Abstract
Objective
Transfusion of red blood cells (RBCs) has been linked to disappointing clinical outcomes in the critically ill, but specific mechanisms of organ dysfunction after transfusion remain poorly understood. We tested the hypothesis that RBC storage impairs the ability of RBCs to release ATP and that impaired ATP-release was injurious in vivo, in part through increased RBC adhesion.
Design
Prospective, controlled, mechanistic study.
Setting
University research laboratory.
Subjects
Human and mouse blood donors; nude mouse transfusion recipients.
Interventions
Manipulation of ATP release, supplemental ATP, and antibodies to RBC and endothelial adhesion receptors were used in vitro and in vivo to probe the roles of released ATP and adhesion in responses to (transfused) RBCs.
Measurements and main results
The ability of stored RBCs to release ATP declined markedly within 14 days after collection, despite relatively stable levels of ATP within the RBCs. Inhibiting ATP release promoted the adhesion of stored RBCs to endothelial cells in vitro and RBC sequestration in the lungs of transfused mice in vivo. Unlike transfusion of fresh human RBCs, stored-RBC transfusion in mice decreased blood oxygenation and increased extravasation of RBCs into the lung’s alveolar airspaces. Similar findings were seen with transfusion of fresh RBCs treated with the ATP-release inhibitors glibenclamide and carbenoxolone. These findings were prevented by either co-infusion of an ATP analog or pre-transfusion incubation of the RBCs with an antibody against the erythrocyte adhesion receptor LW (Landsteiner-Wiener; ICAM-4).
Conclusions
The normal flow of RBCs in pulmonary microvessels depends in part on the release of anti-adhesive ATP from RBCs, and storage-induced deficiency in ATP release from transfused RBCs may promote or exacerbate microvascular pathophysiology in the lung, in part through increased RBC adhesion.
Keywords: erythrocyte, oxygenation, acute lung injury, intercellular adhesion molecule-4 (ICAM-4), Landsteiner-Weiner (LW), endothelium, blood storage
INTRODUCTION
Several recent clinical studies have reported negative clinical outcomes in certain groups of patients receiving transfusion of RBCs for anemia (1–5). For example, in critically ill pediatric or adult patients with anemia, mortality and major morbidity in those randomly assigned to a liberal RBC-transfusion strategy was not superior to those transfused according to a conservative strategy (1, 2). While the uncertainty surrounding optimal transfusion thresholds and storage times continues and has appropriately given rise to new clinical trials examining outcomes as a function of RBC storage duration, a basic and translational reexamination of the “storage lesions” in the RBC is also needed. Functionally relevant biochemical and morphological changes during RBC storage have been recognized for decades. Recently, the recognition that RBCs participate directly and actively, rather than only passively, in the regulation of blood flow and thus O2 delivery has informed this reexamination. For example, we and others reported that early after collection, the ability of RBCs to relax blood vessels in hypoxia is significantly reduced, as are the S-nitrosothiol mediators of this RBC-dependent vasodilator activity (6, 7).
Vasodilation can also be mediated by ATP, and more generally signaling by exported ATP has been demonstrated in a variety of cell types, including the RBC (8, 9). The conduit for ATP release from the RBC appears to be the pannexin 1 hemichannel (10). The efflux of ATP from RBCs can be inhibited by pannexin-1 inhibitors or by the cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor glibenclamide (GLIB, which also blocks ATP-sensitive potassium (K+ATP) channels) (8, 10–13)_ENREF_12. In RBCs stored in additive solutions used currently, ATP levels are well maintained, falling only about 40% by the 42-day expiration (14). But little is known regarding the influence of storage on ATP export from RBCs, or of the resulting functional changes, if any.
We tested the hypotheses that: a) the ability of RBCs to release ATP declines during storage, and b) the consequent impairment in RBC-ATP release promotes endothelial adhesion of RBCs. We also studied the ATP-sensitive sequelae of RBC transfusion in the lung, which is the first organ to encounter transfused RBCs and is frequently adversely impacted, according to clinical studies of outcomes after RBC transfusion (1–3). Collectively, our new findings point to a critical role for ATP exported from the normal RBC in preventing endothelial injury.
MATERIALS AND METHODS
Materials and mice
Reagents were purchased from Sigma (St. Louis, MO) except where otherwise noted. Experiments involving mice were approved by the Duke IACUC, and the collection and use of human blood was approved by the Duke IRB. Nude mice (9–15 weeks) purchased from a Duke core facility were housed under specific pathogen-free (SPF) conditions until the day of an experiment. C57BL6 mice were purchased from Jackson Labs and/or bred at Duke.
Collection and preparation of RBCs
After written informed consent and under an IRB-approved protocol, venous blood was drawn via antecubital vein of healthy volunteers into either Vacutainer tubes or syringes containing either EDTA or sodium heparin (50 units/ml), and kept on ice. Fresh RBCs were separated centrifugally, not leukoreduced except where specifically noted, and washed twice using excess PBS containing 0.1 mM diethylaminetetrapentaacetic acid (DTPA). Stored, leukoreduced RBCs were obtained either from segments of banked RBC units stored by Duke Medical Center Transfusion Services or commercially (Interstate Blood Bank, Memphis TN). The additive solutions used for stored, leukoreduced RBCs were: AS-1 (40% of unit sources), AS-3 (56%), or CPDA (4%). Aliquots were removed aseptically, washed twice with excess PBS (with 0.1 mM DTPA,), and resuspended at a hematocrit of 35% before use.
In vitro ATP release from RBCs
was assayed in Krebs buffer, pH 7.40, bubbled with 21% O2/5% CO2/balance N2 (“normoxia”) or 0% O2/5% CO2/balance N2 (“hypoxia”) 37°C. RBC final concentration was 0.4% hematocrit (i.e., 0.4% vol/vol in PBS). Assays were conducted on the post-centrifugation supernatant using a standard luciferase assay. PO2 measured using a portable O2 electrode calibrated daily (Mettler, Thermo, Pittsburgh, PA) was 5–10 mmHg (~1% O2) under “hypoxic” conditions. The gas flow through the buffer was kept equal under the two conditions. Preliminary experiments indicated that upon exposure to hypoxia, ATP release from RBCs reached a plateau 2 minutes later, and levels at 10 minutes were similar (not shown). We therefore used a 5-minute timepoint in subsequent in vitro studies of the determinants and consequences of RBC ATP release.
RBC exposure to glibenclamide (GLIB) or carbenoxolone (CBX)
GLIB (10 mM stock solution prepared daily) was dissolved in 25 µM NaOH supplemented with 50 mg/ml dextrose as previously described (12), and a final concentration of 10 µM was used. CBX was dissolved in PBS; final concentration was 100 µM in RBC exposures. Following exposure (30 mins, 25°C) to GLIB, CBX, or vehicle, RBCs were washed twice with excess PBS. Preliminary data using the ectonucleotidase (ATPase) inhibitor ARL67156 indicated that extracellular ATPase(s) had little effect on the accumulation of released or added ATP (data not shown) under these conditions, and therefore ATPase inhibitors were not used thereafter.
