Abstract
The pectate lyase (PL; EC 4.2.2.2) secreted by the plant pathogen Erwinia chrysanthemi is induced and catabolite repressed by different concentrations of its own product, digalacturonic acid 4,5-unsaturated at the nonreducing end [u(GalUA)2]. Both activities of u(GalUA)2 depend on its cleavage by oligogalacturonide lyase (OGL; EC 4.2.2.6). This intracellular enzyme converts u(GalUA)2 to the deoxyketuronic acid 4-deoxy-L-threo-5-hexosulose uronic acid, which is then isomerized to 3-deoxy-D-glycero-2,5-hexodiulosonic acid. An OGL-deficient mutant unable to grow on u(GalUA)2 was poorly induced by u(GalUA)2 or by D-galacturonan but produced wild-type levels of PL when supplied with 3-deoxy-D-glycero-2,5-hexodiulosonic acid. PL synthesis in the mutant could also be stimulated by 4,5-unsaturated trigalacturonic acid, from which deoxyketuronic acid is released by another intracellular enzyme. An OGL-deficient mutant that grew slowly on u(GalUA)2 in comparison with the wild-type parent was hyperinduced by u(GalUA)2 unless catabolite repression was relieved by cyclic AMP or imposed by logarithmic growth on glycerol. PL synthesis is also stimulated by saturated digalacturonic acid, which is released from D-galacturonan by another extracellular enzyme, exo-poly-α-D-galacturonosidase (EC 3.2.1.82). Because these dimers stimulate PL synthesis at concentrations (wt/vol) 1/1000th of the concentration required by D-galacturonan, and because an OGL-deficient mutant uninducible by dimers was also uninducible by D-galacturonan, we postulate that PL induction by pectic polymers entails extracellular formation of dimers and subsequent intracellular conversion to deoxyketuronic acids, the apparent inducers of PL.
Keywords: phytopathogenic bacteria, exoenzyme regulation, regulatory mutants, product induction, deoxyketuronic acid
Full text
PDF

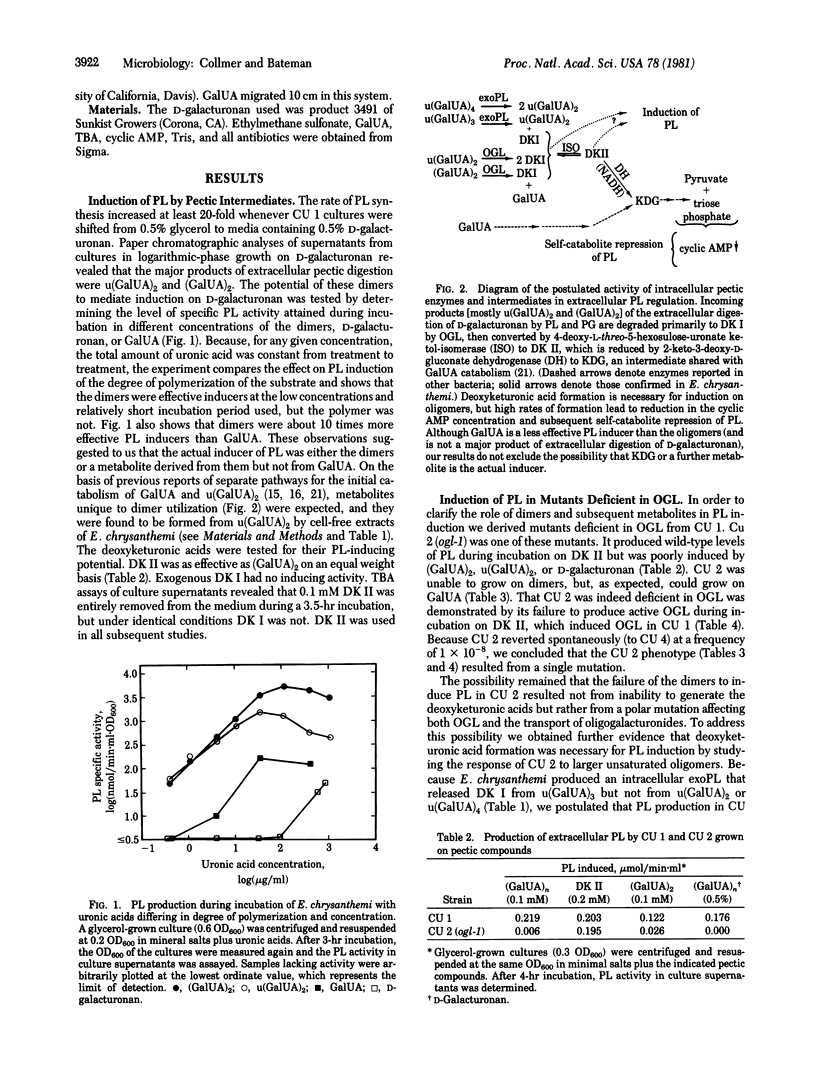
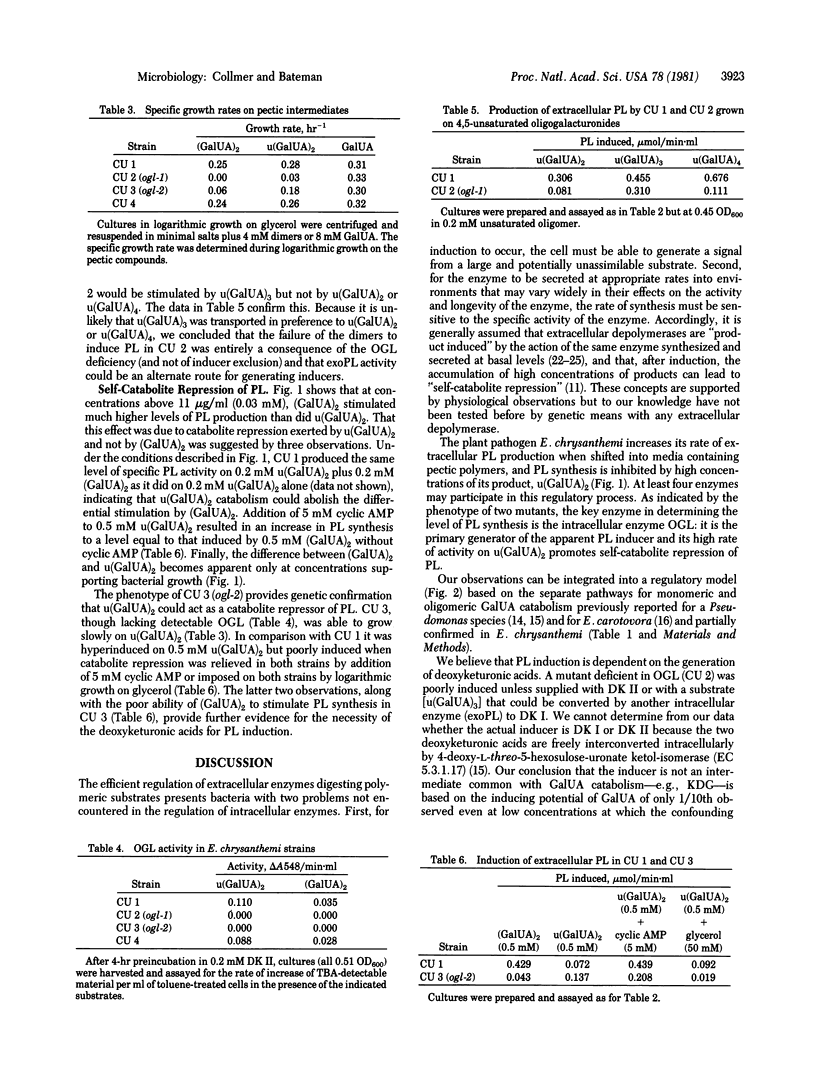

