Abstract
BACKGROUND
The purpose of this study was to assess the technical aspects related to polar body (PB) biopsy, which might have an influence on the results of the microarray comparative genomic hybridization analysis. Furthermore, a comparison was made between two biopsy methods (mechanical and laser).
METHODS
Biopsy of the first and second PB (PB1 and PB2) was performed by mechanical- or laser-assisted biopsy in two different IVF centres. PBs were separately amplified by whole genome amplification.
RESULTS
The method of biopsy, mechanical or laser had no influence on the proportion of successfully biopsied oocytes. Especially, for the PB2, the timing of biopsy after ICSI was directly correlated to amplification efficiency.
CONCLUSIONS
Special care has to be taken with respect to the timing of biopsy of the PB2. Mechanical- and laser-assisted biopsy give the same performance in terms of diagnostic efficiency.
Keywords: aneuploidy, array CGH, biopsy, polar body, oocyte
Introduction
Since its introduction in 1993 (Munne et al., 1993), preimplantation genetic screening (PGS) of chromosome aneuploidy in human-assisted reproduction has been performed predominantly by blastomere biopsy at cleavage stages and interphase fluorescent in situ hybridization (FISH) for 5–8 chromosomes (Gianaroli et al., 1999; Munne et al., 2003). Since it has been shown that the cleavage stage may not be the best approach to identify aneuploidy reliably because of the high incidence of chromosomal mosaicism, the European Society of Human Reproduction and Embryology (ESHRE) Task Force on PGS decided to initiate a pilot study of polar body (PB) biopsy and chromosome copy number analysis using array comparative genomic hybridization (CGH) to analyse the chromosome complement in both the first (PB1) and second (PB2) polar bodies. The clinical results of this study are published separately (Geraedts et al., 2011).
Here, we critically examine how the quality of the results, and hence the diagnosis, depends on aspects of the biopsy procedure.
Materials and Methods
Patients
For this study only ICSI cycles with ejaculated spermatozoa were included. There were no restrictions with respect to the maternal age of the patients, their reproductive history or the number of retrieved or fertilized oocytes.
Except for two cycles in the Bologna centre, in which the analysis was performed on thawed oocytes, all cycles involved PB biopsy and analysis in fresh cycles.
All patient materials were obtained and evaluated with informed patient consent and under approval from the Ethics Committees from both centres. The approval was given in Bologna by the Ethics Committee of the SISMER center and in Bonn by the Local Ethics Committee implemented for all medical research topics by the Medical Faculty at the University of Bonn. Patients had to sign an informed consent prior to entering the study.
Timing strategies
Hormone stimulation, follicular puncture and ICSI were performed according to standard protocols. Contrary to the routine in the centre in Bonn, ovulation induction was initiated at 1a.m. and consequently follicular puncture as well as ICSI were postponed accordingly.
Individual time frames for each centre are shown in Table I.
Table I.
Time frame for a cycle with PB biopsy and array CGH.
| Bonn |
Bologna |
|||
|---|---|---|---|---|
| Oocyte retrieval | Day 0 | Approx. 13:00 | Day 0 | Approx. 8:00 |
| ICSI | Day 0 | 20:30–21:00 | Day 0 | 11:00–11:30 |
| Biopsy/transfer to PCR tubes | Day 1 | 5:20–5:40 | Day 0 | 20:00–20:30a |
| Amplification | Day 1 | 5:45–9:00 | Day 0 | 20:40–23:45 |
| Labelling/concentration | Day 1 | 9:00–12:00 | Day 0–Day 1 | 23:45–03:25 |
| Hybridization | Day 1 | 12:00–15:15 | Day 1 | 3:25–6:30 |
| Washing: | Day 1 | 15:15–15:55 | Day 1 | 6:30–7:05 |
| Scanning/diagnosisb | Day 1 | 16:05–17:05 | Day 1 | 7:05–8:05 |
| Oocyte selection | Day 1 | 17:05 | Day 1 | 8:10 |
aThis is the time adopted for the last 65 consecutive biopsies in Bologna. For the previous oocytes, biopsy was performed 6–7 h after ICSI.
bTime frame for scan and diagnosis for six oocytes corresponding to 12 PBs. Additional six oocytes will require another 70 min.
For the two thawing cycles, cryopreservation had been performed by slow-freezing (Magli et al., 2010) and the timing of thawing and PB biopsy was scheduled to be equivalent to the biopsy in fresh cycles.
Special care was taken during denuding the oocyte with hyaluronidase to remove all the cumulus cells adhering to the zona pellucida with the aim of avoiding DNA contamination during the amplification steps.
