hypothesis that the VDR and HR converge to regulate similar pathways in the hair cycle (Hsieh et al., 2003).
Figure.
The Vitamin D Receptor
The human VDR gene is located on chromosome 12q13.11 and is composed of 14 exons spanning ~64 kbp of DNA. The human VDR protein contains either 427 or 424 amino acids depending upon the presence of a T to C polymorphism (ATG to ACG) in a translational start site (Gross et al., 1996, Saijo et al., 1991). The overall structure of the VDR protein is similar to the other members of the steroid-thyroid-retinoid receptor superfamily. At the N-terminus the VDR has a highly conserved DNA-binding domain (DBD) and in the C-terminal half of the protein a more variable ligand-binding domain (LBD). The VDR LBD contains 12 α-helices (H1–H12) and 3 β-sheets (S1–S3) (Rochel et al., 2000). Helix H12 forms a retractable lid that traps and holds the ligand in position. Ligand binding causes a conformational change in the VDR that promotes heterodimerization with RXR. VDR heterodimerization with RXR involves residues in H9, H10 and an E1 domain that overlaps H4 and H5 within the LBD. An activating function domain 2 (AF-2 domain) residues 416-424 of helix H12 and the region between amino acids 232-272 encompassing H3 and H4 are essential for transactivation (Rochel et al., 2000). The repositioning of helix H12 after ligand binding is critical for the formation of a hydrophobic groove that binds LxxLL motifs (where L is leucine and x is any amino acid) in the nuclear receptor interacting domains of coactivator proteins such as the g160 family of coactivators (SRC-1, SRC-2, SRC-3) and DRIP205 a member of the DRIP complex. Other regions of the VDR also act to recruit coactivator proteins or facilitate contact with proteins associated with the core transcriptional machinery such as TFIIB or the TAFs (Blanco et al., 1995, MacDonald et al., 1995). The VDR-complex together with the general transcription apparatus then drives the transcription of 1,25(OH)2D-responsive genes that ultimately determine the cellular response to the hormone. Resistance to 1,25(OH)2D can be caused by mutations in the VDR DBD that disrupt VDR binding to DNA or mutations in LBD that affect ligand binding, RXR heterodimerization or interactions with coactivators.
Analyses of VDR mutations in HVDRR patients with and without alopecia
Some HVDRR patients have been successfully treated with oral or intravenous calcium therapy, or in a few cases with high dose oral vitamin D therapy. These treatments correct the metabolic abnormalities of hypocalcemia and associated secondary hyperparathyroidism leading to healing of the rickets as assessed by X-ray and bone biopsy. However, despite the normalization of circulating calcium concentration, the correction of all serum biochemistries and reversal of the rickets, an important unresolved medical problem of HVDRR patients is persistent alopecia. The cumulative data indicate that a functional VDR is required for hair growth and that the alopecia is unrelated to the calcium or metabolic abnormalities that cause the rickets. In general, the presence of alopecia in HVDRR patients is correlated with the severity of rickets and the metabolic abnormality (Malloy et al., 1999, Malloy et al., 2011, Marx et al., 1986). HVDRR patients with alopecia are usually more resistant to treatment with vitamin D metabolites and usually require high doses of calcium, either administered intravenously or in some cases, success can be achieved with large oral doses (Ma et al., 2009, Malloy and Feldman, 2010, Malloy et al., 1999, Malloy et al., 2005, Malloy et al., 2011, Malloy et al., 2007, Malloy et al., 2010, Malloy et al., 2004).
The VDR is expressed in the hair follicle but mechanisms by which the VDR regulates hair growth are not well understood (Berger et al., 1988, Stumpf et al., 1979). However a number of insights have been gained from the molecular analysis of the VDR in HVDRR patients with and without alopecia. For example, patients with nonsense mutations that introduce premature stop signals and are totally hormone resistant all have alopecia. These findings indicate that the VDR is required for and is directly involved in hair growth. In addition, patients with missense mutations in the DBD or mutations that interfere with RXR dimerization also have alopecia indicating that DNA binding and heterodimerization are critical functions of the VDR to regulate hair growth. Mutations in the LBD that only reduce VDR binding affinity for 1,25(OH)2D3 are usually not associated with alopecia.
Molecular analyses of the VDR from HVDRR patients that do not develop alopecia almost always have missense mutations in the LBD. Several of these mutations (Arg274Leu, His305Gln, Ile314Ser, and Trp286Arg) reduce the binding affinity of 1,25(OH)2D3 for the VDR. Two of these amino acids Arg274 and His305 make direct contact with 1,25(OH)2D3 (Rochel et al., 2000). Since the patients with the His305Gln (Malloy et al., 1997) and Ile314Ser (Whitfield et al., 1996) mutations were somewhat responsive to vitamin D therapy, it is reasonable to speculate that these VDRs, although exhibiting a 2–8 fold reduction in 1,25(OH)2D3-binding affinity, are still able to exhibit enough activity to prevent the development of alopecia after birth. However, the patients with the Arg274Leu (Kristjansson et al., 1993) and Trp286Arg (Nguyen et al., 2002) mutations that resulted in more than a 1000-fold reduction in 1,25(OH)2D3-binding also did not have alopecia. These findings led us to the hypothesize that 1,25(OH)2D3-binding is not critical for the VDR pathway to regulate hair growth (Malloy et al., 2002). In further support of this hypothesis, alopecia is not found in patients with 1α-hydroxylase deficiency due to mutations in CYP27B1, the gene that encodes the 1α-hydroxylase enzyme that coverts 25(OH)D3 to 1,25(OH)2D3 a condition of severe 1,25(OH)2D3 deficiency, or in other forms of vitamin D deficiency (Kim et al., 2007). Thus alopecia appears to develop independently of the metabolic abnormalities due to defective 1,25(OH)2D3 action and its presence or absence is instead dependent on the presence or absence of functional VDR, even if unliganded.