RBC adhesion assays
Graduated-height flow chambers were used to quantify adhesion of RBCs to human umbilical vein endothelial cells (HUVECs) grown to confluence on glass slides pre-coated with 2% gelatin as described (15). Temperature was maintained at 37°C, and adhesion was quantified by counting the number of adherent cells before and 5 mins after fluidic flow began. % adherence was calculated as 100 × the number of cells adhering after flow begins, divided by the number of cells adhering before flow began. HUVECs washed with PBS containing Ca2+ and Mg2+ were exposed in room air to either fresh or stored packed RBCs at 0.2% hematocrit (i.e., 0.1% vol/vol in PBS with Ca2+ and Mg2+) after RBC washing in PBS with Ca2+ and Mg2+. We did not detect any hemolysis in the effluent from flow chambers in adhesion experiments. In some experiments, RBCs were pretreated with GLIB (final concentration, 10 µM) or pre-incubated with anti-RBC adhesion receptor antibodies prior to adhesion assays. In other experiments HUVECs were exposed to RBCs in the presence of apyrase (5 units/ml in PBS with Ca2+ and Mg2+). In some experiments HUVECs were pre-incubated with extracellular ATP or mAbs as indicated. See also Supplemental Digital Content.
Adhesion receptor antibodies
were obtained from the following sources: anti-LW Ab (mouse polyclonal anti-human, B01P) from Abnova (Taipei, Taiwan); anti-CD44 (mouse monoclonal anti-human, MS-668-PABX) from Thermo Scientific, Fremont, CA), anti-CD47 (mouse monoclonal anti-human, sc-12730) from Santa Cruz Biotechnology (Santa Cruz, CA), (ab3283) from Abcam (Cambridge, MA), and anti-αvβ3 integrin (mouse monoclonal anti-human, LM609) was from Millipore (Temecula, CA). RBCs or HUVECs were incubated with saturating Ab concentrations (25 or 20 µg/ml, respectively) for 45 mins at 25° C or 37°C respectively. Murine myeloma protein P3×63/Ag8 (P3 ascitic fluid, 1:500 dilution) was grown in the Telen lab and used as a non-reactive control immunoglobulin in specified mAb studies.
RBC deformability
was measured in washed RBCs suspended in polyvinylpyrrolidone using an ektacytometer (laser-assisted optical rotational cell analyzer; Mechatronics, Zwaag, The Netherlands) as previously described (7).
RBC transfusion model in nude mice
Nude mice 9–15 weeks of age were anesthetized with sodium pentobarbital, cannulated with a tracheostomy, and positioned on a warming platform. A rodent ventilator delivered room air as 7 µl/g 100 times per minute. Mice were randomly assigned before the experiment to undergo either bronchoalveolar lavage (BAL) or lung harvest after the experiment. A polyethylene catheter was placed in the ventral tail vein and secured. Continuous pulse oximetry measured arterial Hb O2 saturation (MouseOx, rodent-specific pulse oximeter, Starr Life Sciences, Oakmont, PA) and was recorded electronically. For RBC transfusions, 10 µl/g body weight of a 35% hematocrit RBC suspension in PBS was warmed to 37°C, then infused over 10 secs i.v.. Ten minutes after SaO2 had re-equilibrated following RBC transfusion, BAL was performed using 4 serial 1-ml exchanges with PBS containing 0.1 mM DTPA delivered via the tracheostomy at 18 cm H2O pressure and recovered by gravity. BAL fluid (BALF) cell pellets obtained by centrifugation were resuspended in HBSS. Cells were counted using a hemacytometer, fixed on a Cytospin slide for differential WBC counts, or analyzed using a flow cytometer as indicated.
Fluorescent labeling and detection of transfused RBCs
Washed RBCs were labeled with the fluorescent dye Dil (Molecular Probes, OR) as previously described (16) in the presence of Ca++ and Mg++ followed by washing with Ca++- and Mg++-free PBS containing the chelator DTPA (0.1 mM). BALF cellularity or tissues were examined under a fluorescent microscope fitted with a Texas-red filter.
Flow cytometry
was performed on BALF samples after incubation with mouse (anti-TER 119) or human (anti-CD235a)-specific fluorescent antibodies (eBioscience, San Diego, CA) using a FACS™ Canto II Becton-Dickinson instrument (San Jose, CA).
Immunohistology of organs from transfused mice
In pre-selected experiments, lungs from nude mice transfused with Dil-labeled, fresh human RBCs pretreated with vehicle or GLIB were perfused free of intravascular blood cells with PBS (with DTPA, 0.1 mM) and inflation-fixed using 10% formalin immediately following an experiment (~15 mins post-transfusion) and euthanasia. In a separate series of experiments, the lungs, spleen and a kidney were harvested after similar experiments. Frozen tissue sections were counterstained with DAPI, and deposition of RBCs (fluorescence-labeled with Dil prior to transfusion) was identified using a Texas-red filter. Where indicated, channel-specific fluorescence intensity was quantified (Adobe Photoshop CS5).
RBC perfusion of isolated mouse lungs
was performed as previously described (17). Briefly, C57BL6 mice 9–12 weeks of age were anesthetized with ketamine/xylazine, and a Krebs-based perfusate was used at 1.0 ml/min. For RBC perfusion (0.1 ml/min), the total rate was held constant by decreasing the perfusion rate of coinfused buffer. See also Supplemental Digital Content.
Statistical analysis
utilized ANOVA or paired or unpaired t-tests, with post-hoc testing as appropriate. All tests in ANOVA were compared using Tukey’s multiple-comparison test that adjusts for the number of tests within an experimental series. Given the exploratory nature of the study, we did not apply an omnibus correction for multiple testing. Analyses were performed using GraphPad (La Jolla, CA) Prism software. P < 0.05 was considered significant unless otherwise indicated.
RESULTS
Influence of storage on ATP release from human RBCs
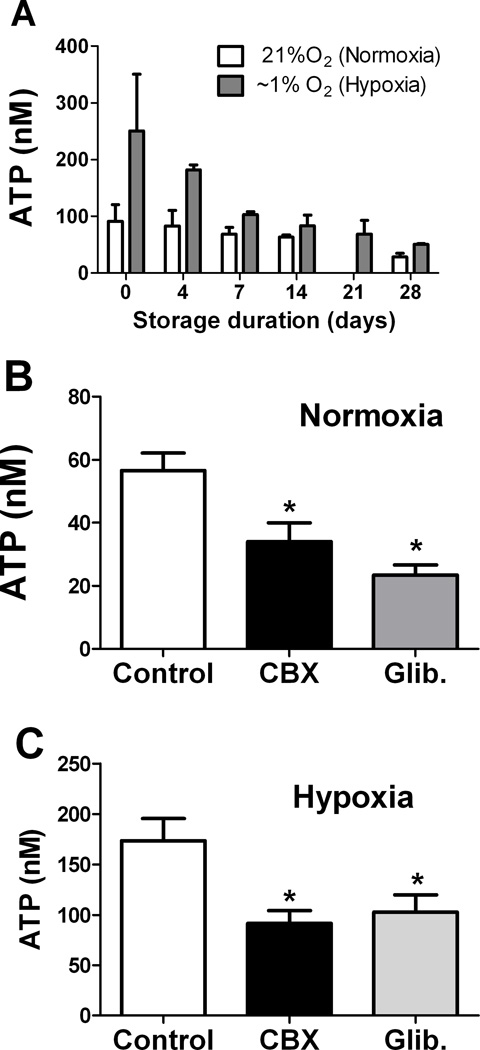
RBC ATP release (extracellular accumulation) was assayed in normoxia (21% O2) and hypoxia (~1% O2) at varying points in time after RBC collection (Fig. 1A). Basal release of ATP (i.e., in normoxia) was evident and could not be accounted for by RBC lysis, which does increase with storage (7). RBCs were washed before assaying ATP release, and RBC lysis (mean ± SEM: 1.44 ± 0.17% in normoxia and 1.27 ± 0.16% in hypoxia) contributed minimally to the extracellular ATP measured in our assays. As previously reported, exposure of RBCs to hypoxia increased ATP release. Under these storage conditions, hypoxia-induced release of ATP declined progressively, and by 7–14 days after collection was less than half that from fresh RBCs (Fig. 1A). Normoxic (basal) ATP release also declined progressively with storage time.