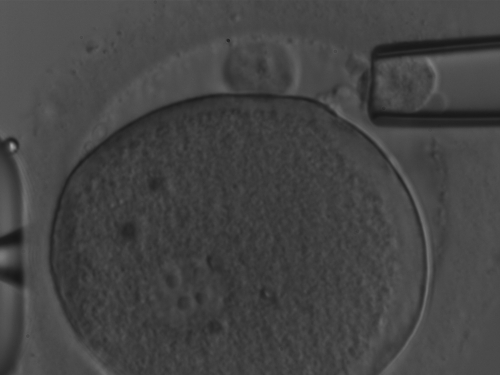
Simultaneous biopsy of both PB1 and PB2 was carried out at 6–9 h after ICSI. At that time, PB2 is still tenuously connected by a cytoplasmic strand to the oocyte and thus can be distinguished in the majority of the cases from PB1 (Fig. 1). More important, spindle remnants are normally no longer present within the connective cytoplasmic strand and therefore there is no risk of accidentally removing chromatids from the oocyte. Generally, pronuclei are already visible at this time (Montag, 2010).
Figure 1.
The presence of a faint but clearly identifiable strand connecting PB2 to the oolemma. The biopsy was performed ∼9 h after ICSI.
PB biopsy
The PB biopsy procedure was performed in dishes prepared with three droplets of 3–5 μl buffered culture medium per oocyte overlaid with pre-equilibrated mineral oil. One droplet was used for the oocyte and the other two for sampling of PB1 and PB2 of the corresponding oocyte after biopsy.
For removal of the PBs, the oocyte was first rotated and held in a position where both the PBs and the meridian of the oocyte were in the same focal plane. In most cases, PB1 and PB2 could be distinguished based on their morphology, as PB2 is normally slightly smaller and regular in shape. In addition, within the time frame used in this study, at biopsy PB2 was still linked to the oocyte with a cytoplasmic strand and hence at a fixed position within the perivitelline space, whereas PB1 could be dislocated by gently pushing the oocyte with the biopsy capillary. Whenever possible, PB2 was rotated to face towards the biopsy capillary to facilitate breaking the connecting cytoplasmic strand. Where there was a significant distance between the position of PB1 and PB2, the oocyte was rotated so that both PBs were in line with the biopsy capillary.
Mechanical biopsy
All PB biopsies in the Bologna centre were performed using mechanical biopsy.
Mechanical biopsy was achieved using a micromanipulator with a double holder that carried a partial zona dissection (PZD) glass microneedle and a PB biopsy capillary. Before starting the procedure, the two microtools were carefully aligned in the same plane as the holding capillary.
To breach the zona pellucida, the oocyte was positioned with the two PBs at the 2–3 o'clock position. The oocyte was then rotated in such a way that the two PBs were vertically oriented along the meridian line that ideally divides the zygote into two almost identical hemispheres at both sides of the viewing field.
A slit of ∼20 μm was made mechanically in the zona pellucida by passing the PZD microneedle through the perivitelline space tangentially to the oocyte, and the cut completed by temporarily releasing the oocyte and repeatedly rubbing the PZD microneedle against the holding capillary (Magli et al., 2006). When the slit was opened, the oocyte was released from the holding capillary and rotated in such a way that the slit was located at the 1 or 5 o'clock position with the two PBs in the same plane on the left. The PBs were then gently aspirated with the biopsy capillary as described later.
Laser-assisted PB biopsy
All biopsies in the Bonn centre were performed using laser-assisted biopsy.
For removal of PB1 and PB2, the oocyte was held in a position where the PBs were located at the 1-o'clock position. Using a non-contact diode laser (Octax, Bruckberg, Germany) an opening of 16–20 μm was drilled with 2–4 laser shots. A flame-polished blunt ended biopsy capillary (Tip MML, Eppendorf, Hamburg, Germany) was then pushed smoothly through the opening and towards PB2. Retrieval was accomplished by sliding the capillary as far as possible over both PBs and they were both aspirated with as little suction as necessary (Montag, 2009).
Once the capillary was removed, PB1 and PB2 were expelled in different droplets of HEPES-buffered medium. For later transfer to the reaction tubes, it was crucial to position each PB exactly in the middle of the corresponding droplet.
Transfer of PBs to the reaction tube
Transfer of PBs into the reaction tube was done in a laminar flow cabinet in order to avoid any contamination of the sample. Each reaction tube was pre-filled with 1.6 μl phosphate-buffered saline (PBS) (BlueGnome, Cambridge, UK). Under visual control in a stereomicroscope, a micropipette (0.1–2.5 μl Research, Eppendorf) was inserted into the droplet containing the PB and 0.4 μl of medium was drawn into the pipette. Some of the medium was expelled over the PB in order to make sure that it was not attached to the bottom of the dish. Next the PB was sucked into the pipette in a total maximum volume of 0.4 μl. The tip of the pipette was briefly dipped into an uncovered drop of sterile PBS to remove any oil derived from the biopsy dish. The pipette was then lowered into the reaction tube until the tip was in contact with the PBS of the tube and the PB was directly expelled into the PBS. The reaction tube was closed immediately and stored on ice in a tube rack until amplification.