In contrast, some patients with missense mutations in the LBD have alopecia. In these cases the mutations (Phe251Cys, Gln259Pro and Arg391Cys) have little or no effect on ligand binding but interfere with RXR heterodimerization (Cockerill et al., 1997, Malloy et al., 2001, Whitfield et al., 1996). Although these mutations cause HVDRR in patients, in studies of cultured fibroblasts from patients in vitro, or in cells transfected with mutant VDR, the 1,25(OH)2D3 resistance could be overcome by treatment with supra-physiological doses of 1,25(OH)2D3 or by over-expression of RXR (Whitfield et al., 1996). These findings indicate that RXR heterodimerization is critical for VDR function in hair development. Furthermore, targeted inactivation of RXR in keratinocytes in mice also caused alopecia further supporting a role for RXR in hair growth (Li et al., 2001).
Perhaps the most interesting discovery providing a critical clue to which mutations cause alopecia came from a patient with HVDRR without alopecia who had a missense mutation that caused a Glu420Lys substitution in the VDR LBD. The mutation is located in the AF-2 domain that is important in binding the LxxLL motifs of coactivators. The Glu420Lys mutation had no effect on ligand binding or RXR heterodimerization but abolished binding of coactivators to the VDR and caused severe vitamin D resistance and all of the metabolic abnormalities associated with HVDRR but not alopecia (Malloy et al., 2002). These findings indicated that coactivator binding is not an essential function of the VDR for hair growth and although the mutation caused HVDRR and all of the metabolic abnormalities associated with a defective VDR, the presence of the defective VDR was sufficient to prevent alopecia. These finding led us to hypothesize that the VDR exhibits a ligand-independent action (that is, an action mediated by the VDR without hormone or by ‘unliganded VDR’) during hair follicle development that is critical for hair differentiation and growth (Malloy et al., 2002).
Findings in CYP27B1 and VDR knockout mice
The role of 1,25(OH)2D3-binding and the VDR in the development of alopecia were also analyzed in mouse models of the human diseases. CYP27B1 knockout mice that are deficient in 1,25(OH)2D3 production and two forms of VDR knockout mice, ones that express a VDR protein that has a deletion of the first zinc-finger module in the VDR DBD and in VDR knockout mice that do not express a VDR protein (Bouillon et al., 2008). In the CYP27B1 knockout mice, abnormalities develop in skeletal, reproductive and immune function (Panda et al., 2001). However, the CYP27B1 knockout mice do not develop alopecia. These results, along with the findings in patients with CYP27B1 mutations, support the conclusion that 1,25(OH)2D3-binding to the VDR is not required for hair development. On the other hand, both forms of VDR knockout mice develop alopecia supporting the hypothesis that the VDR protein and particularly the DNA binding domain of the VDR, are essential for hair growth (Li et al., 1997, Yoshizawa et al., 1997). These VDR knockout mice also develop cysts suggesting an additional action of VDR function on sweat glands and sebum accumulation in skin, not just hair (reviewed in Luderer and Demay, 2010).
Analysis of VDR knockout mice and transgenic mice has led to a series of important findings about the role of the VDR in preventing alopecia (reviewed in Bouillon et al., 2008, Demay, 2006, Demay et al., 2007). It is of interest that many of these findings were also elucidated in the patients with HVDRR (reviewed in Malloy and Feldman, 2010, Malloy et al., 1999, Malloy et al., 2005, Malloy et al., 2011, Malloy et al., 2002). Importantly, Demay and colleagues showed that restricted expression of the intact VDR to keratinocytes was capable of preventing alopecia in the VDR null mice (Chen et al., 2001). Hair follicles reconstituted with keratinocytes from VDR knockout mice demonstrated a defective response to anagen initiation. Hence, alopecia in the VDR-null mice seems to be due to a localized defect within keratinocytes, perhaps involving epithelial-mesenchymal communication that is required for normal hair cycling (Chen et al., 2001). Further study was therefore focused on the keratinocyte as the cell of origin of alopecia and the findings continue to indicate that the alopecia is due to an absence of ligand-independent VDR function in this cell (Skorija et al., 2005).
Subsequent studies were performed to determine which regions of the VDR were required for actions that would prevent alopecia (reviewed in Bouillon et al., 2008, Demay, 2006, Demay et al., 2007). Investigation of mice engineered to lack the first zinc finger of the VDR demonstrated that they express a truncated receptor containing an intact LBD and AF2 domain (Bula et al., 2005). These mice demonstrated the critical requirement of an intact DBD for hair follicle homeostasis and for the prevention of alopecia. These findings in mice mirror the results from HVDRR patients with mutations in the DBD (reviewed in Malloy and Feldman, 2010, Malloy et al., 1999, Malloy et al., 2005, Malloy et al., 2011). Even a single point mutation in a critical amino acid of the DBD can cause HVDRR and alopecia (Malloy et al., 2010).
