Abstract
BACKGROUND
Polycystic ovary syndrome (PCOS) patients typically have 17-hydroxyprogesterone (17OHP) hyperresponsiveness to GnRH agonist (GnRHa) (PCOS-T). The objective of this study was to determine the source of androgen excess in the one-third of PCOS patients who atypically lack this type of ovarian dysfunction (PCOS-A).
METHODS
Aged-matched PCOS-T (n= 40), PCOS-A (n= 20) and controls (n= 39) were studied prospectively in a General Clinical Research Center. Short (4 h) and long (4–7 day) dexamethasone androgen-suppression tests (SDAST and LDAST, respectively) were compared in subsets of subjects. Responses to SDAST and low-dose adrenocorticotropic hormone (ACTH) were then evaluated in all.
RESULTS
Testosterone post-SDAST correlated significantly with testosterone post-LDAST and 17OHP post-GnRHa (r = 0.671–0.672), indicating that all detect related aspects of ovarian dysfunction. An elevated dehydroepiandrosterone peak in response to ACTH, which defined functional adrenal hyperandrogenism, was similarly prevalent in PCOS-T (27.5%) and PCOS-A (30%) and correlated significantly with baseline dehydroepiandrosterone sulfate (DHEAS) (r = 0.708). Functional ovarian hyperandrogenism was detected by subnormal testosterone suppression by SDAST in most (92.5%) PCOS-T, but significantly fewer PCOS-A (60%, P< 0.01). Glucose intolerance was absent in PCOS-A, but present in 30% of PCOS-T (P < 0.001). Most of the PCOS-A cases with normal testosterone suppression in response to SDAST (5/8) lacked evidence of adrenal hyperandrogenism and were obese.
CONCLUSIONS
Functional ovarian hyperandrogenism was not demonstrable by SDAST in 40% of PCOS-A. Most of these cases had no evidence of adrenal hyperandrogenism. Obesity may account for most hyperandrogenemic anovulation that lacks a glandular source of excess androgen, and the SDAST seems useful in making this distinction.
Keywords: glucose intolerance, functional adrenal hyperandrogenism, functional ovarian hyperandrogenism, obesity
Introduction
Polycystic ovary syndrome (PCOS) affects over 5% of reproductive age women, yet the cause of the ovarian dysfunction is unknown (Azziz et al., 2009). Most cases meeting National Institutes of Health (NIH) criteria for the diagnosis (Zawadzki and Dunaif, 1992), (69%) have a typical type of functional ovarian hyperandrogenism in which there is 17-hydroxyprogesterone (17OHP) hyper-responsiveness to LH, as indicated by GnRH agonist (GnRHa) or hCG testing (Ehrmann et al., 1995; Rosenfield, 1999; Hirshfeld-Cytron et al., 2009; Mortensen et al., 2009). These women with functionally typical PCOS (PCOS-T) also demonstrate a subnormal reduction of plasma free testosterone concentration after adrenocortical suppression by a long (4-day) dexamethasone androgen-suppression test (LDAST) (Ehrmann et al., 1992; Rosenfield et al., 2003). These abnormalities seem to be due to an intrinsic abnormality in ovarian theca cell function (Nelson et al., 1999).
In contrast, approximately one-third (31%) of PCOS cases meeting NIH diagnostic criteria have functionally atypical PCOS (PCOS-A) in that 17OHP hyper-responsiveness to LH is lacking (Ehrmann et al., 1992; Mortensen et al., 2009). Detailed characterization of a small sample of this group of women showed that they had significantly subnormal testosterone suppression in response to overnight adrenal suppression by dexamethasone, a lesser degree of ovarian enlargement and a greater degree of adiposity than those who hyperrespond to gonadotrophin stimulation (Hirshfeld-Cytron et al., 2009). It is unclear whether their apparent atypical ovarian dysfunction is intrinsic to the ovary or, rather, secondary to another source of androgen excess, such as functional adrenal hyperandrogenism. Here we report the results of studies to determine the source of androgen excess in functionally atypical PCOS by using a short (4-h) dexamethasone androgen-suppression test (SDAST) and a low-dose ACTH test.
Materials and Methods
Study subjects
Healthy non-hirsute eumenorrheic volunteers who were one-year or more post-menarcheal and in Day 4–10 of their menstrual cycle and PCOS patients who were amenorrheic for >2 months were phenotyped in 2000–2007 as previously reported (Mortensen et al., 2009) and briefly described below. Subjects found to be in the pre-ovulatory or luteal phase of a menstrual cycle were excluded from analysis. Reference ranges were derived from the population of 21 volunteers with ultrasonographically normal ovaries. PCOS was diagnosed by NIH criteria based on an elevated preadmission plasma free testosterone and otherwise unexplained oligo-anovulation (Zawadzki and Dunaif, 1992).
For this report, subjects were classified by post hoc analysis according to menstrual history and biochemical markers of ovarian function. Controls for this analysis were 39 eumenorrheic volunteers with normal ovarian function tests: they were comprised of those who lacked a polycystic ovary (the above reference population) pooled with those whose polycystic ovary was a normal variant judging from baseline plasma free testosterone levels and 17OHP responses to GnRHa in the reference range (Mortensen et al., 2009). Among 88 PCOS patients who had been phenotyped concurrently and were by definition hyperandrogenemic, we age-matched to controls those with elevated versus normal 17OHP responses to a GnRHa test in a 2-to-1 ratio: this yielded a PCOS study cohort of 40 subjects with PCOS-T and 20 with PCOS-A. PCOS-T was defined by a 17OHP > 2SD above the reference population mean peak level 20–24 h post-GnRHa, i.e. >132 ng/dl (Mortensen et al., 2009). Functional adrenal hyperandrogenism in PCOS was defined analogously by a peak dehydroepiandrosterone > 2SD above the mean peak of the reference population (i.e. >1136 ng/dl) post-ACTH.
