Abstract
Sensitivity to stress has long been implicated in the pathogenesis of schizophrenia. It remains unclear, however, which exact mechanisms underlie the progression from vulnerability to psychotic breakdown. For the present study, we hypothesized that the induction of stress would aggravate cognitive biases in schizophrenia. A total of 20 acute and remitted schizophrenia patients and 15 healthy controls were tested with parallel versions of cognitive biases paradigms under 2 laboratory conditions: stress (loud noise, 75 dB) vs no-stress. In the course of both conditions, participants had to fill out a questionnaire that assessed depressive, obsessive–compulsive, and paranoid symptoms. For the patients with acute psychotic symptoms, paranoid but not other psychiatric symptoms were elevated under stress in comparison with no-stress. In contrast, stress somewhat diminished subclinical paranoid symptoms in healthy participants. Jumping to conclusions was evident in schizophrenia under both conditions but significantly more pronounced when stress was applied first in the acute group. A tendency emerged in both acute and remitted patients to attribute events to other people under stress which was not seen in healthy subjects. The present study may serve as a starting point for further research investigating how stress translates vulnerability into acute paranoia and to pinpoint cognitive risk factors that can be modified by treatment.
Keywords: schizophrenia, stress, cognitive biases, jumping to conclusions, noise
Introduction
It is one of the most well-established findings in schizophrenia research that stress triggers or aggravates psychosis.1 Daily hassles2 as well as sustained levels of stress,3 as exerted, for example, by urbanicity,4 migration,5 and increased stress sensitivity,6 easily translate into symptoms such as paranoia in vulnerable individuals. The vulnerability–stress conceptualization of schizophrenia is picked up in almost all psychosocial interventions and is at the core of psychoeducation programs7: However, while valuable as a heuristic model, many of its constituents remain unexamined, especially the modulating mechanisms on the way from stress to psychosis.
Given the large literature suggesting that cognitive distortions, such as reasoning8–10 and attributional biases,11–13 especially jumping to conclusions (JTC) and blaming others for failure, are present in the disorder14–17 and are presumably linked with the positive symptoms of schizophrenia, it is tempting to speculate that these biases are especially sensitive to stress and pave the way to breakdown. Starting with a study by Mujica-Parodi and coworkers,18 this assumption has obtained some empirical support. For example, our group found that healthy participants scoring high on psychosis-prone items showed a direct increase of paranoia under stress, which was mediated by negative emotion.19 However, elevated paranoia was not accompanied by an increase in reasoning biases in this sample.20 In another study,21 healthy participants with higher baseline vulnerability for psychosis showed an increase of paranoia after the induction of anxiety, which was mediated by increased JTC. In yet another study, patients high on delusions showed pronounced hasty decision making under emotional music relative to controls lending further support to the assumption that stress and emotional arousal aggravate cognitive biases.22 Ellett et al.23 reported that patients with persecutory delusions who were randomized either to an urban environment or to a brief mindfulness relaxation task showed enhanced JTC under the former condition.
Although these studies have not produced entirely consistent findings, they underline the potential relevance of cognitive biases for the translation from stress to psychosis. So far, most studies have focused on JTC and did not address other cognitive biases (eg, attributional biases) which tap independent cognitive functions.24 Furthermore, the aforementioned studies used very different induction paradigms and currently we do not know whether general fear, social anxiety, or stress per se impact on cognitive biases the most.
For the present study, we aimed to extend on previous research by investigating the cognitive performance of schizophrenia patients and controls on 2 laboratory conditions, either during sensory stress (noise) or without stress. We chose loud noise as it is a valid and potent stressor.25 We concurrently measured several cognitive biases (eg, JTC, bias against disconfirmatory evidence [BADE]) along with a neurocognitive parameter (nonverbal memory). We assumed that patients would display deviant responses on the no-stress condition for cognitive biases, especially JTC, which would be further aggravated under stress. No changes were expected for cognitive deficits as these are not related to positive but rather to nonacute negative and disorganized symptoms which are less stress sensitive. In order to ascertain the specificity of the association between stress and psychotic symptoms, we assessed other clinical symptoms as well. We predicted that stress would have the strongest impact on paranoid ideation, particularly for the acute patient group.