Skorija et al then developed transgenic mice that expressed VDRs with mutations in either the LBD or the AF2 domain (Skorija et al., 2005). These investigations demonstrated that mutant VDRs, even though incapable of ligand-dependent transactivation, were still able to prevent the development of alopecia (Skorija et al., 2005). Again, these mouse studies recapitulate the findings in HVDRR patients (Malloy et al., 2002). Mutations in children that prevent DNA binding, or VDR-RXR dimerization exhibit alopecia while patients with VDRs that are defective in ligand binding or co-activator binding do not develop alopecia although they do exhibit the metabolic abnormalities of hypocalcemia and rickets (reviewed in Malloy and Feldman, 2010, Malloy et al., 1999, Malloy et al., 2011). Investigation is currently underway to define the mechanisms by which the unliganded VDR maintains hair follicle homeostasis and prevents alopecia.
Role of the Hairless protein (HR) in regulating unliganded VDR actions in the hair follicle
The alopecia associated with HVDRR is clinically and pathologically indistinguishable from the generalized atrichia with papules found in patients with mutations in the hr gene (Ahmad et al., 1998, Bergman et al., 2005, Miller et al., 2001, Zlotogorski et al., 2003). But hr mutations do not cause the metabolic abnormalities or rickets of HVDRR. The hr gene is expressed in many tissues especially in the skin and brain (Cichon et al., 1998). The hr gene product, HR acts as a corepressor and directly interacts with the VDR and suppresses 1,25(OH)2D3-mediated transactivation (Hsieh et al., 2003, Malloy et al., 2009, Wang et al., 2007). Like the VDR, HR is a zinc finger protein suggesting that it interacts with DNA. It has been hypothesized that the role of the VDR in the hair cycle is to repress the expression of a gene(s) in a ligand-independent manner (Hsieh et al., 2003, Malloy and Feldman, 2003, Malloy et al., 2004, Malloy et al., 2002, Skorija et al., 2005, Wang et al., 2007). The ligand-independent activity requires that the VDR heterodimerize with RXR and bind to DNA even if it failed to activate gene transcription (Malloy and Feldman, 2003, Malloy et al., 2002). The corepressor actions of HR may also be required in order for the unliganded VDR to repress gene transcription during the hair cycle.
Studies of naturally occurring mutations in the hr gene that cause alopecia in humans have shown that they do not affect HR association with the VDR but do disrupt association with histone deacetylase 1 (Wang et al., 2007). In addition, mutant HRs with alteration of residues in the Jumonji C domain abolishes or reduces HR transrepressor activity also retain association with VDR (Hsieh et al., 2010, Malloy et al., 2009). The Jumonji C domain is thought to confer lysine demethylase activity that may be important in chromatin remodeling and repress the transcription of VDR target genes that control the hair cycle (Hsieh et al., 2010, Malloy et al., 2009, Wang et al., 2007).
Mutations in the VDR that disrupt the ability of the unliganded VDR to suppress gene transcription are hypothesized to lead to the derepression of a gene(s) whose product, when expressed inappropriately, disrupts the hair cycle that ultimately leads to alopecia (Hsieh et al., 2003, Malloy and Feldman, 2003, Malloy et al., 2004, Malloy et al., 2002, Skorija et al., 2005, Wang et al., 2007). Potential candidates include inhibitors of the Wnt signaling pathway (Beaudoin et al., 2005, Thompson et al., 2006) and PTHrP (Holick, 1985, Hsieh et al., 2003, Hsieh et al., 2010, Peters et al., 2001, Wysolmerski et al., 1994). Overexpression of PTHrP causes alopecia indicating its involvement in the regulation of the hair cycle (Cho et al., 2003, Wysolmerski et al., 1994). Since the VDR is a negative regulator of the PTHrP gene expression (Ikeda et al., 1989), loss of VDR regulation of the PTHrP gene due to mutations in the VDR may lead to the development of alopecia in HVDRR patients.
Transient gene expression assays have demonstrated that the cooperative transcriptional effects of β-catenin and Lef1 are abolished in keratinocytes isolated from VDR-null mice, suggesting a role for the unliganded VDR in canonical Wnt signaling (Cianferotti et al., 2007). Thus, absence of the VDR impairs canonical Wnt signaling in keratinocytes and may be the defect in keratinocyte stem cells that leads to the development of alopecia (Cianferotti et al., 2007). Teichert et al compared the gene expression profiles in the hair follicle of the VDR knockout mice, the CYP27B1 null mice that have no 1α-hydroxylase and are unable to synthesize 1,25(OH)2D3 and the Rhino mice that have alopecia due to the absence of the hairless protein caused by a nonsense mutation in the hairless gene (Teichert et al., 2010). In the VDR knockout and Rhino mice a number of genes were down-regulated in the hedgehog, WNT, FGF and TGFβ pathways, but not in CYP27B1 knockout mice. When the VDR knockout mice were treated with an agonist that activates the hedgehog pathway hair follicle cycling was partially restored, suggesting a role for the hedgehog pathway in the regulation of hair follicle cycling by the unliganded VDR (Teichert et al., 2010).