These studies were approved by the University of Chicago Institutional Review Board and were performed after obtaining appropriate informed consent and assent in the case of minors.
Study protocol
Phenotyping was performed in the University of Chicago General Clinical Research Center according to an inpatient protocol previously published in detail (Mortensen et al., 2009). Baseline early morning plasma sex steroids and steroid intermediates, mean gonadotrophin and insulin levels during an oral glucose tolerance test, pelvic ultrasonography and gonadotrophin and steroid responses to a GnRHa test (leuprolide acetate 10 µg/kg, maximum 1.0 mg) were previously reported in the controls and the larger group from which the PCOS cohorts were derived (Mortensen et al., 2009).
This report focuses on the following previously unanalyzed aspects of the protocol. The inpatient protocol began with early morning baseline sampling, after which an afternoon SDAST was performed by administering dexamethasone 0.25 mg/m2 orally at 1200 h and obtaining a ‘basal’ sample for steroids 4-h later (1600 h). Immediately after the basal sampling, ACTH1-24 (1.0 µg/1.7 m2 IV) was administered, and blood was sampled for steroids 15 and 30 m later (Dickstein et al., 1997), prior to GnRHa testing.
LDAST was performed as an outpatient study: dexamethasone 0.5 mg was administered every 6 h for 4 days, or for 7 days in women > 100 kg, with sampling shortly after an early morning dose on the subsequent day. This test was performed as a preadmission procedure in 54 PCOS subjects > 2 months prior to their inpatient study and in a convenience subset of 14 consenting normoandrogenic volunteers (93% adults) during the follicular phase of the cycle following admission.
Laboratory methods
All serum was promptly frozen and stored at −20°C for up to one month for steroid and peptide assays in the University of Chicago Hospital Laboratories; assays in each individual were performed in a batch. Steroid assays were performed by immunoassays and steroid-binding assays as previously reported (Mortensen et al., 2009) with 11–12% precision. Methodologic details and comparisons of total and free testosterone, androstenedione and 17OHP assays to commercially available liquid chromatography-tandem mass spectrometry assays are provided in the Supplementary data. Gonadotrophins were measured by immunofluorometric assays (Delphia®, Wallach, Finland). Insulin resistance was indexed by homeostatic model assessment, and composite insulin sensitivity index was computed from glucose tolerance test data as previously reported (Mortensen et al., 2009).
Data analysis
The primary analysis involved using receiver-operating characteristic (ROC) curve methods to compare the control group to PCOS subjects with and without elevated 17OHP post-GnRHa, i.e. PCOS-T and PCOS-A, respectively. The Wilcoxon rank-sum test was used to determine whether the area under the ROC curve was significantly different from 0.5 (indicating a marker with accuracy no better than expected by chance), and the areas under ROC curves were compared by a non-parametric method (DeLong et al., 1988). Cut-points with ≥95% specificity for distinguishing PCOS from controls were derived from ROC curve analysis and were applied to subsequent analyses.
Univariate linear regression analysis was performed to examine associations between various factors. Data distributions were normalized using logarithmic transformation, if necessary, before analysis by paired or unpaired Student t-tests or one-way analysis of variance using Tukey's post hoc comparisons. Data analyses were performed using Excel (Microsoft Corp.), Prism (GraphPad Software, Inc.) and Stata Version 11 (StataCorp., College Station, TX, USA) programs. Results are expressed as mean ± SEM except as otherwise specified; two-tailed P< 0.05 was considered statistically significant.
Results
Baseline characteristics of PCOS-T and PCOS-A
PCOS-A had significantly milder hyperandrogenemia, lower sex hormone-binding globulin (SHBG) and higher BMI than PCOS-T (Table I). PCOS-A baseline LH (5.7 ± 0.7 U/l) was intermediate between that of PCOS-T and controls (7.0 ± 0.8 versus 3.5 ± 0.3 U/l), which differed significantly (P< 0.01). No baseline steroid level provided clinically useful discrimination between these two types of PCOS. Abnormal glucose tolerance was strikingly more prevalent in PCOS-T (30%, three-quarters of which was impaired glucose tolerance, one-quarter type 2 diabetes mellitus) than in the other groups (P < 0.001), and glucose intolerance was absent in PCOS-A. Exclusive of glucose-intolerant subjects, insulin resistance according to the homeostatic model assessment and insulin sensitivity indices was greater in both PCOS groups than in controls (P< 0.05), but similar in both PCOS groups in spite of the higher BMI of PCOS-A.
Table I.
Baseline (8AM) characteristics of study groups (mean ± SEM).
| Group | Age (year) | BMI (kg/m2) | Total testosteronea (ng/dl) | Free testosteronea (pg/ml) | Androstene-dionea (ng/dl) | 17OHPa (ng/dl) | DHEASa (µg/dl) | SHBG (nM) |
|---|---|---|---|---|---|---|---|---|
| Controls (n = 39) | 23.9 ± 1.4 | 26.4 ± 1.1††† | 25.9 ± 1.6††† | 5.6 ± 0.3††† | 85.1 ± 4.7††† | 39 ± 3.7††† | 84 ± 6.7†† | 27.9 ± 1.8††† |
| Reference range | 13.3–34.4 | 18.5–37.9 | 15–53 | 3.0–9.0 | 51–165 | 22–89 | 30–180 | 11–47 |
| PCOS-T (n = 40) | 20.7 ± 1.1 | 34.6 ± 1.4 | 72.6 ± 4.1 | 22 ± 1.7 | 194 ± 12 | 67 ± 3.9 | 132 ± 13 | 14.1 ± 1.4 |
| PCOS-A (n = 20) | 22.1 ± 1.6 | 42.2 ± 2.9††*** | 50.1 ± 4.1†††*** | 16 ± 1.5††*** | 122 ± 11†††** | 35 ± 3.3††† | 134 ± 18* | 12.3 ± 2.5*** |
SHBG, sex hormone-binding globulin testosterone-binding capacity.
aConversion multipliers to SI units: Total testosterone 0.0347 (nmol/l), free testosterone 3.47 (pmol/l), androstenedione 0.0349 (nmol/l), 17OHP 0.0303 (nmol/l), DHEAS 0.0271 (µmol/l).