Methods
Participants
Twenty schizophrenia patients were recruited from the psychosis units of the University Medical Center Hamburg. Patients met criteria for schizophrenia and schizoaffective disorder as determined by the Mini International Neuropsychiatric Interview (MINI).26 According to the MINI criteria, 13 of the patients were currently in remission. None of the patients had a macroscopic neurological disorder or substance dependence. All patients were on stable antipsychotic medication. We recruited 15 healthy participants via an established subject pool who did not have any axis I diagnosis as assessed with the MINI and had had no prior contact with mental health institutions (for sociodemographic characteristics, see table 1).
Table 1.
Sociodemographic and Psychopathological Baseline Variables
| Variable | Healthy (n = 15) | Schizophrenia (n = 20) | Statistics |
| Age | 37.07 (9.78) | 34.55 (9.22) | t = 0.78, P > .4 |
| Sex (male/female) | 6/9 | 10/10 | χ2 = .34, P > .5 |
| Years of school education | 12.00 (1.46) | 11.25 (1.68) | t = 1.38, P > .1 |
| Previous admissions | — | 4.45 | — |
| OCI-R | 7.60 (5.95) | 16.30 (12.27) | t = 2.77, P = .01 |
| Paranoia Checklist | 4.13 (4.08) | 19.45 (16.15) | t = 4.07, P < .001 |
| Depression Scale | 15.93 (11.88) | 20.45 (46.16) | t = 0.37, P > .7 |
| Perceived Stress Scale | 15.40 (5.69) | 21.90 (7.67) | t = 2.76, P = .009 |
| CAPE positive | 1.35 (0.16) | 1.80 (0.39) | t = 4.70, P < .001 |
| CAPE negative | 1.79 (0.27) | 2.15 (0.34) | t = 3.43, P = .001 |
| CAPE depression | 1.87 (0.29) | 2.36 (0.54) | t = 3.49, P = .001 |
Note: df = 33 for t-test comparisons. CAPE, Community Assessment of Psychic Experiences; OCI-R, Obsessive–Compulsive Inventory-Revised.
Design and Procedure
The experiment was conducted as a randomized repeated-measures design. All participants underwent an assessment under 2 conditions within a time interval of 1–7 days: stress vs no-stress. During each session, they had to complete parallel versions of various cognitive tasks as well as the symptom rating scales. In the stress condition, stress was induced by loud construction noise via headphones (75 dB) except for the respective instruction phase. The no-stress condition was identical in administration except that no noise was applied.
Parallel versions of the experiments and the clinical questionnaires (randomized item order) were randomized to the conditions. The order of the assessment was the same in each condition. Participants who first underwent the stress condition will be referred to as “stress first” group, whereas participants who first underwent the no-stress condition second will be referred to as the “stress second” group (see table 2).
Table 2.
Protocol of Investigation (Stress Test without baseline)
| Time (minutes) | Tasks |
| 0 | Instruction |
| 8 | VAS(1) > instruction BADE > [noise on—start] > execution > BADE |
| 28 | VAS(2) > [noise out] > complex figure instruction > [noise on] > execution complex copy and immediate recall |
| 40 | VAS(3) > [noise out] > instruction fish task > [noise on] > execution fish task |
| 54 | VAS(4) > [noise out] > instruction complex figure delayed > [noise on] > execution complex figure delayed > [noise out] > instruction questionnaires > [noise on] > execution POD |
| 68 | VAS(5) > [noise out] > instruction IPSAQ-R > [noise on] > execution IPSAQ-R |
| 90 | End of IPSAQ-R > VAS (6) > [noise out—end] |
Note: BADE, bias against disconfirmatory evidence; IPSAQ-R, Internal, Personal, and Situational Attributions Questionnaire; POD, Questionnaire on Paranoid, Obsessive–Compulsive, and Depressive Symptoms; VAS, Visual Analog Scale.
Instruments
Baseline Assessments.
To assess the baseline stress level, the Perceived Stress Scale (PSS)27 was administered which measures the degree to which situations in one’s life are appraised as stressful. It has shown satisfactory reliability and concurrent as well as discriminate validity.
In addition, the Community Assessment of Psychic Experiences (CAPE) questionnaire28 was administered before the experimental procedure to assess the extent of psychotic, negative, and depressive symptoms.
Assessments during the Experimental Conditions.
Symptomatology
A combined questionnaire on paranoid, obsessive–compulsive, and depressive symptoms was administered in random item order. Participants had to rate the extent to which each item applies “at the moment.” It consisted of the following 3 instruments:
The Paranoia Checklist29 is an 18-item scale capturing ideas of persecution and reference. The German version has good internal consistency and convergent validity.19
The Obsessive–Compulsive Inventory-Revised (OCI-R)30 was administered to evaluate the frequency and distress experienced by OC symptoms across 6 subscales. The OCI-R is sensitive to change,31 and both the original30,32 and the German version33 have good psychometric properties.