Successful PB transfer was checked by drawing the medium up and down in the capillary tip under visual control.
Fragmented PBs
As the PB1 and PB2 were biopsied simultaneously 6–9 h after ICSI, PB1s were occasionally fragmented, whereas PB2s remained in a single cell configuration. Fragmented PB2s could be retrieved as an entity as the fragments usually stick together. However, once the fragments were released in another medium droplet, special care was to be taken during transfer into the PCR tube, as this step bears a certain risk of dislocating the fragments. PB2 could still be distinguished from PB1 fragments due to its typical morphology and the presence of a cytoplasmic bridge linking PB2 to the oolemma.
Whole genome amplification and array CGH
The amplification, labelling, hybridization and analysis procedure have been published in the parallel paper (Geraedts et al., 2011).
Results
The biopsy methods used, laser-assisted in Bonn and mechanical in Bologna, did not affect the proportion of successfully biopsied oocytes. The only difference was a slightly longer time associated with the mechanical biopsy (Table I).
Time frame of a treatment cycle with PB biopsy and 12 h chromosomal analysis
When analysed per centre, the total amplification rates of all PBs were 97% in Bonn (245/252) and 92% in Bologna (183/200, P< 0.025) (Table II). The amplification results obtained for PB1 were identical for both centres (95 versus 94%, respectively), while for PB2 there was a significant difference (99% in Bonn versus 89% in Bologna; P < 0.005).
Table II.
Results of PB amplification and diagnosis.
| Total | Bonn | Bologna | P Bonn versus Bologna | |
|---|---|---|---|---|
| Number of cycles (patients) | 42 (41) | 20 (19) | 22 (22) | — |
| Age (mean ± SD), years | 40.0 ± 2.9 | 39.9 ± 3.3 | 40.2 ± 2.6 | — |
| Number of biopsied oocytes (mean ± SD) | 226 (5.4 ± 3.3) | 126 (6.3 ± 4.1) | 100 (4.5 ± 2.1) | — |
| Number of biopsied PBs | 452 | 252 | 200 | — |
| Number of amplified PBs (%) | 428 (95) | 245 (97) | 183 (92) | <0.025 |
| Number of diagnosed PBs | 419 | 237 | 182 | |
| (%/biopsied) | (93) | (94) | (91) | |
| (%/amplified) | (98) | (97) | (99) | |
| Number of biopsied PB1 | 226 | 126 | 100 | — |
| Number of amplified PB1 (%) | 214 (95) | 120 (95) | 94 (94) | — |
| Number of diagnosed PB1 | 212 | 118 | 94 | |
| (%/diagnosed) | (94) | (94) | (94) | |
| (%/amplified) | (99) | (98) | (100) | |
| Number of biopsied PB2 | 226 | 126 | 100 | — |
| Number of amplified PB2 (%) | 214 (95) | 125 (99) | 89 (89) | <0.005 |
| Number of diagnosed PB2 | 207 | 119 | 88 | |
| (%/biopsied) | (92) | (94) | (88) | |
| (%/amplified) | (97) | (95) | (99) |
Following detailed examination of differences in the protocol steps for PB2 analysis, the timing of biopsy was found to be different between Bonn and Bologna, i.e. biopsy was carried out 2–3 h earlier in Bologna when compared with Bonn. Based on these considerations, that became apparent during an interim analysis, the biopsy of the last 65 consecutive oocytes in Bologna was delayed by 2–3 h with respect to the original protocol and was performed ∼9 h after ICSI following the time schedule of Bonn. The corresponding proportion of amplified PB2 in this series was 95% (62/65), which was similar to that obtained in the other centre, while it was 77% (27/35) in the first series (P< 0.025).
In Bonn, no changes were made in the biopsy timing and the amplification rate remained constant during all the experiments.
Discussion
From the results of this study, it is possible to draw some relevant conclusions regarding the technical aspects involved in PB biopsies. It was confirmed that the technique is applicable in an IVF setting providing the results in 12–13 h.
This timeframe overcomes one of the main drawbacks of CGH, i.e. that until very recently, this technique was not compatible with a fresh transfer especially when performing the biopsy at the blastocyst stage. The protocol is quite flexible, and longer incubation periods can be adopted to make the procedure fit into normal working hours. The two biopsy methods used in this study, laser and mechanical, did not affect the proportion of successfully biopsied oocytes confirming that the act of biopsy, when performed by a skilled practitioner, does not apparently damage the oocyte. The only difference was the slightly longer time needed for mechanical biopsy due to the fact that to open the zona pellucida, the oocyte had to be detached from the holding pipette and, when the slit was complete, it had to be relocated with the opening at the 2 or 5 o'clock position to permit the entry of the biopsy needle. It could be speculated that the mechanical procedure is a compromise between a longer time of exposure to the external environment and a more natural method compared with the use of localized heat denaturation using a laser beam.