††P values versus PCOS-T: <0.01.
†††P values versus PCOS-T: <0.001.
*P values versus controls: <0.05.
**P values versus controls: <0.01.
***P values versus controls: <0.001.
Validation of SDAST: comparison with LDAST
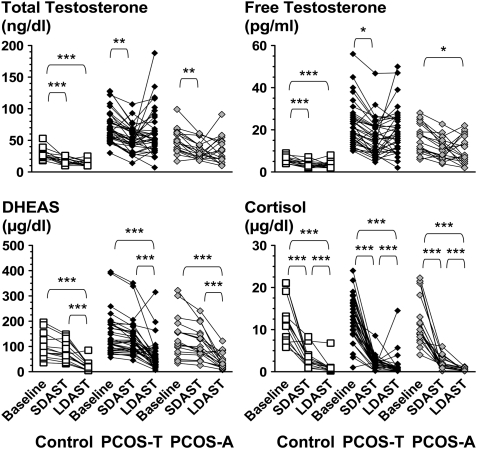
SDAST and LDAST were compared in subsets of controls and PCOS subjects (Fig. 1). SDAST and LDAST suppressed testosterone similarly, by respective averages of 40–46% for volunteers and 28–11% for PCOS and testosterone levels in response to SDAST and LDAST correlated well (r = 0.672, P< 0.0001). Bland–Altman analysis showed no significant bias in the mean difference between the two tests (e.g. free testosterone bias 0.01 ± 6.7, SD, pg/ml). The mean ± SD for free testosterone in controls was 3.45 ± 1.6 and 3.48 ± 1.6 pg/ml for SDAST and LDAST, respectively; these LDAST results were consistent with the historically established normal range (<8 pg/ml) (Ehrmann et al., 1992).
Figure 1.
Testosterone (top left panel), free testosterone (top right panel), DHEAS (bottom left panel) and cortisol (bottom right panel) at baseline and in response to SDAST and LDAST. Testosterone was suppressed promptly and maximally by SDAST in controls and PCOS, with no further response to LDAST. DHEAS did not fall significantly during SDAST, but was suppressed by LDAST in all groups. Cortisol fell in response to SDAST and further in response to LDAST in all groups. *P< 0.05, **P< 0.01 and ***P< 0.001 respectively. To convert cortisol to µmol/l, multiply by 0.0276.
However, the short test did not suppress the adrenal steroids dehydroepiandrosterone sulfate (DHEAS) and cortisol as much as the longer test (Fig. 1). The DHEAS fall only achieved significance in response to the long test, at which point it fell by 77 ± 5.2% from baseline in controls and 58 ± 4.5% in PCOS; there was no difference in DHEAS levels in response to the 4- or 7-day LDAST. Cortisol concentrations fell significantly in response to SDAST and fell further in response to LDAST.
Testosterone levels after SDAST and LDAST were more disparate than expected from assay precision; for example, the results of these tests were >50% different from their average in about one-third of subjects. This disparity seems to mainly indicate biologic variability in ovarian function. To a small extent (5.6% of subjects), such disparities appear to arise from lack of compliance with the longer test regimen, judging from failure of both DHEAS and cortisol to fall more on LDAST than SDAST.
Detection of functional ovarian hyperandrogenism by SDAST
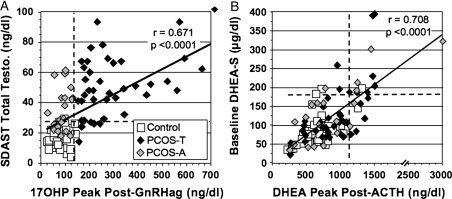
Testosterone in response to SDAST (Fig. 2A) correlated well with 17OHP post-GnRHa. This indicates that both tests detect related aspects of functional ovarian hyperandrogenism.
Figure 2.
Relationships between outcomes of benchmark and screening tests for ovarian and adrenal androgenic function. (A) Relationship between outcomes of tests of ovarian androgenic function (GnRHa test and SDAST). (B) Relationship between outcomes of tests of adrenal androgenic function (peak DHEA) response to low-dose ACTH and baseline DHEAS level. The dotted lines delineate the upper limit of normal (2SD above mean of the reference population) for each test; the upper limit of normal for DHEAS is based on back-transformation of logarithmically transformed data.
The reference range for total testosterone in response to SDAST was 15.4 ± 5.9 (SD) ng/dl (95th percentile = 27 ng/dl). A post-SDAST testosterone level > 27 ng/dl was positive in 92.5% of PCOS-T subjects; thus, SDAST outcome was highly concordant with GnRHa-test outcome in identifying the functional ovarian hyperandrogenism of PCOS-T (Table II). Consistent with this, ROC curve analysis indicated that a post-SDAST total testosterone level > 27 ng/dl had 95% specificity and 90% sensitivity for identifying PCOS-T (P< 0.001).
Table II.