VDR Gene analysis in wild-type, HVDRR and APL fibroblasts
We also examined gene expression in fibroblasts from normals, from patients with HVDRR with alopecia and patients with APL having a mutation in hr. In a first analysis, in the absence of calcitriol, we compared the gene expression profiles of normal fibroblasts to fibroblasts that are devoid of VDR due to a premature stop mutation (Y295X) from an HVDRR patient with alopecia. Genes that were de-repressed in the mutant fibroblasts that do not express VDR vs. normal fibroblasts with WT VDR are candidates for ligand-independent silencing by the VDR. Table 1 shows a partial list of the genes that exhibited elevated expression in HVDRR fibroblasts compared to normal fibroblasts in the absence of calcitriol. There were 57 genes that were elevated in the HVDRR mutant fibroblasts vs. normal fibroblasts. These genes represent potential targets for silencing by the unliganded VDR. Interestingly, expression of the Wnt pathway inhibitor, SFRP1, was substantially elevated in the mutant cells. This finding is highly significant since Wnt signaling is critical for many developmental processes including hair. It is worth noting that SFRP1 is also an important regulator of osteogenesis (Gaur et al., 2005) and some APL patients exhibit delayed bone growth (Kruse et al., 1999). The expression of the nuclear receptors, Nurr-1 and Nor-1, were also elevated in the HVDRR mutant fibroblasts. These nuclear receptors belong to a set of early response genes that have been shown to be involved in bone homeostasis and brain development (Maruyama et al., 1998).
Table 1.
Partial Microarray Data Comparing HVDRR Mutant (VDR−/−) Vs. Wildtype Fibroblasts (VDR+/+) in the Absence of Calcitriol.
| De-repressed Genes In The HVDRR Mutant Fibroblasts | Fold Increase | |
|---|---|---|
| Gene Name | Gene Symbol | VDR null |
| Nurr-1 | NR4A2 | 14.0 |
| Fibroblast growth factor 7 (keratinocyte growth factor) | FGF7 | 11.4 |
| Nor-1 | NR4A3 | 10.8 |
| Parathyroid hormone like hormone (also known as PTHrP) | PTHLH | 6.2 |
| Involucrin | IVL | 6.0 |
| Secreted frizzled-related protein 1 | SFRP1 | 5.6 |
| Prostaglandin-endoperoxidase synthase 2 (COX-2) | PTGS2 | 4.0 |
In a second microarray analysis, we compared the gene expression profile of fibroblasts from an APL patient (HR null) to normal fibroblasts. Approximately 112 genes were elevated in the HR null vs. normal fibroblasts. This list of genes was then compared to the list of genes identified in the VDR null fibroblasts from an HVDRR patient with alopecia to identify genes that were common to both APL and HVDRR with alopecia. This list contained 37 genes in common. Table 2 shows a partial listing of the potential targets for gene silencing by VDR and HR. TRα is particularly interesting because of its role in development and as a partner for HR. Also of special interest were FABP5, SOX9 and FGF13 that are important in hair growth and skin regeneration (Kawano et al., 2004, Madsen et al., 1992, Vidal et al., 2005). SOX9 is also important in chondrogenesis and sexual development (Marshall and Harley, 2000).
Table 2.
Partial Microarray Data of Differentially Regulated Genes in Fibroblasts From Patients With HVDRR with Alopecia (VDR−/−) and Patients with APL (HR−/−) Compared To Wildtype Fibroblasts (VDR+/+, HR+/+).
| Genes Elevated In Common In HVDRR And APL Fibroblasts | Fold Increase | ||
|---|---|---|---|
| Gene Name | Gene Symbol | VDR null | HR null |
| Thyroid hormone receptor, alpha (TRα) | THRA | 9.8 | 8.8 |
| Phospholipase A2, group IVA (cytosolic, calcium-dependent) | PLA2G4A | 9.2 | 6.6 |
| Synaptotagmin XIV | SYT14 | 6.2 | 5.8 |
| Matrix metalloproteinase 3 (stromelysin 1, progelatinase) | MMP3 | 6.2 | 4.6 |
| Fatty acid binding protein 5 (psoriasis-associated) | FABP5 | 6.2 | 4.4 |
| SRY (sex determining region Y)-box 9 (campomelic dysplasia) | SOX9 | 5.4 | 6.4 |
| Fibroblast growth factor 13 | FGF13 | 4.4 | 4.0 |
Conclusions
The data on the VDR mutations in HVDRR patients combined with the findings in the 1α-hydroxylase and VDR knockout mouse models, suggest that the role of the VDR in the hair cycle is to represses the expression of some gene(s) in a ligand-independent manner. This activity requires RXR heterodimerization and DNA binding but not interaction with coactivators. HR may also be required as a co-repressor for this negative regulatory activity of the VDR. The VDR is a negative regulator of a number of genes and the loss of its suppressor activity by the unliganded VDR could potentially lead to the derepression of those genes that could ultimately lead to alopecia. Some of the genes with suspected involvement in the process include Wnt inhibitors such as SFRP1 and inhibitors of PTHrP.