The comparison of the results between the two laboratories revealed that the timing of PB biopsy plays a key role for the achievement of a diagnosis. For the purpose of the study, it was decided to remove the two PBs simultaneously and the timing was decided in the two centres according to their experience of PB analysis by FISH. For practical reasons, Bonn decided to do the biopsy early in the morning ∼9 h post injection, while in Bologna the procedure was done late in the evening, initially at 6–7 h post injection. The interval of 6 h after ICSI was considered to be necessary to allow completion of anaphase of the second meiotic division and to be sufficient to provide good quality results as confirmed by FISH studies. Nevertheless, the process of whole genome amplification revealed that there is a significant difference in the DNA status of PB2s biopsied at 6 h or at 9 h post injection. As demonstrated by the rate of amplification in the two different conditions, DNA was more accessible to random primers and Taq polymerase at 9 h post insemination when chromosomes had most likely completed telophase (Table II). Accordingly, when the strategy in Bologna was adjusted to later biopsy timing, the amplification rate between the two centres was identical.
As for all cases involving DNA amplification, especially from a single cell, the presence of exogenous DNA has to be systematically avoided. However, our results clearly show that if it is easy to control the biopsy of PBs where every single step is done under visual control, oocyte collection, especially in combination with the use of Pronase, is associated with a higher risk of contamination by cumulus cell DNA, which might be released during the enzymatic treatment.
Authors’ roles
All authors designed the study. C.M., M.M., M.K. and L.M. performed the microarray experiments. S.R. performed the concordance analysis. C.M., M.M. and J.G. wrote the drafts of the manuscript. A.H.H., C.M., M.M. and S.R. completed it. All authors modified and improved it.
Conflict of interest
All authors have completed the ICMJE conflict of interest disclosure form, and have no conflicts to declare.
Funding
Funded by ESHRE, the European Society of Human Reproduction and Embryology, Grimbergen, Belgium. Funding to pay the Open Access publication charges for this article was provided by the European Society of Human Reproduction and Embryology (ESHRE).
Acknowledgements
The authors thank the IVF teams at Bonn and Bologna (especially Prof. Dr Katrin van der Ven, Prof. Dr Hans van der Ven and Dr Anna P. Ferraretti) for their cooperation and support throughout the study.
References
- Geraedts J, Montag M, Magli MC, Repping S, Handyside A, Staessen C, Harper J, Schmutzler A, Collins J, Goossens V, et al. Polar body array CGH for prediction of the status of the corresponding oocyte. Part I: clinical results . Hum Reprod. 2011 doi: 10.1093/humrep/der294. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaroli L, Magli MC, Ferraretti AP, Munne S. Preimplantation diagnosis for aneuploidies in patients undergoing in vitro fertilization with a poor prognosis: identification of the categories for which it should be proposed. Fertil Steril. 1999;72:837–844. doi: 10.1016/s0015-0282(99)00377-5. [DOI] [PubMed] [Google Scholar]
- Magli MC, Ferraretti AP, Crippa A, Lappi M, Feliciani E, Gianaroli L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil Steril. 2006;86:629–635. doi: 10.1016/j.fertnstert.2006.02.083. [DOI] [PubMed] [Google Scholar]
- Magli MC, Lappi M, Ferraretti AP, Capoti A, Ruberti A, Gianaroli L. Impact of oocyte cryopreservation on embryo development. Fertil Steril. 2010;93:510–516. doi: 10.1016/j.fertnstert.2009.01.148. [DOI] [PubMed] [Google Scholar]
- Montag M, van der Ven K, van der Ven H. Polar body biopsy. In: Harper J, editor. Preimplantation Genetic Diagnosis. 2nd edn. Cambridge University Press; 2009. pp. 166–174. [Google Scholar]
- Montag MMK, Nikolov A, van der Ven H. Time-lapse based evaluation of human oocytes from ICSI up to the pronuclear stage. Hum Reprod. 2010;25i:180. [Google Scholar]
- Munne S, Lee A, Rosenwaks Z, Grifo J, Cohen J. Diagnosis of major chromosome aneuploidies in human preimplantation embryos. Hum Reprod. 1993;8:2185–2191. doi: 10.1093/oxfordjournals.humrep.a138001. [DOI] [PubMed] [Google Scholar]
- Munne S, Sandalinas M, Escudero T, Velilla E, Walmsley R, Sadowy S, Cohen J, Sable D. Improved implantation after preimplantation genetic diagnosis of aneuploidy. Reprod Biomed Online. 2003;7:91–97. doi: 10.1016/s1472-6483(10)61735-x. [DOI] [PubMed] [Google Scholar]