Relationship of SDAST and ACTH test outcomes in PCOS-T and PCOS-A.
| PCOS-Ta | ACTH test | ACTH test |
| Positiveb | Negative | |
| SDAST positivea | 10 (25%) | 27 (67.5%) |
| SDAST negative | 1 (2.5%) | 2 (5%) |
| PCOS-A | ACTH test | ACTH test |
| Positiveb | Negative | |
| SDAST positivea | 3 (15%) | 9 (45%) |
| SDAST negative | 3 (15%) | 5 (25%) |
Values are expressed as number (% of total).
aFunctional ovarian hyperandrogenism in PCOS is indicated by either the presence of PCOS-T (17OHP hyper-response to GnRHa test) or a positive SDAST.
bFunctional adrenal hyperandrogenism in PCOS is indicated by a positive ACTH test.
ROC curve analysis indicated that this post-SDAST total testosterone cut-off level (>27 ng/dl) had 95% specificity and 60% sensitivity for identifying PCOS-A (P< 0.001). Thus, SDAST detected functional ovarian hyperandrogenism in most (60%) PCOS-A, though to a significantly lesser extent than in PCOS-T (P< 0.01). Although the testosterone response to SDAST was significantly different between PCOS-T and PCOS-A (P< 0.001), the relatively high testosterone level (>60 ng/dl) required for 95% specificity yielded poor sensitivity (35%).
The accuracy for detecting functional ovarian hyperandrogenism in PCOS with total testosterone as the SDAST end-point was as good as or better than the use of free testosterone (P= 0.284), androstenedione (P= 0.049) or 17OHP (P< 0.001). While total testosterone concentrations post-SDAST were markedly different in controls from that in both types of PCOS, the percent falls (averages of 40% for controls and 27–30% for the two types of PCOS) were not significantly different.
Detection of functional adrenal hyperandrogenism in PCOS by low-dose ACTH test
Functional adrenal hyperandrogenism, as defined by an elevated dehydroepiandrosterone level in response to ACTH, was similarly prevalent in PCOS-T (27.5%) and PCOS-A (30%) (Table II). A baseline DHEAS > 180 µg/dl, the upper limit of the reference range (Fig. 2B), was highly specific (94%), though only 59% sensitive, for differentiating between controls and those with functional adrenal hyperandrogenism according to ROC curve analysis. Although the absolute fall in testosterone and the relative (%) fall in testosterone and androstenedione in response to SDAST were subnormal in PCOS (P< 0.05), they were not significant predictors of functional adrenal hyperandrogenism according to ROC curve analysis (data not shown). 17-Hydroxypregnenolone and dehydroepiandrosterone responses correlated well (r = 0.773, P< 0.001), and 17-hydroxypregnenolone was the only other assayed steroid to rise excessively in response to ACTH in dehydroepiandrosterone hyper-responders (Table III, FAH-positive). Although androstenedione and testosterone peak responses to ACTH were significantly higher in PCOS patients with normal dehydroepiandrosterone responsiveness than in controls (Table III, FAH-negative), these responses were above the reference range in only 5% of this group.
Table III.
Low-dose ACTH1-24 (1.0 µg/1.7 m2) test results in controls and PCOS subjects according to functional adrenal hyperandrogenism status.a Mean ± SEM (5–95th percentiles reference range). Dexamethasone 0.25 mg/m2 was given 4 h before the basal sample. All steroid intermediate levels rose significantly to peak at 15–30 min.
| Progesterone (ng/dl) |
17-hydroxyprogesterone (ng/dl) |
11-deoxycortisol (ng/dl) |
Cortisol (µg/dl) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal | Peak | Rise | Basal | Peak | Rise | Basal | Peak | Rise | Basal | Peak | Rise | |
| FAH-pos | 25 ± 0.7 | 34 ± 3.4 | 11 ± 4.0 | 39 ± 6.2 | 106 ± 11 | 68 ± 7.9 | 26 ± 1.4 | 153 ± 11 | 127 ± 10 | 1.7 ± 0.0 | 19 ± 0.9 | 17 ± 0.8 |
| FAH-neg | 25 ± 0.9 | 31 ± 1.7 | 5.5 ± 1.1 | 45 ± 3.1*** | 113 ± 6.8 | 67 ± 6.5 | 26 ± 0.9 | 162 ± 8.7 | 136 ± 8.8 | 1.9 ± 0.3 | 18 ± 0.7 | 16 ± 0.7 |
| Controls | 24 ± 0.3 | 35 ± 1.8 | 10 ± 1.7 | 24 ± 0.2† | 98 ± 7.1 | 73 ± 7.1 | 25 ± 0.3 | 176 ± 10 | 151 ± 10 | 2.3 ± 0.3 | 18 ± 1.4 | 16 ± 1.1 |
| Reference range | <25 | <25–57 | 0–14 | <25 | 54–195 | 30–107 | ≤26 | 90–251 | 66–227 | 0.2–4.0 | 12–33 | 11–37 |
| 17-hydroxypregnenolone (ng/dl) |
Dehydroepiandrosterone (ng/dl) |
Androstenedione (ng/dl) |
Testosterone (ng/dl) |
|||||||||
| Basal | Peak | Rise | Basal | Peak | Rise | Basal | Peak | Rise | Basal | Peak | Rise | |
| FAH-pos | 30 ± 2.8 | 991 ± 79 | 960 ± 76 | 284 ± 14 | 1440 ± 106 | 1089 ± 41 | 139 ± 19 | 223 ± 25 | 84 ± 10 | 45.6 ± 6.1 | 53 ± 6.6 | 8.7 ± 1.4 |
| FAH-neg | 31 ± 2.9 | 672 ± 39††† | 641 ± 39††† | 209 ± 17**†† | 723 ± 37††† | 514 ± 34††† | 131 ± 8.0*** | 182 ± 10*** | 51 ± 4.9†† | 47.4 ± 2.9*** | 56 ± 3.4*** | 9.3 ± 0.8* |
| Controls | 27 ± 2.0 | 650 ± 45††† | 623 ± 44††† | 154 ± 7.9††† | 645 ± 40††† | 491 ± 35††† | 62 ± 3.5††† | 111 ± 6.0††† | 49 ± 5.0†† | 15.7 ± 1.1††† | 22 ± 1.2††† | 6.2 ± 0.8 |
| Reference range | ≤25 | 336–1212 | 312–1188 | 98–192 | 286–1012 | 201–814 | 33–89 | 70–207 | 23–128 | 10–27 | 12–30 | 0–14 |
Conversion to SI units: Progesterone × 0.0318 = nmol/l, 11-deoxycortisol × 0.0289 = nmol/l, 17-hydroxypregenolone × 0.0316 = nmol/l, dehydroepiandrosterone × 0.0347 = nmol/l.