Figure 1. Basal and stimulated release of ATP from RBCs declines during conventional storage.
(A) RBCs sampled at varying times after collection and storage were added to chambers filled with Krebs buffer and bubbled with normoxic (21% O2) or hypoxic (~1% O2) gas. Extracellular ATP was measured by the luciferase assay in the supernatant of centrifuged samples. Normoxic ATP release was not assayed at 21 days. (B, C) Influence of glibenclamide (Glib.) or carbenoxolone (CBX) on ATP release from fresh RBCs in (B) normoxia or (C) hypoxia. * = p<0.05 compared to control; ANOVA with Tukey’s was used. n=3–6 for each timepoint and condition.
Inhibitors of ATP release
The decline in ATP release could not be explained simply by the established decline in RBC ATP content, which remains substantial even at the 42-day expiration date for conventional storage solutions(14, 18)_ENREF_17. Both GLIB, an inhibitor of CFTR and K+ ATP channels, and the pannexin 1 hemichannel-inhibitor CBX (10) inhibited ATP release from fresh RBCs during normoxia (Fig. 1B) and hypoxia (Fig. 1C), consistent with published reports (19, 20). Similar results were seen whether GLIB was dissolved in a DMSO or in a solvent containing dextrose and sodium hydroxide, and neither GLIB vehicle alone inhibited RBC release of ATP (not shown).
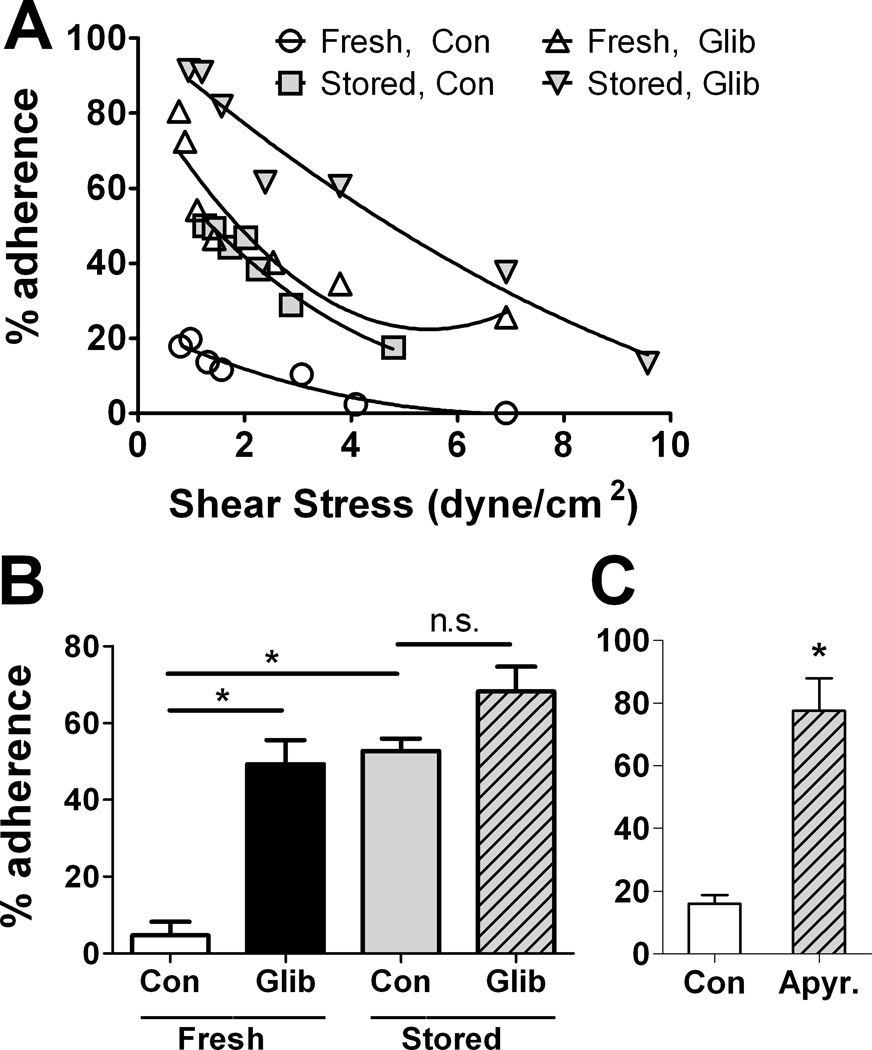
Adhesion of fresh and stored RBCs: influence of GLIB and apyrase
Fresh and stored human RBCs treated with vehicle or with the ATP-release inhibitor GLIB were tested for their ability to maintain adhesion to HUVECs established under static conditions when exposed to varying shear stresses. Adhesion of fresh RBCs to HUVECs was lower than that of stored RBCs (Fig. 2). At a shear stress of 2 dynes/cm2, the mean percentage of adherent stored RBCs was markedly higher relative to the mean percentage of adherent fresh RBCs, which was very low (Fig. 2B). Inhibition of ATP release by GLIB increased the adhesion of fresh RBCs (Fig. 2A and B). In stored RBCs, the pro-adhesive effect of GLIB was statistically significant but small in magnitude (Fig. 2B). In the presence of ATP-hydrolyzing extracellular apyrase, the adherence of fresh human RBCs to HUVECs was markedly increased at varying shear stresses (not shown), relative to exposure to vehicle alone (mean results at 2 dynes/cm2 are shown in Fig. 2C). Together, these findings indicate a role for exported ATP in preventing the endothelial adhesion of healthy RBCs.
Figure 2. Influence of storage and ATP release on the adhesion of RBCs to cultured endothelial cells.
HUVECs adherent to gelatin-coated glass slides were exposed to either stored (35–42 days) or fresh human RBCs treated or not with 10 µm glibenclamide. Adhesion was measured at varying shear stresses as described. (A) shows results from a typical experiment, and (B) shows the mean % adhesion (+ SEM) at a shear stress of 2 dyne/cm2; (n=3). (C) shows the influence of apyrase (5 U/ml) on adhesion of fresh human RBCs to HUVECs at 2 dyne/cm2 (mean ± SEM; n=3). * = p<0.05 the pairwise comparison. ANOVA with Tukey’s was used in (B) and paired t-test in (C).