The General Depression Scale (GDS)34 is a German 20-item self-report questionnaire tapping depressive features. The GDS shows high internal consistency and is strongly correlated with other indexes of depression.
Six times during each condition (see table 1), participants had to complete a Visual Analog Scale (VAS) assessing the subjective experience of stress by making a mark on a 10-cm line for 4 stress items.
Cognitive Biases
JTC was determined using a variant of the so-called beads task.10 The revised version24,35 is easier to comprehend than the original task and controls for effects of motivation. Participants were shown 2 lakes with fish of 2 different colors (lake 1: 80% color A and 20% color B and vice versa for lake 2). Participants were told that fish would be caught successively from only one lake and were asked to judge from which lake the fish were caught. In total, 10 fish were drawn for all subjects in a seemingly random but actually fixed order. Participants were asked to give an estimate for each fish. In one version, lake 1 was favored (sequence: A-A-A-B-A-A-A-A-B-A). In the other version, lake 2 was favored (sequence: B-B-B-A-B-B-B-B-A-B). A decision after only one fish indicated JTC. At each of the 10 stages, a decision could be made, withdrawn, or changed.
The BADE task was adapted from earlier versions36,37 and entailed 16 delusion-neutral scenarios each consisting of 3 successive statements each providing additional information that increasingly disambiguates the scenario. Along with the statements 4 interpretations appeared. Each scenario had one true interpretation, 2 lure interpretations, and one absurd interpretation. The true interpretation appears implausible initially but gains plausibility when additional information is revealed. In contrast, the lure interpretations appear plausible initially but loose plausibility afterward. After presentation of each statement, participants could update ratings. Two parallel computerized versions of the BADE task were administered. The BADE score used in the study was computed as the change score (first vs final sentence) for lure interpretations. Thus, this parameter reflects the extent to which participants corrected their estimates for lure interpretations.
Attributional style was assessed using a variant of the Internal, Personal, and Situational Attributions Questionnaire (IPSAQ).38 The original IPSAQ consists of 32 items describing 16 positive and 16 negative self-referent social situations. Participants are required to put themselves in the position of someone experiencing a certain event and to write down the most likely cause for the situation. The participant then has to estimate the relative contribution of internal, external-personal, or situational factors in percentages. The original task contains satisfactory psychometric properties.38 For the present investigation, we split the original item set into 2 parallel versions of each 16 items.
Nonverbal Memory
The Rey–Osterrieth and Taylor Complex Figure Tests were employed to measure nonverbal memory. The participant is first required to copy a complex figure. Immediately thereafter and 20 min later, the figure has to be redrawn from memory.39
Psychophysiological Responses
During the entire trial, the heart rate (beats per minute) was measured every 2 min via a wireless signal transmission device.
Strategy of Data Analysis
We adopted a mixed ANOVA with Condition (stress vs no-stress) as the within-subject factor and Group (schizophrenia, healthy) and Sequence (stress applied first or second) as the between-subject factors. These 3 factors will be referred to as the standard set of factors in the following and complemented by additional factors depending on the experimental variable. The report of significant analyses will be confined to the core effects involving the Group factor.
Results
Sociodemographic and Clinical Variables
As can be seen in table 1, the groups did not differ with respect to any of the background variables. Patients had elevated CAPE scores which were in the expected range according to prior studies.40 The baseline stress level, as assessed with the PSS, was elevated in patients.
Effect of Stress on Psychopathology
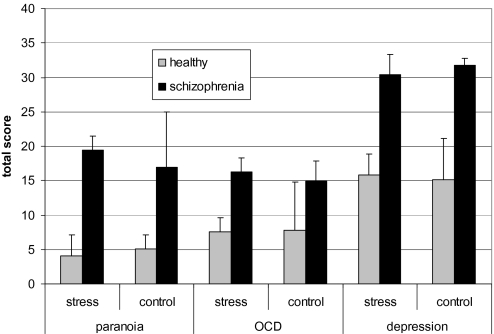
A 4-way mixed ANOVA was computed: Psychopathology (Paranoia Checklist, OCI-R, GDS) and Condition (stress vs no-stress) served as the within-subject factors and Group (schizophrenia, healthy) and Sequence (stress condition applied first or second) were the between-subject factors. As expected, the main effect of Group was significant, F(1,31) = 16.18, P < .001, η2partial = .34. As can be derived from figure 1, the schizophrenia group had overall higher scores than the healthy participants. In addition, the Group × Sequence interaction was significant, F(1,31) = 4.62, P = .01, η2partial = .13: Patients in the stress first condition had overall higher psychopathology scores than in the stress second condition. This was qualified by a significant 3-way interaction of Psychopathology × Condition × Group, F(2,62) = 4.55, P = .01, η2partial = .13. Figure 1 shows that patients were more paranoid under stress than under no-stress, and this was slightly opposite for healthy participants. For depression, this response pattern was inverted: Stress led to an increase of depression in healthy participants and a decrease of depression in patients. Results remained essentially unchanged when the patient sample was split into acute vs remitted participants.