aPCOS patients divided into those with (FAH-positive) or without (FAH-negative) elevated dehydroepiandrosterone peak in response to ACTH.
*P values versus controls: <0.05.
**P values versus controls: <0.01.
***P values versus controls: <0.001.
†P values versus FAH-positive: <0.05.
††P values versus FAH-positive: <0.01.
†††P values versus FAH-positive: <0.001.
Heterogeneity of ovarian and adrenal androgenic function in PCOS-A
Most (60%) subjects with PCOS-A had abnormal SDAST results, i.e. evidence of functional ovarian hyperandrogenism. Their baseline free testosterone levels were similar to that of PCOS-T. Of this subgroup, 25% had coincident functional adrenal hyperandrogenism.
On the other hand, a sizeable minority (40%) had no evidence of an ovarian source for the hyperandrogenism by either the SDAST or GnRHa test (Table II). A minority of these PCOS-A cases had evidence of isolated functional adrenal hyperandrogenism: 3/8 (37.5%) had hyper-responsiveness of DHEA, with or without an elevated baseline DHEAS level (Table IV). Their BMI was lower than that of the PCOS-A subgroup with abnormal SDAST (P= 0.05), as was their baseline free testosterone (P< 0.01).
Table IV.
Baseline characteristics in the heterogeneous PCOS-A subgroups (mean ± SD).
| Age (year) | BMI (kg/m2) | WCa (cm) | Hirsutism scoreb | Free Ta (pg/ml) | SHBG (nM) | A'dionea (ng/dl) | DHEAS (µg/dl) | LH (U/l) | HOMAa | ISIa |
|---|---|---|---|---|---|---|---|---|---|---|
| PCOS-A with abnormal SDAST (n = 12) | ||||||||||
| 20.3 ± 6.7 | 46.1 ± 12.1 | 127 ± 25 | 6.1 ± 4.8 | 19.8 ± 5.3 | 9.0 ± 12 | 136 ± 53 | 137 ± 92 | 6.2 ± 2.9 | 6.5 ± 6.0 | 3.1 ± 3.1 |
| PCOS-A with normal SDAST and abnormal ACTH test (n = 3) | ||||||||||
| 20.3 ± 5.8 | 29.5 ± 4.8* | 86.5 ± 9.8 | 5.7 ± 7.4 | 10.0 ± 2.6** | 20.0 ± 4.5 | 120 ± 10 | 167 ± 62 | 5.7 ± 2.4 | 2.2 ± 1.4 | 4.5 ± 2.1 |
| PCOS-A with normal SDAST and normal ACTH test (n= 5) | ||||||||||
| 22.3 ± 7.7 | 43.9 ± 9.1 | 111 ± 28 | 7.6 ± 8.8 | 9.4 ± 2.2*** | 12.8 ± 9.6 | 92.8 ± 50 | 83 ± 52 | 4.5 ± 3.6 | 5.3 ± 4.1 | 2.7 ± 2.2 |
| Reference range: | ||||||||||
| 13.3–34.4 | 18.5–37.9 | 61–106 | 0–5 | 3.0–9.0 | 11–47 | 51–165 | 30–180 | 1.5–5.6 | 0.7–5.9 | 1.4–10.3 |
WC, waist circumference; T, testosterone; A'dione, androstenedione; HOMA, homeostatic model assessment index; ISI, insulin sensitivity index.
bHirsutism score according to Ferriman–Gallwey.
*P = 0.05.
**P < 0.01.
***P < 0.001 versus group I. (PCOS-A with abnormal SDAST).
Most (5/8 = 62.5%) of the PCOS-A with normal ovarian function tests had no detectable glandular source for androgen excess: they had endocrinologically idiopathic hyperandrogenism (Table IV). Their baseline free testosterone level was significantly lower than that of the SDAST-positive PCOS-A subgroup (P< 0.001), and 3/5 were not hirsute. Notable was the finding that obesity was the one feature common to all in this subgroup (BMI 34.4–56.4). Most (4/5) had central obesity, and all had insulin resistance according to either abnormal homeostatic model assessment or insulin sensitivity index. However, the degree of obesity and the metabolic profile of this subgroup were not significantly different from that of the other PCOS-A subgroups.
To control for the possibility that obesity masks the steroidogenic abnormalities of PCOS, a secondary analysis was performed comparing the subset of PCOS-A with positive SDAST and BMI ≥ 40 kg/m2 (n= 10) and PCOS-T with BMI ≥ 40 kg/m2 (n= 10). In the absence of a significant difference in BMI (P= 0.12), the major steroidogenic changes persisted (P< 0.05, data not shown). Total testosterone and androstenedione remained significantly higher at baseline and after SDAST in PCOS-T than in PCOS-A, while free testosterone became similarly elevated, 24.3 ± 1.9 versus 19.4 ± 1.9 pg/ml at baseline and 17.4 ± 2.3 versus 13.9 ± 1.5 pg/ml post-SDAST. These levels tended to be higher than in the remainder of their respective groups with BMI < 40 kg/m2, the baseline free testosterone significantly so (P< 0.05). Peak ovarian steroids (estradiol, progesterone, 17OHP, androstenedione and dehydroepiandrosterone) of PCOS-T remained higher in response to GnRHa, while the proportion of subjects with elevated testosterone post-SDAST remained high in both subgroups (100% of PCOS-T and 75% of PCOS-A), and the proportion of subjects with dehydroepiandrosterone hyper-responses to ACTH remained similar between subgroups (20% of PCOS-T, 33% of PCOS-A).