Although HVDRR is a rare disease, studies of these cases and the VDR knock out and transgenic animal models have revealed many aspects of vitamin D biology including its roles in calcium metabolism, bone homeostasis and hair differentiation. The children with HVDRR, so-called “experiments of nature” have furthered our knowledge of VDR function and led to advances in the understanding of vitamin D action in humans. The molecular and metabolic analyses of the consequences of mutations in the VDR have helped to elucidate the structure and function of the VDR and have led to advances in the therapy of HVDRR, a complicated and severe disease of childhood. Further study of the cases of HVDRR as they mature and get older will no doubt continue to shed light on the role of the VDR in the development or prevention of various human diseases considered to be related to vitamin D deficiency (Malloy et al., 2011).
Acknowledgments
We thank Lihong Peng for her assistance in performing the gene arrays comparing the VDR and HR mutant cells and normal cells. We are grateful to the physicians from around the world who have collaborated with us and allowed us to study their HVDRR patients. We are indebted to the families affected by this disease who have consented to our involvement in the diagnosis and treatment of their abnormalities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad W, Faiyaz ul Haque M, Brancolini V, Tsou HC, ul Haque S, Lam H, Aita VM, Owen J, de Blaquiere M, Frank J, Cserhalmi-Friedman PB, Leask A, McGrath JA, Peacocke M, Ahmad M, Ott J, Christiano AM. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998;279:720–724. doi: 10.1126/science.279.5351.720. [DOI] [PubMed] [Google Scholar]
- Ahmad W, Irvine AD, Lam H, Buckley C, Bingham EA, Panteleyev AA, Ahmad M, McGrath JA, Christiano AM. A missense mutation in the zinc-finger domain of the human hairless gene underlies congenital atrichia in a family of Irish travellers. Am J Hum Genet. 1998;63:984–991. doi: 10.1086/302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsan S, Garabedian M, Liberman UA, Eil C, Bourdeau A, Guillozo H, Grimberg R, Le Deunff MJ, Lieberherr M, Guimbaud P, Broyer M, Marx SJ. Rickets and alopecia with resistance to 1,25-dihydroxyvitamin D: two different clinical courses with two different cellular defects. Journal of Clinical Endocrinology and Metabolism. 1983;57:803–811. doi: 10.1210/jcem-57-4-803. [DOI] [PubMed] [Google Scholar]
- Beaudoin GM, 3rd, Sisk JM, Coulombe PA, Thompson CC. Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc Natl Acad Sci U S A. 2005;102:14653–14658. doi: 10.1073/pnas.0507609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger U, Wilson P, McClelland RA, Colston K, Haussler MR, Pike JW, Coombes RC. Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. Journal of Clinical Endocrinology and Metabolism. 1988;67:607–613. doi: 10.1210/jcem-67-3-607. [DOI] [PubMed] [Google Scholar]
- Bergman R, Schein-Goldshmid R, Hochberg Z, Ben-Izhak O, Sprecher E. The alopecias associated with vitamin D-dependent rickets type IIA and with hairless gene mutations: a comparative clinical, histologic, and immunohistochemical study. Arch Dermatol. 2005;141:343–351. doi: 10.1001/archderm.141.3.343. [DOI] [PubMed] [Google Scholar]
- Blanco JC, Wang IM, Tsai SY, Tsai MJ, O’Malley BW, Jurutka PW, Haussler MR, Ozato K. Transcription factor TFIIB and the vitamin D receptor cooperatively activate ligand-dependent transcription. Proc Natl Acad Sci U S A. 1995;92:1535–1539. doi: 10.1073/pnas.92.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliziotes M, Yergey AL, Nanes MS, Muenzer J, Begley MG, Viera NE, Kher KK, Brandi ML, Marx SJ. Absent intestinal response to calciferols in hereditary resistance to 1,25-dihydroxyvitamin D: documentation and effective therapy with high dose intravenous calcium infusions. Journal of Clinical Endocrinology and Metabolism. 1988;66:294–300. doi: 10.1210/jcem-66-2-294. [DOI] [PubMed] [Google Scholar]
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bula CM, Huhtakangas J, Olivera C, Bishop JE, Norman AW, Henry HL. Presence of a truncated form of the vitamin D receptor (VDR) in a strain of VDR-knockout mice. Endocrinology. 2005;146:5581–5586. doi: 10.1210/en.2005-0806. [DOI] [PubMed] [Google Scholar]
- Chen CH, Sakai Y, Demay MB. Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology. 2001;142:5386–5389. doi: 10.1210/endo.142.12.8650. [DOI] [PubMed] [Google Scholar]
- Cho YM, Woodard GL, Dunbar M, Gocken T, Jimenez JA, Foley J. Hair-cycle-dependent expression of parathyroid hormone-related protein and its type I receptor: evidence for regulation at the anagen to catagen transition. J Invest Dermatol. 2003;120:715–727. doi: 10.1046/j.1523-1747.2003.12147.x. [DOI] [PubMed] [Google Scholar]
- Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9428–9433. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichon S, Anker M, Vogt IR, Rohleder H, Putzstuck M, Hillmer A, Farooq SA, Al-Dhafri KS, Ahmad M, Haque S, Rietschel M, Propping P, Kruse R, Nothen MM. Cloning, genomic organization, alternative transcripts and mutational analysis of the gene responsible for autosomal recessive universal congenital alopecia. Hum Mol Genet. 1998;7:1671–1679. doi: 10.1093/hmg/7.11.1671. [DOI] [PubMed] [Google Scholar]
- Cockerill FJ, Hawa NS, Yousaf N, Hewison M, O’Riordan JL, Farrow SM. Mutations in the vitamin D receptor gene in three kindreds associated with hereditary vitamin D resistant rickets. J Clin Endocrinol Metab. 1997;82:3156–3160. doi: 10.1210/jcem.82.9.4243. [DOI] [PubMed] [Google Scholar]
- Demay MB. Mechanism of vitamin D receptor action. Annals of the New York Academy of Sciences. 2006;1068:204–213. doi: 10.1196/annals.1346.026. [DOI] [PubMed] [Google Scholar]
- Demay MB, MacDonald PN, Skorija K, Dowd DR, Cianferotti L, Cox M. Role of the vitamin D receptor in hair follicle biology. The Journal of steroid biochemistry and molecular biology. 2007;103:344–346. doi: 10.1016/j.jsbmb.2006.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D, Malloy PJ, Krishnan AV, Balint E. Vitamin D: biology, action, and clinical implications. In: Marcus R, et al., editors. Osteoporosis. 3. Academic Press; San Diego: 2007. pp. 317–382. [Google Scholar]
- Fraher LJ, Karmali R, Hinde FR, Hendy GN, Jani H, Nicholson L, Grant D, O’Riordan JL. Vitamin D-dependent rickets type II: extreme end organ resistance to 1,25-dihydroxy vitamin D3 in a patient without alopecia. European Journal of Pediatrics. 1986;145:389–395. doi: 10.1007/BF00439245. [DOI] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- Gross C, Eccleshall TR, Malloy PJ, Villa ML, Marcus R, Feldman D. The presence of a polymorphism at the translation initiation site of the vitamin D receptor gene is associated with low bone mineral density in postmenopausal Mexican-American women. J Bone Miner Res. 1996;11:1850–1855. doi: 10.1002/jbmr.5650111204. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, Jurutka PW. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev. 2008;66:S98–112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Benderli A, Levy J, Vardi P, Weisman Y, Chen T, Feldman D. 1,25-Dihydroxyvitamin D resistance, rickets, and alopecia. American Journal of Medicine. 1984;77:805–811. doi: 10.1016/0002-9343(84)90516-3. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Gilhar A, Haim S, Friedman-Birnbaum R, Levy J, Benderly A. Calcitriol-resistant rickets with alopecia. Archives of Dermatology. 1985;121:646–647. [PubMed] [Google Scholar]
- Holick MF. Vitamin D resistance and alopecia. A causal or casual relationship? Archives of Dermatology. 1985;121:601–603. [PubMed] [Google Scholar]
- Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR, Thompson CC. Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem. 2003;278:38665–38674. doi: 10.1074/jbc.M304886200. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Slater SA, Whitfield GK, Dawson JL, Hsieh G, Sheedy C, Haussler CA, Haussler MR. Analysis of hairless corepressor mutants to characterize molecular cooperation with the vitamin D receptor in promoting the mammalian hair cycle. J Cell Biochem. 2010;110:671–686. doi: 10.1002/jcb.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Lu C, Weir EC, Mangin M, Broadus AE. Transcriptional regulation of the parathyroid hormone-related peptide gene by glucocorticoids and vitamin D in a human C-cell line. J Biol Chem. 1989;264:15743–15746. [PubMed] [Google Scholar]
- Kawano M, Suzuki S, Suzuki M, Oki J, Imamura T. Bulge- and basal layer-specific expression of fibroblast growth factor-13 (FHF-2) in mouse skin. J Invest Dermatol. 2004;122:1084–1090. doi: 10.1111/j.0022-202X.2004.22514.x. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Kaplan LE, Perwad F, Huang N, Sharma A, Choi Y, Miller WL, Portale AA. Vitamin D 1alpha-hydroxylase gene mutations in patients with 1alpha-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:3177–3182. doi: 10.1210/jc.2006-2664. [DOI] [PubMed] [Google Scholar]
- Kristjansson K, Rut AR, Hewison M, O’Riordan JL, Hughes MR. Two mutations in the hormone binding domain of the vitamin D receptor cause tissue resistance to 1,25 dihydroxyvitamin D3. Journal of Clinical Investigation. 1993;92:12–16. doi: 10.1172/JCI116539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse R, Cichon S, Anker M, Hillmer AM, Barros-Nunez P, Cantu JM, Leal E, Weinlich G, Schmuth M, Fritsch P, Ruzicka T, Propping P, Nothen MM. Novel Hairless mutations in two kindreds with autosomal recessive papular atrichia. J Invest Dermatol. 1999;113:954–959. doi: 10.1046/j.1523-1747.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Kumagai T, Uetsuji N, Tsugawa S, Oyanagi K, Chiba Y, Minami R, Nakao T. Vitamin D dependent rickets: decreased sensitivity to 1,25-dihydroxyvitamin D. European Journal of Pediatrics. 1981;137:307–311. doi: 10.1007/BF00443263. [DOI] [PubMed] [Google Scholar]