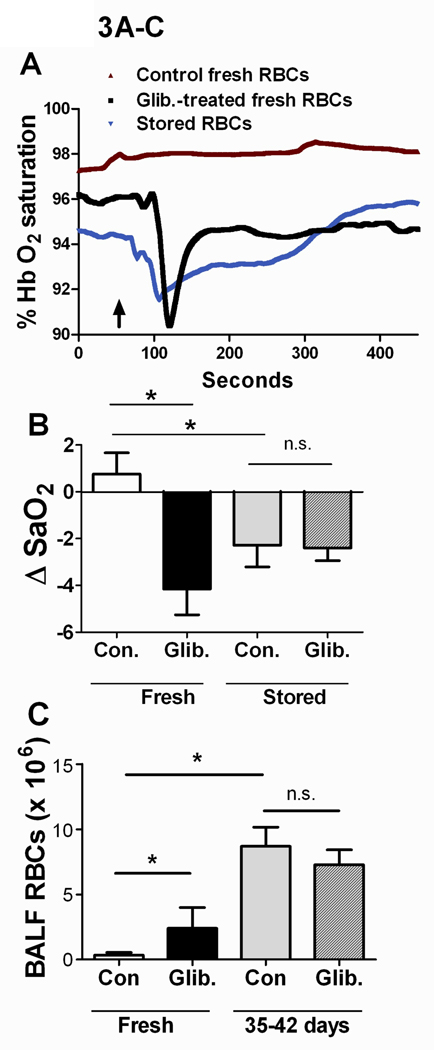
Influence of RBC storage and ATP-release on blood oxygenation and BAL cellularity in vivo
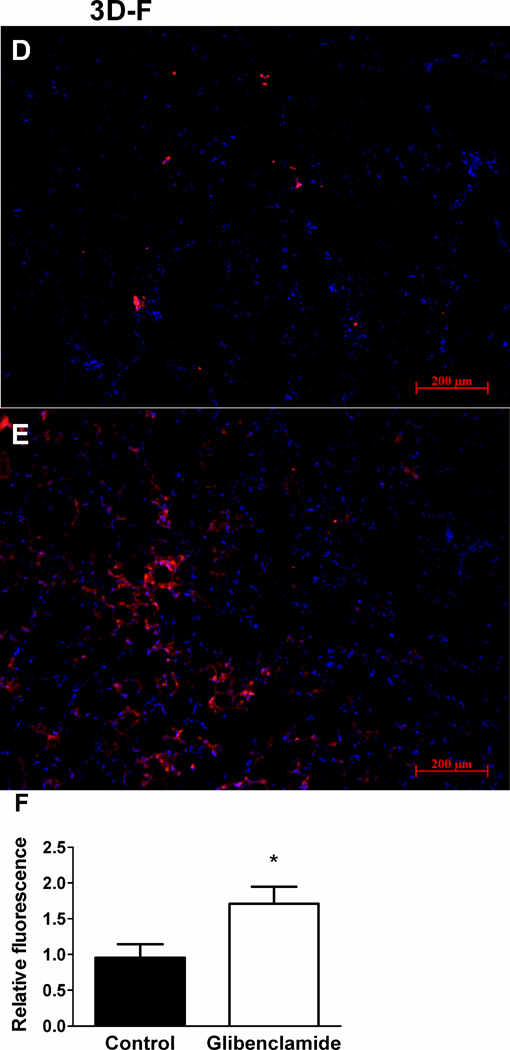
In a nude-mouse model of RBC transfusion, blood oxygenation (SaO2, Fig. 3A–B) fell in response to transfusion of untreated stored (35–42 days), but not fresh, human RBCs. The decreases in oxygenation – which are also seen with greater frequency in patients transfused with older or more RBC units in some studies (1–3) - typically took place beginning 40–60 seconds after transfusion, with recovery to or near baseline levels 4–6 minutes after transfusion (Fig. 3A). We tested whether the effect of RBC storage on blood oxygenation in mice could be due to deficient ATP release by transfusing GLIB-treated fresh RBCs (in which ATP release is depressed). Decreases in SaO2 were seen during transfusion of fresh human RBCs pre-treated with the ATP-release inhibitors GLIB (Fig. 3A–B) or CBX (data not shown). Infusion of either the second (final) wash from GLIB-treated RBCs or a dose of glibenclamide equivalent to that used for RBC exposures did not lead to decreases in SaO2 (not shown). The decreases in SaO2 seen in mice transfused with either GLIB-treated fresh RBCs or untreated 35–42-day stored RBCs were accompanied by extravasation of RBCs into the alveoli, as reflected in increased RBC counts in the BAL fluid (Fig. 3C). Transfusion of leukofiltered, washed, fresh RBCs led to decreases in oxygenation only if the RBCs were pretreated with GLIB (data not shown). Thus, our finding that transfusion of washed (leukofiltered) RBCs stored 35–42 days, but not fresh, washed, untreated RBCs, led to impaired oxygenation and lung RBC extravasation cannot be explained by an effect of leukoreduction. BALF accumulation of leukocytes in transfused mice did not differ as a function of RBC storage or ATP-release inhibition, and predominantly reflected (resident) macrophages in all cases (Supp. Fig. S1). When fresh-RBC transfusates were pre-exposed to the fluorescent dye Dil, sequestration of fluorescent RBCs within the lung was increased by prior exposure to GLIB, vs. vehicle (Fig. 3D–F). In a separate series of RBC-transfusion experiments, we harvested the spleen, lungs and kidney from mice. The results indicate that sequestration of transfused human RBCs in the mouse kidney was minimal, relative to that seen in the lung or spleen. In addition, no obvious differences were seen in either the spleen or the kidney in the sequestration of transfused RBCs as a function of RBC pre-exposure to GLIB (n=4 for GLIB vs. n=3 for vehicle), as was prominent in the lung (Fig. S2). Although the results do not suggest ATP-sensitive, extrapulmonary adhesion of transfused RBCs definitive conclusions cannot be made from these new data, given the limited number of experiments performed.
Figure 3. Influence of storage and ATP release in a mouse model of the pulmonary sequelae of RBC transfusion.
(A) Typical recordings of arterial Hb O2 saturation in mice transfused with either stored (35–42 days) human RBCs, untreated (Con) fresh human RBCs, or fresh human RBCs treated with 10 µm glibenclamide (Glib.). RBC transfusions (over 10 seconds) began where indicated by arrow. (B) Mean (+ SEM) peak changes in oxygenation in nude mice after transfusion of stored or fresh, human RBCs treated or not (Con) with Glib. n=6–13. (C) Mean (+ SEM) RBC counts in the bronchoalveolar lavage fluid (BALF) of nude mice after the indicated transfusions (n=5–10). (D,E) Typical fluorescence micrographs, and (F) mean (+ SEM, n=8) fluorescence intensity in the lungs of nude mice transfused with fresh, Dil-labeled RBCs pretreated (E) or not (D) with the ATP-release inhibitor GLIB. Lungs were counterstained with DAPI (blue). * =p<0.05; ANOVA with Tukey’s was used.
RBC ATP release and deformability
Conceivably, the in vitro or in vivo effects of glibenclamide (GLIB) or carbenoxolone (CBX) could be mediated by changes in RBC deformability, which is sensitive to intracellular ATP and is known to decline during storage (21). However, GLIB did not change the intracellular ATP concentration in stored or fresh RBCs (not shown and (12)). To test for a possible rheological action of GLIB or CBX, we measured RBC deformability in their presence and absence. Relative to exposure to vehicle alone (or no exposure, not shown), neither GLIB nor CBX altered the ability of RBCs to deform in response to a physiological range of shear stresses (Supp. Data Fig. S3). A similar lack of effect of GLIB treatment on RBC rheology was reported using a different assay (RBC filterability)(12).