Discussion
This study demonstrates that the testosterone response to SDAST and LDAST are similar and SDAST correlates well with the 17OHP response to GnRHa testing across study groups of PCOS and normal women. Furthermore, SDAST outcome is highly concordant with GnRHa-test outcome in PCOS-T. These findings indicate that SDAST is useful for determining the presence of the functional ovarian hyperandrogenism of PCOS. We further demonstrate that PCOS-A is a functionally heterogeneous group: while functional ovarian hyperandrogenism was detected in most PCOS-A by SDAST, it was found in significantly fewer PCOS-A than in PCOS-T. Most of the PCOS-A cases with normal testosterone suppression in response to SDAST (5/8) were obese and had no evidence of adrenal hyperandrogenism. These results are compatible with the possibility that obesity itself may produce hyperandrogenic anovulation, thus mimicking PCOS. These findings have practical implications for determining the source of androgen excess in obese women.
The short dexamethasone androgen-suppression test as a test for the functional ovarian hyperandrogenism of PCOS-T
We have validated the SDAST in PCOS and controls by demonstrating that it suppresses testosterone levels, unlike DHEAS levels, as much as does LDAST. The rapid suppression of testosterone is explicable by its rapid turnover [plasma clearance rate of ≥ 20 l/h (Southren et al., 1967)], with about half being derived from circulating androstenedione, which is cleared even faster (Horton and Tait, 1966; Bardin and Lipsett, 1967). In contrast, DHEAS turnover is very slow (plasma clearance ∼0.3 l/h), and its metabolism in the peripheral circulation contributes very little to testosterone production (Sandberg et al., 1964; Kirschner et al., 1973).
Testosterone levels post-SDAST correlated well with 17OHP responses to GnRHa. Elevation of plasma total testosterone (>27 ng/dl) 4-h after noontime dexamethasone administration detected 92.5% of PCOS-T cases with 95% specificity. These findings indicate that both these tests detect related aspects of functional ovarian hyperandrogenism, which in PCOS-T appears to result from dysregulation of steroidogenesis that prominently involves cytochrome P450c17 (17-hydroxylase/17,20-lyase) up-regulation (Ehrmann et al., 1995; Nelson et al., 1999).
The low-dose ACTH test for the functional adrenal hyperandrogenism of PCOS-T
Of the PCOS-T group, 27% had dehydroepiandrosterone hyper-responsiveness to low-dose ACTH. This type of functional adrenal hyperandrogenism has been postulated to be the adrenal manifestation of the dysregulated steroidogenesis that underlies the thecal cell dysfunction of PCOS (Ehrmann et al., 1995). The prevalence of dehydroepiandrosterone hyper-responsiveness in this series is less than the 46% prevalence seen in our previous series of PCOS patients tested with high-dose ACTH (Ehrmann et al., 1992; Ehrmann et al., 1995). Also contrasting with our earlier findings is the lack of significant 17OHP and 11-deoxycortisol hyper-responsiveness to ACTH. These differences are probably due to our use of a low ACTH dose, which would be expected to identify the extent to which androgen excess arises from physiologic ACTH stimulation (Dickstein et al., 1997; Bridges et al., 1998). These results are consistent with the results of the direct comparison of low- and high-dose ACTH tests in PCOS (Colak et al., 2002).
Functional heterogeneity in PCOS-A
SDAST indicates that 60% of PCOS-A (12/20) had functional ovarian hyperandrogenism although they lacked the 17OHP hyper-responsiveness to LH that characterizes PCOS-T. Thus, SDAST detects both typical and atypical types of functional ovarian hyperandrogenism. Of this subgroup with atypical functional ovarian hyperandrogenism, 25% had the same type of functional adrenal hyperandrogenism that is found in PCOS-T, i.e. dehydroepiandrosterone hyper-responsiveness to ACTH. This finding suggests that the ovarian dysfunction of these women is related to that of PCOS-T. On the other hand, two of these cases were previously found to lack the hyper-responsiveness to submaximal hCG stimulation that is characteristic of PCOS-T (Hirshfeld-Cytron et al., 2009), which suggests an atypical type of ovarian dysfunction.
In 15% of PCOS-A (3/20) hyperandrogenism was attributable to adrenal androgenic hyper-responsiveness to ACTH. This subgroup had isolated functional adrenal hyperandrogenism without a discernable ovarian source for their androgen excess. This PCOS-A subgroup was significantly less obese than the above PCOS-A subgroup with functional ovarian hyperandrogenism.
The other 25% of PCOS-A (5/20) lacked evidence of either functional ovarian hyperandrogenism or functional adrenal hyperandrogenism. This subgroup literally has ‘idiopathic hyperandrogenism’, which has ordinarily been associated with otherwise asymptomatic hirsutism, but here it is associated with anovulatory symptoms (Rosenfield, 2005). What might explain their combination of mild hyperandrogenemia and anovulation? We postulate that it is related to all being obese.