- Laufer D, Benderly A, Hochberg Z. Dental pathology in calcitirol resistant rickets. Journal of Oral Medicine. 1987;42:272–275. [Google Scholar]
- Li M, Chiba H, Warot X, Messaddeq N, Gerard C, Chambon P, Metzger D. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 2001;128:675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman UA, Samuel R, Halabe A, Kauli R, Edelstein S, Weisman Y, Papapoulos SE, Clemens TL, Fraher LJ, O’Riordan JL. End-organ resistance to 1,25-dihydroxycholecalciferol. Lancet. 1980;1:504–506. doi: 10.1016/s0140-6736(80)92763-4. [DOI] [PubMed] [Google Scholar]
- Luderer HF, Demay MB. The vitamin D receptor, the skin and stem cells. J Steroid Biochem Mol Biol. 2010;121:314–316. doi: 10.1016/j.jsbmb.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Ma NS, Malloy PJ, Pitukcheewanont P, Dreimane D, Geffner ME, Feldman D. Hereditary vitamin D resistant rickets: Identification of a novel splice site mutation in the vitamin D receptor gene and successful treatment with oral calcium therapy. Bone. 2009;45:743–746. doi: 10.1016/j.bone.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PN, Sherman DR, Dowd DR, Jefcoat SC, Jr, DeLisle RK. The vitamin D receptor interacts with general transcription factor IIB. J Biol Chem. 1995;270:4748–4752. doi: 10.1074/jbc.270.9.4748. [DOI] [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99:299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D. Hereditary vitamin D resistant rickets caused by a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest. 1997;99:297–304. doi: 10.1172/JCI119158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Feldman D. Hereditary 1,25-Dihydroxyvitamin D-resistant rickets. Endocr Dev. 2003;6:175–199. doi: 10.1159/000072776. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Feldman D. Genetic disorders and defects in vitamin D action. Endocrinol Metab Clin N Am. 2010;39:333–346. doi: 10.1016/j.ecl.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev. 1999;20:156–188. doi: 10.1210/edrv.20.2.0359. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Pike JW, Feldman D. Hereditary 1,25-dihydroxyvitamin D resistant rickets. In: Feldman D, et al., editors. Vitamin D. 2. Elsevier, cc; 2005. pp. 1207–1238. [Google Scholar]
- Malloy PJ, Tiosano D, Feldman D. Hereditary 1,25-dihydroxyvitamin D resistant rickets. In: Feldman D, et al., editors. Vitamin D. 3. Elsevier; San Diego: 2011. pp. 1197–1232. [Google Scholar]
- Malloy PJ, Wang J, Jensen K, Feldman D. Modulation of vitamin d receptor activity by the corepressor hairless: differential effects of hairless isoforms. Endocrinology. 2009;150:4950–4957. doi: 10.1210/en.2009-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Wang J, Peng L, Nayak S, Sisk JM, Thompson CC, Feldman D. A unique insertion/duplication in the VDR gene that truncates the VDR causing hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Arch Biochem Biophys. 2007;460:285–292. doi: 10.1016/j.abb.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Wang J, Srivastava T, Feldman D. Hereditary 1,25-dihydroxyvitamin D-resistant rickets with alopecia resulting from a novel missense mutation in the DNA-binding domain of the vitamin D receptor. Molecular Genetics and Metabolism. 2010;99:72–79. doi: 10.1016/j.ymgme.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy PJ, Xu R, Cattani A, Reyes L, Feldman D. A unique insertion/substitution in helix H1 of the vitamin D receptor ligand binding domain in a patient with hereditary 1,25-dihydroxyvitamin D-resistant rickets. J Bone Miner Res. 2004;19:1018–1024. doi: 10.1359/jbmr.2004.19.6.1018. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Xu R, Peng L, Clark PA, Feldman D. A novel mutation in helix 12 of the vitamin D receptor impairs coactivator interaction and causes hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia. Mol Endocrinol. 2002;16:2538–2546. doi: 10.1210/me.2002-0152. [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Zhu W, Zhao XY, Pehling GB, Feldman D. A novel inborn error in the ligand-binding domain of the vitamin D receptor causes hereditary vitamin D-resistant rickets. Mol Genet Metab. 2001;73:138–148. doi: 10.1006/mgme.2001.3181. [DOI] [PubMed] [Google Scholar]
- Marshall OJ, Harley VR. Molecular mechanisms of SOX9 action. Mol Genet Metab. 2000;71:455–462. doi: 10.1006/mgme.2000.3081. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Tsukada T, Ohkura N, Bandoh S, Hosono T, Yamaguchi K. The NGFI-B subfamily of the nuclear receptor superfamily (review) Int J Oncol. 1998;12:1237–1243. doi: 10.3892/ijo.12.6.1237. [DOI] [PubMed] [Google Scholar]