Influence of extracellular ATP on adhesion of GLIB-treated fresh RBCs to endothelial cells
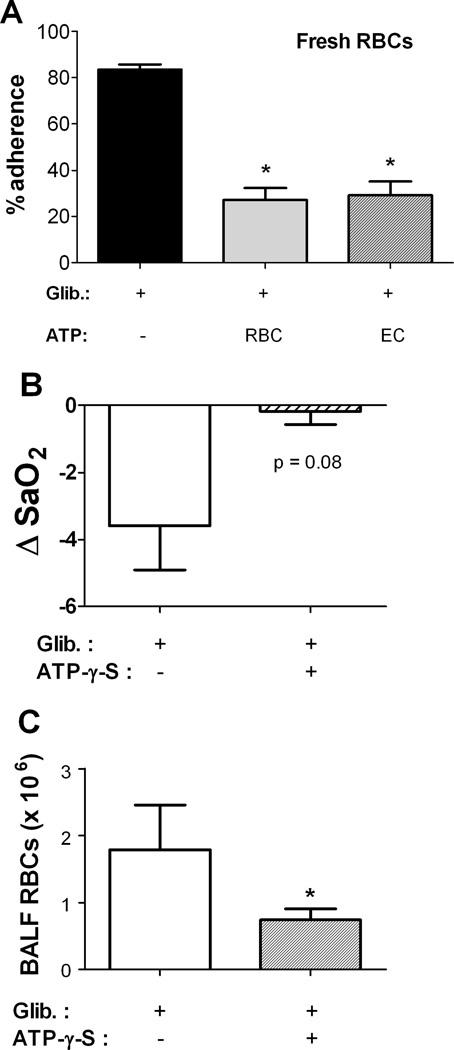
To confirm that the pro-adhesive effect of GLIB was in fact due to the suppressed release of ATP from RBCs (as opposed to another, nonspecific effect), we compared adhesion of GLIB-treated fresh human RBCs in the absence or presence of authentic ATP added to the RBC suspension or applied to the HUVECs just before adhesion assays. The adhesion to HUVECs of GLIB-treated RBCs treated was markedly reduced when the RBC suspension contained supplemental (extracellular) ATP (Fig. 4A), consistent with an effect of GLIB specific to its inhibition of ATP release, and suggesting that in control experiments the released ATP (or a metabolite) was active. The reversal of the GLIB effect by ATP was identical when HUVECs were exposed to ATP just before exposure to GLIB-treated RBCs, also consistent with an extracellular action of RBC-derived ATP to inhibit adhesion of RBCs to ECs.
Figure 4. (A) Extracellular ATP prevents glibenclamide (GLIB)-induced RBC adhesion to endothelial cells.
Adhesion of GLIB-treated fresh RBCs to HUVECs was measured in the absence or presence of ATP applied extracellularly (final conc. 1 µM). Fresh RBCs incubated with 10 µm GLIB were washed in PBS. Just before adhesion assays, ATP was applied either to the RBC suspension (RBCs + ATP) or to the HUVECs. Results are mean % adhesion (+ SEM) at a shear stress of 2 dyne/cm2 from 3 experiments. * = p<0.05 (differs significantly from adhesion to HUVECs of RBCs treated with GLIB alone). (B–C), Influence of co-infusion of an ATP analog on (B) changes in oxygenation and (C) BALF RBC counts in nude mice transfused with glibenclamide (GLIB)-treated fresh human RBCs. p=0.08 for Con vs. Glib in (A); * indicates p<0.05 for comparison (ANOVA with Tukey’s) with GLIB treatment alone. n=6.
Influence of extracellular ATP on responses to transfusion of GLIB-treated human RBCs in vivo in mice
Nude mice were transfused with GLIB-treated fresh human RBCs supplemented with the stable, non-hydrolyzable ATP analog ATP-γ-S at a calculated final concentration in plasma of 1 µM, approximating the increase in plasma ATP predicted by in vitro RBC-ATP experiments. (Infused ATP itself is vulnerable to hydrolysis by ectonucleotidases in vivo). With co-infusion of ATP-γ-S, blood oxygenation was preserved, in contrast with the effect of transfusing GLIB-treated fresh RBCs alone and consistent with the inhibition of ATP release accounting for the GLIB effect in vivo (Fig. 4B). By contrast, inclusion in the RBC suspension of the ATP precursor/metabolite adenosine did not attenuate the decrease in blood oxygenation seen in response to transfusion of GLIB-treated fresh human RBCs (not shown). Increases in BALF RBCs were also blunted when extracellular ATP-γ-S was infused together with the GLIB-treated RBCs (Fig. 4C). Taken together, these results suggest (but do not directly demonstrate) that ATP release from RBCs acts to limit the adhesion of transfused RBCs.
Effect of RBC storage on vascular resistance in RBC-perfused lungs
Perfusion of isolated mouse lungs allows direct measurement of changes in vascular resistance, and eliminates leukocyte-RBC interactions because the lungs are first perfused free of native WBCs and RBCs (17). In lungs perfused with human RBCs suspended in a physiological, isotonic Krebs buffer, increasing duration of RBC storage correlated directly with increases in both basal PAP, reflecting PVR (Fig. S4), and with the increases in mean PAP during hypoxia (HPV, not shown). The effect of RBC storage on PAP recapitulates that of pretreatment of fresh RBCs with GLIB (increased PVR and HPV) demonstrated previously in a similar model (22). These data are consistent with the hypothesis that the RBC-storage-dependent increases in PAP involve the progressive loss of the ability of stored RBCs to release ATP. _ENREF_26
Identity of surface receptor(s) mediating ATP-sensitive adhesion
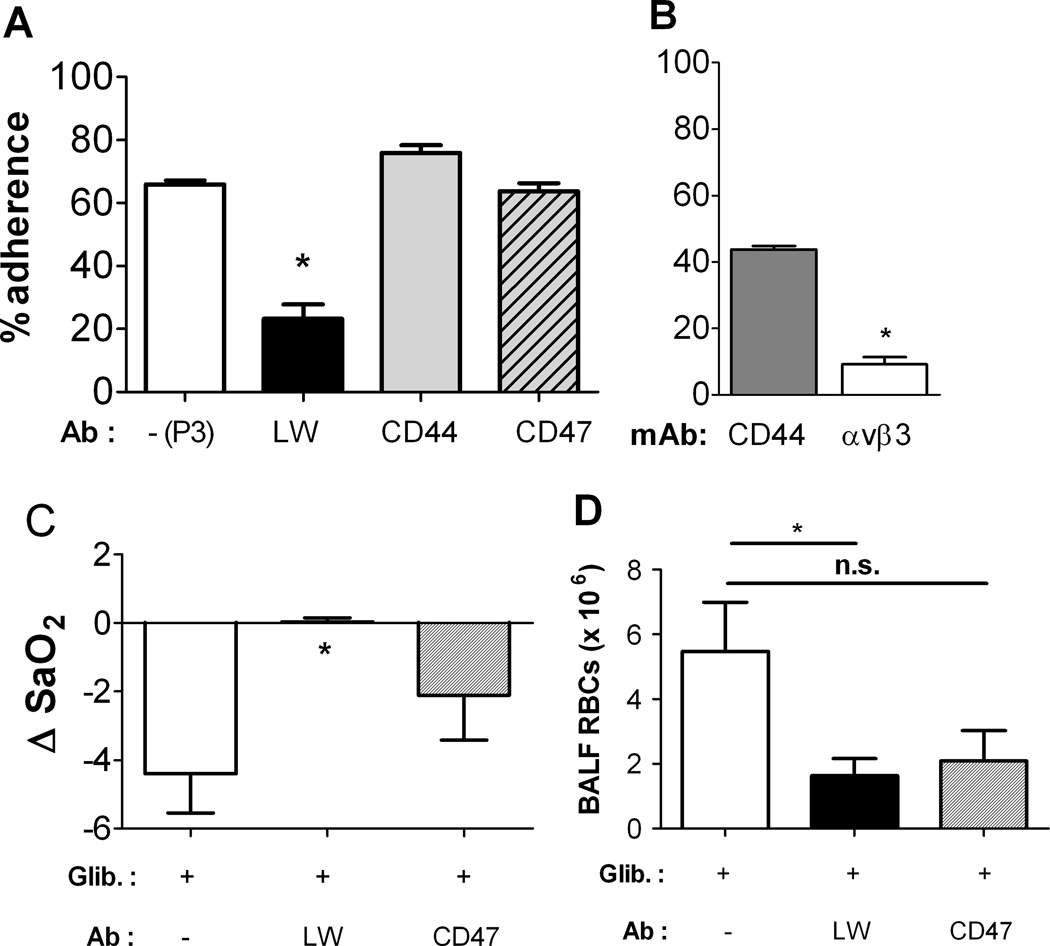
The influence of antibodies to candidate erythrocyte adhesion receptors was examined in fresh human RBCs treated with the ATP-release inhibitor GLIB (Fig. 5A). Incubation of RBCs with antibody (Ab) to the adhesion receptor LW (ICAM-4) largely prevented the GLIB-induced increases in endothelial adhesion, whereas RBC exposure to Abs to CD44 or CD47 (hyaluronan and thrombospondin receptors, respectively) or the nonreactive monoclonal murine IgG P3 had no effect relative to GLIB treatment alone (Fig. 5A). Exposure of HUVECs to an anti-αvβ3 mAb, but not to an anti-CD44 mAb, prevented endothelial adhesion of GLIB-treated fresh human RBCs (Fig. 5B).