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatterjee A. K., Starr M. P. Donor strains of the soft-rot bacterium Erwinia chrysanthemi and conjugational transfer of the pectolytic capacity. J Bacteriol. 1977 Dec;132(3):862–869. doi: 10.1128/jb.132.3.862-869.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Ingle M. B., Erickson R. J. Bacterial alpha-amylases. Adv Appl Microbiol. 1978;24:257–278. [PubMed] [Google Scholar]
- KILGORE W. W., STARR M. P. Catabolism of galacturonic and glucuronic acids by Erwinia carotovora. J Biol Chem. 1959 Sep;234:2227–2235. [PubMed] [Google Scholar]
- Keegstra K., Talmadge K. W., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: III. A Model of the Walls of Suspension-cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973 Jan;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran F., Nasuno S., Starr M. P. Oligogalacturonide trans-eliminase of Erwinia carotovora. Arch Biochem Biophys. 1968 Jun;125(3):734–741. doi: 10.1016/0003-9861(68)90508-0. [DOI] [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. I. Enzymatic formation of 4-deoxy-L-threo-5-hexoseulose uronic acid. J Biol Chem. 1963 May;238:1571–1583. [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. II. Formation and metabolism of 3-deoxy-D-glycero-2, 5-hexodiulosonic acid. J Biol Chem. 1963 May;238:1577–1583. [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexová-Benková L., Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Tsuyumu S. "Self-catabolite repression" of pectate lyase in Erwinia carotovora. J Bacteriol. 1979 Feb;137(2):1035–1036. doi: 10.1128/jb.137.2.1035-1036.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyumu S. Inducer of pectic acid lyase in Erwinia carotovora. Nature. 1977 Sep 15;269(5625):237–238. doi: 10.1038/269237a0. [DOI] [PubMed] [Google Scholar]
- WARREN L. Thiobarbituric acid spray reaction for deoxy sugars and sialic acids. Nature. 1960 Apr 16;186:237–237. doi: 10.1038/186237a0. [DOI] [PubMed] [Google Scholar]
- Zucker M., Hankin L. Regulation of pectate lyase synthesis in Pseudomonas fluorescens and Erwinia carotovora. J Bacteriol. 1970 Oct;104(1):13–18. doi: 10.1128/jb.104.1.13-18.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