- Marx SJ, Bliziotes MM, Nanes M. Analysis of the relation between alopecia and resistance to 1,25-dihydroxyvitamin D. Clinical Endocrinology. 1986;25:373–381. doi: 10.1111/j.1365-2265.1986.tb01703.x. [DOI] [PubMed] [Google Scholar]
- Marx SJ, Spiegel AM, Brown EM, Gardner DG, Downs RW, Jr, Attie M, Hamstra AJ, DeLuca HF. A familial syndrome of decrease in sensitivity to 1,25-dihydroxyvitamin D. Journal of Clinical Endocrinology and Metabolism. 1978;47:1303–1310. doi: 10.1210/jcem-47-6-1303. [DOI] [PubMed] [Google Scholar]
- Miller J, Djabali K, Chen T, Liu Y, Ioffreda M, Lyle S, Christiano AM, Holick M, Cotsarelis G. Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol. 2001;117:612–617. doi: 10.1046/j.0022-202x.2001.01438.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Adiceam P, Kottler ML, Guillozo H, Rizk-Rabin M, Brouillard F, Lagier P, Palix C, Garnier JM, Garabedian M. Tryptophan missense mutation in the ligand-binding domain of the vitamin D receptor causes severe resistance to 1,25-dihydroxyvitamin D. J Bone Miner Res. 2002;17:1728–1737. doi: 10.1359/jbmr.2002.17.9.1728. [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EM, Foitzik K, Paus R, Ray S, Holick MF. A new strategy for modulating chemotherapy-induced alopecia, using PTH/PTHrP receptor agonist and antagonist. The Journal of investigative dermatology. 2001;117:173–178. doi: 10.1046/j.0022-202x.2001.01410.x. [DOI] [PubMed] [Google Scholar]
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000;5:173–179. doi: 10.1016/s1097-2765(00)80413-x. [DOI] [PubMed] [Google Scholar]
- Rosen JF, Fleischman AR, Finberg L, Hamstra A, DeLuca HF. Rickets with alopecia: an inborn error of vitamin D metabolism. Journal of Pediatrics. 1979;94:729–735. doi: 10.1016/s0022-3476(79)80139-0. [DOI] [PubMed] [Google Scholar]
- Saijo T, Ito M, Takeda E, Huq AH, Naito E, Yokota I, Sone T, Pike JW, Kuroda Y. A unique mutation in the vitamin D receptor gene in three Japanese patients with vitamin D-dependent rickets type II: utility of single-strand conformation polymorphism analysis for heterozygous carrier detection. American Journal of Human Genetics. 1991;49:668–673. [PMC free article] [PubMed] [Google Scholar]
- Skorija K, Cox M, Sisk JM, Dowd DR, MacDonald PN, Thompson CC, Demay MB. Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol Endocrinol. 2005;19:855–862. doi: 10.1210/me.2004-0415. [DOI] [PubMed] [Google Scholar]
- Sockalosky JJ, Ulstrom RA, DeLuca HF, Brown DM. Vitamin D--resistant rickets: end-organ unresponsiveness to 1,25(OH)2D3. Journal of Pediatrics. 1980;96:701–703. doi: 10.1016/s0022-3476(80)80748-7. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206:1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- Teichert A, Elalieh H, Bikle D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. J Cell Physiol. 2010;225:482–489. doi: 10.1002/jcp.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CC, Sisk JM, Beaudoin GM., 3rd Hairless and Wnt signaling: allies in epithelial stem cell differentiation. Cell Cycle. 2006;5:1913–1917. doi: 10.4161/cc.5.17.3189. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Wang J, Malloy PJ, Feldman D. Interactions of the vitamin D receptor with the corepressor hairless: analysis of hairless mutants in atrichia with papular lesions. J Biol Chem. 2007;282:25231–25239. doi: 10.1074/jbc.M702939200. [DOI] [PubMed] [Google Scholar]
- Whitfield GK, Selznick SH, Haussler CA, Hsieh JC, Galligan MA, Jurutka PW, Thompson PD, Lee SM, Zerwekh JE, Haussler MR. Vitamin D receptors from patients with resistance to 1,25-dihydroxyvitamin D3: point mutations confer reduced transactivation in response to ligand and impaired interaction with the retinoid X receptor heterodimeric partner. Mol Endocrinol. 1996;10:1617–1631. doi: 10.1210/mend.10.12.8961271. [DOI] [PubMed] [Google Scholar]
- Wysolmerski JJ, Broadus AE, Zhou J, Fuchs E, Milstone LM, Philbrick WM. Overexpression of parathyroid hormone-related protein in the skin of transgenic mice interferes with hair follicle development. Proc Natl Acad Sci U S A. 1994;91:1133–1137. doi: 10.1073/pnas.91.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- Zlotogorski A, Ahmad W, Christiano AM. Congenital atrichia in five Arab Palestinian families resulting from a deletion mutation in the human hairless gene. Hum Genet. 1998;103:400–404. doi: 10.1007/s004390050840. [DOI] [PubMed] [Google Scholar]
- Zlotogorski A, Hochberg Z, Mirmirani P, Metzker A, Ben-Amitai D, Martinez-Mir A, Panteleyev AA, Christiano AM. Clinical and pathologic correlations in genetically distinct forms of atrichia. Arch Dermatol. 2003;139:1591–1596. doi: 10.1001/archderm.139.12.1591. [DOI] [PubMed] [Google Scholar]
- Zlotogorski A, Panteleyev AA, Aita VM, Christiano AM. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2002;118:887–890. doi: 10.1046/j.1523-1747.2001.01767.x. [DOI] [PubMed] [Google Scholar]