These findings are compatible with the possibility that obesity itself can account for the combination of mild hyperandrogenemia and anovulation, mimicking PCOS. Obesity can account for excess peripheral formation of testosterone independently of PCOS (Strain et al., 2003; Taponen et al., 2003; Quinkler et al., 2004). Adipocytes convert circulating androstenedione to testosterone via type 5 17β-hydroxysteroid dehydrogenase, which is up-regulated by insulin (Quinkler et al., 2004; Du et al., 2009). The expression of this enzyme in subcutaneous fat correlates with BMI and falls with weight loss in simple obesity. Obesity may also directly cause ovulatory dysfunction via suppression of LH independently of hyperandrogenemia (Holte et al., 1994; Arroyo et al., 1997; Pagan et al., 2006; Jain et al., 2007; Rosenfield and Bordini, 2010).
The alternative explanation, that obesity masks the functional steroidogenic abnormalities of PCOS, seems unlikely for a number of reasons. For one thing, all test doses were administered on a body-size basis. For another, obesity is well-known to exacerbate both the functional ovarian and adrenal hyperandrogenism of PCOS, seemingly via the compensatory hyperinsulinemia that accompanies insulin resistance (Ehrmann et al., 1995; Rosenfield, 1996), and factors that lessen insulin resistance normalize ovarian function (Nestler and Jakubowicz, 1996; Ehrmann et al., 1997). Furthermore, the major steroidogenic differences between PCOS-T and PCOS-A persisted in a secondary analysis of morbidly obese subgroups of PCOS-T and PCOS-A.
While obesity seemed to account for only a small proportion (8.3%) of our hyperandrogenic anovulation study cohort, which was comprised of women presenting to endocrine and gynecology clinics for hirsutism and/or anovulatory symptoms, it may account for many women in the bariatric surgery population who seem to have PCOS (Eid et al., 2005; Escobar-Morreale et al., 2005). We have previously reported that PCOS-A is relatively hyposensitive to gonadotrophins (Hirshfeld-Cytron et al., 2009), which would seem to have implications for ovulation induction. It remains to be determined to what extent the postulated ‘pseudo-PCOS’ of obesity accounts for this relative gonadotrophin hyposensitivity.
Practical clinical implications of this research
These studies suggest that SDAST is potentially a useful tool that could be employed in an outpatient setting to help distinguish patients with the pseudo-PCOS of obesity from PCOS itself. It must be kept in mind that the SDAST is not likely to be useful in distinguishing PCOS from other virilizing disorders, however. Our limited experience with SDAST in non-classic congenital adrenal hyperplasia (unpublished data) suggests that it is not as accurate as LDAST in determining the adrenal source of androgen in this disorder.
While a testosterone level < 28 ng/dl in response to SDAST in an obese woman with hyperandrogenic anovulation suggests the cause to be obesity rather than PCOS, it must be kept in mind that results differ among assays by an average of ∼6–26%, as demonstrated here (Supplementary data) and reported by others (Legro et al., 2010). Higher degrees of variation would be expected from platform methods (Miller et al., 2004; Wang et al., 2004), and there is no uniform reference assay for testosterone, or other androgens, for that matter (Wartofsky and Handelsman, 2010).
We conclude that PCOS is functionally heterogeneous: the common type (PCOS-T) is hypersensitive to gonadotrophins; a minority type (PCOS-A) is relatively gonadotrophin-hyposensitive, possibly due in part to obesity mimicking PCOS. Obesity is suggested as the cause of hyperandrogenic anovulation, rather than PCOS if baseline testosterone elevation is mild and, more specifically, if the testosterone level is suppressed to a normal extent in response to SDAST.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
All authors contributed to study design, data acquisition, analysis and/or interpretation of data; drafting or critically revising the article; and approval of the final submitted product.
Funding
This research was supported in part by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54-041859) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and RR-00055 and UL1RR024999 from the National Center for Research Resources.
Supplementary Material
Acknowledgements
The referrals of gynecology clinic patients by Dr Anthony Caruso is appreciated. Our special thanks to Neal Scherberg, Ph.D. and Kiang-Teck J. Yeo, Ph.D. of the University of Chicago Hospital Laboratories for providing the assay comparison data.
References
- Arroyo A, Laughlin GA, Morales AJ, Yen SSC. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab. 1997;82:3728–3733. doi: 10.1210/jcem.82.11.4377. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Bardin C, Lipsett M. Testosterone and androstenedione blood production rates in normal women and women with idiopathic hirsutism or polycystic ovaries. J Clin Invest. 1967;46:891–902. doi: 10.1172/JCI105588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges NA, Hindmarsh PC, Pringle PJ, Honour JW, Brook CG. Cortisol, androstenedione (A4), dehydroepiandrosterone sulphate (DHEAS) and 17 hydroxyprogesterone (17OHP) responses to low doses of (1–24)ACTH. J Clin Endocrinol Metab. 1998;83:3750–3753. doi: 10.1210/jcem.83.10.5315. [DOI] [PubMed] [Google Scholar]
- Colak R, Kelestimur F, Unluhizarci K, Bayram F, Sahin Y, Tutus A. A comparison between the effects of low dose (1 microg) and standard dose (250 microg) ACTH stimulation tests on adrenal P450c17alpha enzyme activity in women with polycystic ovary syndrome. Eur J Endocrinol. 2002;147:473–477. doi: 10.1530/eje.0.1470473. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Dickstein G, Spigel D, Arad E, Shechner C. One microgram is the lowest ACTH dose to cause a maximal cortisol response. There is no diurnal variation of cortisol response to submaximal ACTH stimulation. Eur J Endocrinol. 1997;137:172–175. doi: 10.1530/eje.0.1370172. [DOI] [PubMed] [Google Scholar]