Fig. 1.
The 3-Way ANOVA Interaction of Group × Psychopathology × Condition Turned Out to be Significant. While paranoid symptoms somewhat decreased in healthy participants under stress, stress elevated paranoia in the schizophrenia sample. A slightly opposite response pattern was found for depressive symptoms. Results are displayed as means and SEs.
Effect of Stress on Reasoning Biases
For the beads task, the Group effect was significant, F(1,31) = 4.06, P = .05, η2partial = .12. As expected, overall more JTC occurred in the patient group. When we split the sample into acute vs remitted patients, the Group × Condition interaction was significant, F(2,29) = 5.90, P = .007, η2partial = .29: acute patients showed an increase of JTC in the stress relative to the no-stress condition (43% vs 0%), whereas other participants showed the opposite response pattern (remitted: 15% vs 31%; healthy: 0% vs 7%). There was a trend for a 3-way interaction involving Sequence, F(2,29) = 3.04, P = .06, η2partial = .17, indicating that this response pattern was more pronounced for the stress first condition.
For the BADE, none of the effects involving Group achieved significance (P > .3, η2partial < .04).
For the Internal, Personal, and Situational Attributions Questionnaire (IPSAQ-R), we added 2 within-subject factors to the standard set of factors: Scenario (positive, negative) and Attribution (“I,” “others”; the percentage for the response option “circumstances” was not entered as an additional step as it can be derived from the percentages of the 2 other steps). The 3-way interaction of Group × Condition × Attribution was significant, F(1,31) = 6.94, P = .01, η2partial = .18. Healthy participants attributed events (irrespective of valence) less often to others, whereas the patients considered others more responsible under stress than without stress. Results remained essentially unchanged when the patient sample was split into acute vs remitted participants.
Nonverbal Memory
For the memory test, we added another within-subject factor to the standard set of factors: Reproduction Trial (copy figure, immediate, and delayed recall). The main effect of Group achieved significance, F(1,31) = 0.18, P = .005, η2partial = .23, reflecting decreased overall performance by the patients. This was qualified by a Group × Reproduction Trial interaction, F(2,62) = 6.91, P = .002, η2partial = .18: While the groups performed similar on the copy trial, patients were far worse for the 2 recall trials. Results remained essentially unchanged when the patient sample was split into acute vs remitted participants.
Heart Rate
For analyzing the heart rate data, we added Time (first vs final heart rate) as additional factor to the standard set of factors. Only the Group factor achieved significance, F(1,31) = 7.59, P = .01, η2partial = .20: Patients (M = 89.79) had higher heart rates than controls (M = 78.96). Results remained essentially unchanged when the patient sample was split into acute vs remitted participants. The mean heart rates were significantly correlated with the respective subjective stress rating and the PSS (at least r = .35, P < .05).
VAS
In addition to the standard set of factors, Trial (6 successive measurements per condition) was incorporated for the analysis of subjective stress. Lending support to the validity of the paradigm, the effect of Condition was significant, F(1,31) = 12.34, P = .001, η2partial = .28: The stress condition was perceived as more stressful (M = 3.79) than the no-stress condition (M = 2.58). The effect of Group, F(1,31) = 24.15, P < .001, η2partial = .44, but not the interaction of Group × Condition was significant, P > .5, η2partial = .01: Patients were overall more stressed by the experimental situation than controls (M = 4.46 vs 1.91). Results remained essentially unchanged when the patient sample was split into acute vs remitted participants: acute patients were not significantly more stressed (M = 4.95) than remitted patients (M = 4.29, t(18) = 0.66, P > .5).