Figure 5. (A) ATP-sensitive RBC adhesion to cultured endothelial cells is mediated by LW (ICAM-4).
Adhesion of glibenclamide (GLIB)-treated fresh RBCs to HUVECs was measured in the absence or presence of antibodies (Abs) to the RBC adhesion receptors LW, CD44, or CD47. RBCs were first incubated with one antibody (20–25 µg/ml) for 45 min followed by washing twice in PBS. *, differs significantly from adhesion of GLIB-treated RBCs to HUVECs in the absence of the mAbs (p<0.05). (B), Influence of exposure of HUVECs to a mAb vs. either CD44 or αvβ3 integrin on endothelial adhesion of GLIB-treated RBCs at a shear stress of 2 dyne/cm2; n=3. * p<0.05 by paired t-test. (C, D) Role of the RBC adhesion receptor LW in changes in oxygenation and BALF RBC counts. Nude mice were transfused with GLIB-treated, fresh human RBCs exposed or not to Abs against the adhesion receptors LW, CD44, or CD47; or with the nonreactive immunoglobulin P3. (B) Changes in SaO2 in nude mice after transfusion of GLIB-treated fresh RBCs. (C) RBC counts in the BALF of nude mice after the indicated transfusions. Mean + SEM; n = 3–7. * indicates p<0.05 for comparison vs. GLIB treatment alone. ANOVA with Tukey’s was used.
Influence of adhesion-receptor Abs on decreases in oxygenation and BALF RBCs in mice transfused with GLIB-exposed RBCs
We compared changes in oxygenation and BALF RBC accumulation in mice transfused with GLIB-treated fresh RBCs exposed to Abs against the adhesion receptors LW (ICAM-4) or CD47. Exposure to anti-LW Ab, but not to the anti-CD47 Ab, mitigated the decrease in oxygenation (Fig. 5C) and prevented the increase in BALF RBCs (Fig. 5D) seen in mice transfused with RBCs exposed to the ATP-release inhibitor.
Flow cytometric analysis of bronchoalveolar accumulation of cells after RBC transfusion
Species-specific fluorescent antibodies were used to enumerate human vs. mouse RBCs in the BALF of transfused mice. The percentages of human RBCs and native mouse RBCs were determined in mouse BALF by gating on the RBCs. After transfusion with fresh human RBCs treated with the ATP-release inhibitor GLIB, the excessive RBCs accumulated in BALF (compared with RBC counts in BALF after transfusion of vehicle-exposed RBCs) were predominantly murine (Supp. Fig. S5). Pre-transfusion incubation of RBCs with antibodies to the adhesion receptor LW (ICAM-4) largely prevented the GLIB-dependent increases in murine RBCs in BALF (Fig. S5). Transfused (human) RBCs were undetectable in BALF under control conditions, appeared only rarely after GLIB-treated RBC transfusions, and were absent after pre-transfusion of GLIB-treated RBCs exposed to the LW Ab (Fig. S5).
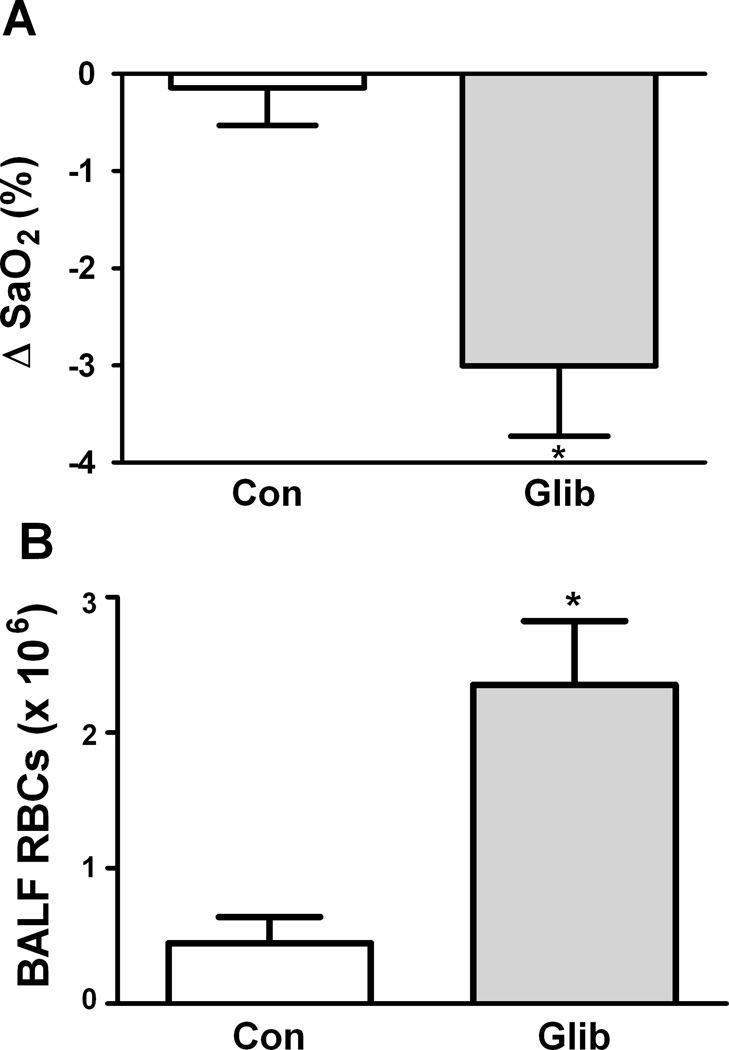
Role of ATP release in responses to transfusion of murine RBCs
We performed experiments to determine whether the effect of ATP-release inhibition on responses to transfusion of human RBCs was attributable in part to an effect related to the larger size of human RBCs relative to mouse lung microvessels. Nude mice were transfused with either GLIB-treated or control (vehicle-exposed) fresh RBCs from C57BL6 wild-type mice. Although generally smaller than those seen in response to human RBCs, decreases in blood oxygenation were also seen when murine RBCs were transfused (Fig. 6A). Greater BALF RBC counts were also seen after transfusion of GLIB-treated, vs. vehicle-treated murine RBCs (Fig. 6B). This further suggests that the effect on blood oxygenation is caused by impaired ATP release from RBCs, and indicates that under the conditions used, the larger size of human RBCs alone did not account for the changes in blood oxygenation we observed.
Figure 6. Influence of ATP-release and LW on the pulmonary sequelae of transfusion of mouse RBCs.
(A) Mean (+ SEM) changes in SaO2 in nude mice after transfusion of glibenclamide (Glib.)-treated or control (Con) fresh RBCs from C57BL6 mice. * = p<0.05 for Con. vs. Glib. (B) Mean (+ SEM) RBC counts in the BALF of nude mice after the indicated transfusions. * p<0.05 (paired t-tests). n = 5–7.