- Du X, Rosenfield RL, Qin K. KLF15 is a transcriptional regulator of the human 17β-hydroxysteroid dehydrogenase type 5 gene. A potential link between regulation of testosterone production and fat stores in women. J Clin Endocrinol Metab. 2009;94:2594–2601. doi: 10.1210/jc.2009-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF, Sheikh Z. Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med. 1992;327:157–162. doi: 10.1056/NEJM199207163270304. [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocrin Rev. 1995;16:322–353. doi: 10.1210/edrv-16-3-322. [DOI] [PubMed] [Google Scholar]
- Ehrmann D, Schneider D, Sobel B, Cavaghan M, Imperial J, Rosenfield R, Polonsky K. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2108–2116. doi: 10.1210/jcem.82.7.4069. [DOI] [PubMed] [Google Scholar]
- Eid GM, Cottam DR, Velcu LM, Mattar SG, Korytkowski MT, Gosman G, Hindi P, Schauer PR. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:77–80. doi: 10.1016/j.soard.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millan JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005;90:6364–6369. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Cytron J, Barnes RB, Ehrmann DA, Caruso A, Mortensen MM, Rosenfield RL. Characterization of functionally typical and atypical types of polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1587–1594. doi: 10.1210/jc.2008-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holte J, Bergh T, Gennarelli G, Wide L. The independent effects of polycystic ovary syndrome and obesity on serum concentrations of gonadotrophins and sex steroids in premenopausal women. Clin Endocrinol (Oxf) 1994;41:473–481. doi: 10.1111/j.1365-2265.1994.tb02578.x. [DOI] [PubMed] [Google Scholar]
- Horton R, Tait JF. Androstenedione production and interconversion rates measured in peripheral blood and studies on the possible site of its conversion to testosterone. J Clin Invest. 1966;45:301. doi: 10.1172/JCI105344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92:2468–2473. doi: 10.1210/jc.2006-2274. [DOI] [PubMed] [Google Scholar]
- Kirschner MA, Sinhamahapatra S, Zucker IR, Loriaux L, Nieschiag E. The production, origin and role of dehydroepiandrosterone and Δ5-androstenediol as androgen prehormones in hirsute women. J Clin Endocrinol Metab. 1973;37:183–189. doi: 10.1210/jcem-37-2-183. [DOI] [PubMed] [Google Scholar]
- Legro RS, Schlaff WD, Diamond MP, Coutifaris C, Casson PR, Brzyski RG, Christman GM, Trussell JC, Krawetz SA, Snyder PJ, et al. Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95:5305–5313. doi: 10.1210/jc.2010-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 2004;89:525–533. doi: 10.1210/jc.2003-030680. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Ehrmann DA, Littlejohn E, Rosenfield RL. Asymptomatic volunteers with a polycystic ovary are a functionally distinct but heterogeneous population. J Clin Endocrinol Metab. 2009;94:1579–1586. doi: 10.1210/jc.2008-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13:946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- Pagan YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab. 2006;91:1309–1316. doi: 10.1210/jc.2005-2099. [DOI] [PubMed] [Google Scholar]
- Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity - a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183:331–342. doi: 10.1677/joe.1.05762. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Editorial: evidence that idiopathic functional adrenal hyperandrogenism is caused by dysregulation of adrenal steroidogenesis and that hyperinsulinemia may be involved. J Clin Endocrinol Metab. 1996;81:878–880. doi: 10.1210/jcem.81.3.8772543. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Ovarian and adrenal function in polycystic ovary syndrome. Endocrinol Metab Clin N Am. 1999;28:265–293. doi: 10.1016/s0889-8529(05)70070-0. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Clinical practice. Hirsutism [Comment in: N Engl J Med. 2006 Apr 6;354(14):1533–5; author reply 1533–5] N Engl J Med. 2005;353:2578–2588. doi: 10.1056/NEJMcp033496. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Bordini B. Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity. Brain Res. 2010;1364:186–197. doi: 10.1016/j.brainres.2010.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield RL, Barnes RB, Ehrmann DA, Toledano AY. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:6115. doi: 10.1210/jc.2003-031357. [DOI] [PubMed] [Google Scholar]
- Sandberg E, Gurpide E, Lieberman S. Quantitative studies on the metabolism of dehydroisoandrosterone sulfate. Biochemistry. 1964;3:1256–1267. doi: 10.1021/bi00897a013. [DOI] [PubMed] [Google Scholar]
- Southren AL, Gordon GG, Tochimoto S, Pinzon G, Lane DR, Stypulkowski W. Mean plasma concentration, metabolic clearance and basal plasma production rates of testosterone in normal young men and women using a constant infusion procedure: effect of time of day and plasma concentration on the metabolic clearance rate of testosterone. J Clin Endocrinol Metab. 1967;27:686–694. doi: 10.1210/jcem-27-5-686. [DOI] [PubMed] [Google Scholar]
- Strain GW, Zumoff B, Miller LK, Rosner W. Sex difference in the effect of obesity on 24-h mean serum gonadotropin levels. Horm Metab Res. 2003;35:362–366. doi: 10.1055/s-2003-41358. [DOI] [PubMed] [Google Scholar]
- Taponen S, Martikainen H, Jarvelin MR, Laitinen J, Pouta A, Hartikainen AL, Sovio U, McCarthy MI, Franks S, Ruokonen A. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab. 2003;88:141–147. doi: 10.1210/jc.2002-020982. [DOI] [PubMed] [Google Scholar]
- Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- Wartofsky L, Handelsman DJ. Standardization of hormonal assays for the 21st century. J Clin Endocrinol Metab. 2010;95:5141–5143. doi: 10.1210/jc.2010-2369. [DOI] [PubMed] [Google Scholar]
- Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens J, Haseltine F, Merriam G, editors. Polycystic Ovary Syndrome. Cambridge, MA: Blackwell Scientific Publications; 1992. pp. 377–384. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.