Discussion
The present study aimed to shed light on the interrelationships of paranoid ideation, stress, and cognitive biases in schizophrenia. As expected, stress increased paranoid symptoms in schizophrenia patients, irrespective of symptom status. In marked contrast, stress somewhat diminished subclinical paranoid symptoms in the healthy participants. It appears as if the healthy participants (correctly) attributed any psychological changes during the stress condition to contextual factors (ie, noise).
The study confirms prior findings15,41 indicating that patients with schizophrenia are hastier in their decision making than controls. As expected,22,23 this response pattern was increased under stress, particularly in acute patients and—in line with another study42—when stress was applied first. Moreover, stress increased external-personal attributions in schizophrenia patients, as measured with the IPSAQ-R, which was not the case in healthy subjects.
Whether the observed effects of stress on JTC are primary or secondary due to stress regulation strategies (eg, hasty decision making in order to decrease cognitive load) needs further exploration. In a prior study, we inferred that the association between anxiety and paranoia was moderated by the extent of baseline symptoms and mediated by an increase in the tendency to jump to conclusions: Threat-related interpretations, evoked by state anxiety, will be more rapidly accepted in the presence of a tendency to jump to conclusions. The excess of external-personal responses in schizophrenia patients on the IPSAQ-R under stress could reflect source confusion: tension and arousal exerted by stress may have been misattributed as tension due to other persons. If this speculation holds true, potential misattributions may become even more prevalent in a real-life stressful situation, in which other people are directly involved in contrast to a laboratory situation. We also suspect that the impact of stress on cognitive biases will be most pronounced when stressors are less explicit. Stress obviously did not affect all cognitive parameters similarly: neither the BADE nor nonverbal memory was differentially affected by stress in the 2 groups.
Subjective stress appraisal at baseline and during the 2 conditions as well as heart rates revealed that patients felt generally more stressed than healthy participants. This result supports the clinical observation that patients experience even minor challenges as stressful which has been associated with enhanced emotional reactivity.6 Although the heart rate was significantly correlated with subjective stress parameters, it cannot entirely be ruled out that some heart rate increase was caused by antipsychotic medication.43
Several shortcomings of the present study must be conceded. First, the sample was small although our within-subject design has advantages over cross-sectional designs regarding power. We are currently conducting a larger trial which will also allow to look for moderating and mediating variables as in a previous study on subclinical paranoia.19 Secondly, we only used one stressor. Thus, further investigations should clarify whether stress in general or specific stressors are pertinent for the emergence and/or maintenance of symptoms and the aggravation of cognitive biases.
To conclude, the results suggest that sensory stress selectively aggravates decision-making and attributional biases as well as psychotic symptoms in (acute) schizophrenia patients. In contrast, stress somewhat increased depressive responses and decreased external attributions in healthy subjects. If replicated, interventions aimed at relapse prevention might benefit from including emotion regulation strategies and teaching patients ways to counter cognitive biases under stress.44
Acknowledgments
Declaration of interests: None. The present study was part of the diploma theses of P.B. and S.S.
References
- 1.Nuechterlein KH, Dawson ME. A heuristic vulnerability/stress model of schizophrenic episodes. Schizophr Bull. 1984;10:300–312. doi: 10.1093/schbul/10.2.300. [DOI] [PubMed] [Google Scholar]
- 2.Myin-Germeys I, Van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. doi: 10.1016/j.cpr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Cutting LP, Aakre JM, Docherty NM. Schizophrenic patients' perceptions of stress, expressed emotion, and sensitivity to criticism. Schizophr Bull. 2006;32:743–750. doi: 10.1093/schbul/sbl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiser M, Van Os J, Reichenberg A, et al. Social and cognitive functioning, urbanicity and risk for schizophrenia. Br J Psychiatry. 2007;191:320–324. doi: 10.1192/bjp.bp.106.031328. [DOI] [PubMed] [Google Scholar]