DISCUSSION
Changes in human RBCs during conventional storage are numerous and complex, and despite extensive investigation there is little established regarding causal links between any particular biochemical or functional change and the suboptimal clinical outcomes following RBC transfusion (1–4). Recent studies have focused on storage-induced loss of RBC-dependent vasoactivity (6, 7), whereby RBCs respond to falling O2 tension or mechanical stress by increasing blood flow so that O2 delivery rises appropriately (8, 19, 23–25).
The principal new findings from this study are that the inducible and basal release of ATP from human RBCs declines as a function of storage time, and that this ATP-related RBC “storage lesion” results in increased endothelial adhesion of RBCs in vitro and sequestration of RBCs in the lungs of transfused mice in vivo. The proadhesive loss of the RBC’s ability to release ATP is a newly identified mechanism by which transfusion of older, banked RBCs may contribute to declines in O2 transport relative to younger, fresh RBCs. Indeed, transfusion of older RBCs produced modest hypoxemia, likely reflecting interference with the normal uptake of O2 by RBCs – either native or transfused – sequestered in the lung. This RBC storage lesion – like the recognized losses of vasodilator SNOs (7) and O2-affinity-regulating 2,3-BPG – may therefore interfere with normal O2 delivery in the transfused patient. More generally, these data demonstrate a novel role for ATP released from the normal RBC as an antiadhesive agent.
Impaired ATP release as a novel RBC storage lesion
Progressive loss of ATP content during RBC storage, although reduced with the use of current additive solutions, is well documented and has been implicated in the progressive cytoskeletal and morphological changes in RBCs and the storage-induced declines in RBC deformability. The decline in RBC deformability could impair microvascular blood flow. However, while storage-induced decreases in ATP content are well established, our study is the first to directly examine whether the ability of RBCs to export ATP declines accordingly (or faster) during storage. The storage-induced progressive loss of the RBC’s ability to release ATP upon stimulation and the related increase in RBC adhesion to endothelium together represent a novel RBC “storage lesion” potentially amenable to correction.
Actions of ATP exported by the RBC
Ellsworth, Sprague, and others have demonstrated that RBCs release ATP in response to falling pO2 or mechanical deformation; the released ATP presumably functions to produce local vasodilation as needed to ensure adequate O2 delivery (8). This promotes unfettered passage of the RBC through microvessels, a process that frequently requires that the RBC deform. In the present study, we demonstrate that released ATP also normally acts to prevent or limit the adhesion of RBCs to the endothelium. Additionally, in mice transfused with RBCs treated with either of 2 chemically and mechanistically distinct ATP-release inhibitors, - sequestration of RBCs within the lung was seen. ATP-sensitive adhesion of RBCs to endothelial cells in vitro and sequestration of RBCs in vivo were largely prevented by supplemental, extracellular ATP, confirming that the pro-adhesive actions of GLIB resulted from the inhibition of ATP release, rather than from another, nonspecific effect. Loss of the ability of stored RBCs to export ATP may therefore promote RBC trapping in the microcirculation of the lung or other organs, impeding blood flow. These new findings point to a previously unrecognized cellular pathway potentially involved in the initiation or progression of tissue injury when RBCs are transfused after longer storage times.
Proadhesive effects of RBC storage
Storage-induced increases in adherence of normal human RBCs to endothelial cells in vitro have been reported, and vary with leukoreduction, endothelial cell source and activation status, and other factors (26–28). Our finding of increased adhesion of stored RBCs (relative to fresh) is consistent with some reports (27, 28), and this study is the first describing a link between deficient ATP release and RBC adhesion. _ENREF_7_ENREF_7 We demonstrated that an Ab to the erythrocytic adhesion receptor LW/ICAM-4 selectively blocked the induced increases in RBC adhesion in vitro and decreases in blood oxygenation in vivo secondary to ATP-release inhibition, consistent with a role for LW in the ATP-sensitive, increased adhesion of RBCs. We showed that addition of ATP to HUVECs prevented GLIB-induced RBC adhesion, and that ATP-sensitive RBC adhesion to HUVECs appears to involve the endothelial αvβ3 integrin, a known counterreceptor for LW/ICAM-4 previously shown to mediate adhesion to endothelium in vitro and in vivo (15, 29). In the previous work, LW-dependent adhesion could be upregulated by either activation of the RBC LW receptor or activation of the target endothelial cells. Precisely how ATP exported by the RBC limits LW-dependent adhesion to endothelial cells is unknown, and is the subject of ongoing investigation.
RBC export of ATP and extravasation
Loss of the ability of RBCs to release ATP – either as a consequence of conventional storage or via pharmacological inhibition – was associated with lung sequestration of RBCs and extravasation of RBCs into the airspaces. The depressed oxygenation and increase in BALF RBC count after transfusion of stored or GLIB-treated human RBCs in nude mice appeared to depend little or none on the species difference, because a) gas exchange changes and BALF RBC counts were minimal in mice transfused with fresh, untreated human RBCs, and b) nude mice transfused with GLIB-treated murine RBCs also exhibited the abnormal pulmonary phenotype. Thus, inter-species incompatibility did not compromise the ability of our mouse model to allow the study of how adverse and potentially significant changes during the banking of human RBCs may promote adverse and disappointing clinical outcomes in transfused human patients.
We observed that when human RBCs with impaired capacity to release ATP were transfused into nude mice, the extravasating RBCs were overwhelmingly murine (native) in origin (≥1000:1 ratio of mouse:human RBCs in BAL fluid). The preferential extravasation of native RBCs may partly reflect the difference in RBC size (human > mouse), and is poorly explained by the post-transfusion circulating mouse RBC:human RBC ratio, which was approximately 20 (data not shown). In nude mice transfused with human RBCs, therefore, the adherent RBCs are generally not the ones that extravasate. Pulmonary edema is recognized clinically when red color is noted in respiratory secretions. The underlying increases in the permeability of the pulmonary capillary endothelium permit the entry of not only water and plasma, but also circulating red and white blood cells, but the specific mechanisms governing RBC extravasation are poorly defined. ATP is a known regulator of endothelial permeability, and it is possible that ATP released from RBCs modulates extravasation and injurious accumulation of RBCs in the alveoli. Neutrophils (PMNs) can also release ATP which can, in turn, modulate PMN-endothelial adhesion (30, 31); but whether ATP exported from the RBC may modulate PMN adhesion (and vice versa) is unknown. Further studies are needed to determine the precise nature of and possible links between ATP-sensitive increases in RBC adhesion, endothelial permeability in the lung, and RBC extravasation into the alveolar airspaces.
Organic phosphate repletion in stored RBCs
There has recently been renewed scientific and clinical interest in exploiting established techniques for the augmentation of organic phosphates within RBCs as a means to improve the O2-delivery function of older stored RBCs (32). Whereas the FDA-approved solution Rejuvesol was originally developed principally to restore 2,3-BPG in order to normalize O2 affinity in the stored RBC, exposure of older RBCs to this solution also raises intra-RBC levels of ATP. Thus this approach could also improve the flow of stored RBCs, because RBC ATP is considered critical to RBC properties such as deformability that influence RBC flow through arterioles, capillaries, and venules. Given our novel findings that ATP release from stored RBCs is markedly reduced and that RBC ATP appears to prevent excessive RBC adhesion to endothelial cells, an additional potential application of Rejuvesol would be to limit the pro-adhesive properties of RBCs transfused after weeks of storage. Specifically, whereas declines in overall intra-RBC ATP are modest even after 42 days of storage, it is unknown whether declines in ATP occur more rapidly in particular compartments, such as the RBC membrane (33, 34). Given the complexity of the total “storage lesion”, ATP repletion alone may be insufficient to improve outcomes or even ATP release, the decline of which may involve other changes such as the progressive increases in O2-affinity of RBCs during storage (35), which would be expected to limit ATP release (36). Future studies are needed to test such treatment.
Clinical implications of RBC adhesion after transfusion
Increased adhesion of RBCs might contribute to adverse pathophysiology in settings where RBC transfusion has proven disappointing, such as pediatric and adult critical illness, acute coronary syndromes, and cardiac surgery. In addition to promoting regional decreases in blood flow or even vaso-occlusion, RBC adherence to endothelium may initiate or propagate inflammatory events through local release of reactive oxygen species (37)_ENREF_14_ENREF_32_ENREF_32, by decreasing local availability of bioactive NO species, or through other mechanisms. The antiadhesive actions of RBC-derived ATP may assume greater importance under conditions of endothelial injury. In critically ill patients, therefore, RBC transfusion may represent a “second hit” following a priming event. Further work is needed to determine whether interference with the normal release from RBCs of antiadhesive ATP might contribute to the unexpectedly increased morbidity and mortality in transfused patients; additional work in relevant animal models is also needed, including testing relevant therapeutics.
Conclusions
The normal flow of RBCs in the pulmonary circulation depends in part on the release of anti-adhesive ATP from RBCs. Storage-induced deficiency in ATP release from transfused RBCs may promote microvascular pathophysiology in the lung, in part through increased RBC adhesion mediated by the LW/ICAM-4 receptor on RBCs and the endothelial αvβ3 integrin. By this mechanism, excessive adhesion of RBCs transfused after storage may contribute to the increased incidence of respiratory failure and other adverse outcomes after transfusion in the critically ill patient.
Supplementary Material
Acknowledgements
The work was supported by grants from the NIH (HL-079915 to MJT) and Veterans Affairs (1I01BX000281-01A1 to TJM). We appreciate the technical contributions of Mays Ali, Melissa Mulherin, and Dr. Lin Shan. For helpful discussions, we thank Dr. Eduardo Lazarowski of the University of North Carolina (Chapel Hill) and Drs. Nicholas Bandarenko and Joseph Bonaventura (Duke). We thank Dr. Maragatha Kuchibhatla for statistical review of the manuscript. Drs. Paul Noble and Elliott Bennett-Guerrero (Duke) kindly provided access to a flow cytometer and ektacytometer, respectively. We thank Roberta Arney, Mary Lee Campbell, Martha Rae Combs, and Dr. Bandarenko of Duke Hospital Transfusion Services for providing “segments” from banked RBC units and some of the fresh blood samples we used.
This study was funded, in part, by the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 2.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 3.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 4.Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 5.Tinmouth A, Fergusson D, Yee IC, et al. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JD, Ahearn GS, Angelo M, et al. S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proc Nat Acad Sci USA. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Nat Acad Sci USA. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellsworth ML, Forrester T, Ellis CG, et al. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 9.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locovei S. Pannexin 1 in erythrocytes: Function without a gap. Proc Nat Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 12.Sprague RS, Ellsworth ML, Stephenson AH, et al. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 13.Sridharan M, Adderley SP, Bowles EA, et al. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–H1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess JR, Rugg N, Knapp AD, et al. The role of electrolytes and pH in RBC ASs. Transfusion. 2001;41:1045–1051. doi: 10.1046/j.1537-2995.2001.41081045.x. [DOI] [PubMed] [Google Scholar]
- 15.Zennadi R, Chien A, Xu K, et al. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112:3474–3483. doi: 10.1182/blood-2008-01-134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zennadi R, Moeller BJ, Whalen EJ, et al. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood. 2007;110:2708–2717. doi: 10.1182/blood-2006-11-056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doctor A, Platt R, Sheram ML, et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Nat Acad Sci USA. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogman CF, Lof H, Meryman HT. Storage of red blood cells with improved maintenance of 2,3-bisphosphoglycerate. Transfusion. 2006;46:1543–1552. doi: 10.1111/j.1537-2995.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 19.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand. 2000;168:551–559. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 20.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe LC. The membrane and the lesions of storage in preserved red cells. Transfusion. 1985;25:185–203. doi: 10.1046/j.1537-2995.1985.25385219897.x. [DOI] [PubMed] [Google Scholar]
- 22.Sprague RS, Ellsworth ML, Stephenson AH, et al. ATP: the red blood cell link to NO and local control of the pulmonary circulation. AmJPhysiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 23.Crawford JH, Isbell TS, Huang Z, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia L, Bonaventura C, Bonaventura J, et al. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 25.McMahon TJ, Moon RE, Luchsinger BP, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 26.Bratosin D, Leszczynski S, Sartiaux C, et al. Improved storage of erythrocytes by prior leukodepletion: flow cytometric evaluation of stored erythrocytes. Cytometry. 2001;46:351–356. doi: 10.1002/cyto.10005. [DOI] [PubMed] [Google Scholar]
- 27.Luk CS, Gray-Statchuk LA, Cepinkas G, et al. WBC reduction reduces storage-associated RBC adhesion to human vascular endothelial cells under conditions of continuous flow in vitro. Transfusion. 2003;43:151–156. doi: 10.1046/j.1537-2995.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 28.Anniss AM, Sparrow RL. Variable adhesion of different red blood cell products to activated vascular endothelium under flow conditions. Am J Hematol. 2007;82:439–445. doi: 10.1002/ajh.20837. [DOI] [PubMed] [Google Scholar]
- 29.Kaul DK, Liu XD, Zhang X, et al. Peptides based on alphaV-binding domains of erythrocyte ICAM-4 inhibit sickle red cell-endothelial interactions and vaso-occlusion in the microcirculation. Am J Physiol Cell Physiol. 2006;291:C922–C930. doi: 10.1152/ajpcell.00639.2005. [DOI] [PubMed] [Google Scholar]
- 30.Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 31.Dawicki DD, McGowan-Jordan J, Bullard S, et al. Extracellular nucleotides stimulate leukocyte adherence to cultured pulmonary artery endothelial cells. Am J Physiol. 1995;268:L666–L673. doi: 10.1152/ajplung.1995.268.4.L666. [DOI] [PubMed] [Google Scholar]
- 32.Raat NJ, Hilarius PM, Johannes T, et al. Rejuvenation of stored human red blood cells reverses the renal microvascular oxygenation deficit in an isovolemic transfusion model in rats. Transfusion. 2009;49:427–434. doi: 10.1111/j.1537-2995.2008.02002.x. [DOI] [PubMed] [Google Scholar]
- 33.Proverbio F, Hoffman JF. Membrane compartmentalized ATP and its preferential use by the Na,K-ATPase of human red cell ghosts. J Gen Physiol. 1977;69:605–632. doi: 10.1085/jgp.69.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman JF, Dodson A, Proverbio F. On the functional use of the membrane compartmentalized pool of ATP by the Na+ and Ca++ pumps in human red blood cell ghosts. J Gen Physiol. 2009;134:351–361. doi: 10.1085/jgp.200910270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogman CF, Meryman HT. Storage parameters affecting red blood cell survival and function after transfusion. Transfus Med Rev. 1999;13:275–296. doi: 10.1016/s0887-7963(99)80058-3. [DOI] [PubMed] [Google Scholar]
- 36.Jagger JE, Bateman RM, Ellsworth ML, et al. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- 37.Kiefmann R, Rifkind JM, Nagababu E, et al. Red blood cells induce hypoxic lung inflammation. Blood. 2008;111:5205–5214. doi: 10.1182/blood-2007-09-113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.