- 5.Cantor-Graae E, Selten J-P. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 6.Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- 7.Lincoln TM, Wilhelm K, Nestoriuc Y. Effectiveness of psychoeducation for relapse, symptoms, knowledge, adherence and functioning in psychotic disorders: a meta-analysis. Schizophr Res. 2007;96:232–245. doi: 10.1016/j.schres.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Lincoln TM, Ziegler M, Mehl S, Rief W. The Jumping to Conclusions bias in delusions: specificity and changeability. J Abnorm Psychol. 2010;119:40–49. doi: 10.1037/a0018118. [DOI] [PubMed] [Google Scholar]
- 9.Moritz S, Woodward TS. Jumping to conclusions in delusional and non-delusional schizophrenic patients. Br J Clin Psychol. 2005;44:193–207. doi: 10.1348/014466505X35678. [DOI] [PubMed] [Google Scholar]
- 10.Garety PA, Hemsley DR, Wessely S. Reasoning in deluded schizophrenic and paranoid patients. Biases in performance on a probabilistic inference task. J Nerv Ment Dis. 1991;179:194–201. doi: 10.1097/00005053-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lincoln TM, Mehl S, Exner C, Lindenmeyer J, Rief W. Attributional style and persecutory delusions. Evidence for an event independent and state specific external-personal attribution bias for social situations. Cognit Ther Res. 2010;34:297–302. [Google Scholar]
- 12.Kinderman P, Bentall RP. A new measure of causal locus: the Internal, Personal and Situational Attributions Questionnaire. Pers Individ Dif. 1996;20:261–264. [Google Scholar]
- 13.Bentall RP, Kaney S, Dewey ME. Paranoia and social reasoning: an attribution theory analysis. Br J Clin Psychol. 1991;30:13–23. doi: 10.1111/j.2044-8260.1991.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 14.Bell V, Halligan PW, Ellis HD. Explaining delusions: a cognitive perspective. Trends Cogn Sci. 2006;10:219–226. doi: 10.1016/j.tics.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Freeman D. Suspicious minds: the psychology of persecutory delusions. Clin Psychol Rev. 2007;27:425–457. doi: 10.1016/j.cpr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. Br J Clin Psychol. 1999;38:113–154. doi: 10.1348/014466599162700. [DOI] [PubMed] [Google Scholar]
- 17.van der Gaag M. A neuropsychiatric model of biological and psychological processes in the remission of delusions and auditory hallucinations. Schizophr Bull. 2006;32:113–122. doi: 10.1093/schbul/sbl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mujica-Parodi LR, Corcoran C, Greenberg T, Sackeim HA, Malaspina D. Are cognitive symptoms of schizophrenia mediated by abnormalities in emotional arousal? CNS Spectr. 2002;7:58–69. doi: 10.1017/s1092852900022276. [DOI] [PubMed] [Google Scholar]
- 19.Lincoln TM, Peter N, Schäfer M, Moritz S. Impact of stress on paranoia: an experimental study of moderators and mediators. Psychol Med. 2009;7:1129–1139. doi: 10.1017/S0033291708004613. [DOI] [PubMed] [Google Scholar]
- 20.Lincoln TM, Peter N, Schäfer M, Moritz S. From stress to paranoia: an experimental investigation of the moderating and mediating role of reasoning biases. Psychol Med. 2010;40:169–173. doi: 10.1017/S003329170999095X. [DOI] [PubMed] [Google Scholar]
- 21.Lincoln TM, Lange J, Burau J, Exner C, Moritz S. The effect of state anxiety on paranoid ideation and jumping to conclusions. An experimental investigation. Schizophr Bull. 2010;36:1140–1148. doi: 10.1093/schbul/sbp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moritz S, Veckenstedt R, Randjbar S, et al. Decision making under uncertainty and mood induction: further evidence for liberal acceptance in schizophrenia. Psychol Med. 2009;39:1821–1829. doi: 10.1017/S0033291709005923. [DOI] [PubMed] [Google Scholar]
- 23.Ellett L, Freeman D, Garety PA. The psychological effect of an urban environment on individuals with persecutory delusions: the Camberwell walk study. Schizophr Res. 2008;99:77–84. doi: 10.1016/j.schres.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Moritz S, Veckenstedt R, Hottenrott B, Woodward TS, Randjbar S, Lincoln TM. Different sides of the same coin? Intercorrelations of cognitive biases in schizophrenia. Cogn Neuropsychiatry. doi: 10.1080/13546800903399993. In press. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 28.Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- 29.Freeman D, Garety PA, Bebbington PE, et al. Psychological investigation of the structure of paranoia in a non-clinical population. Br J Psychiatry. 2005;186:427–435. doi: 10.1192/bjp.186.5.427. [DOI] [PubMed] [Google Scholar]
- 30.Foa EB, Huppert JD, Leiberg S, et al. The Obsessive-Compulsive Inventory: development and validation of a short version. Psychol Assess. 2002;14:485–496. [PubMed] [Google Scholar]
- 31.Abramowitz JS, Tolin D, Diefenbach G. Measuring change in OCD: sensitivity of the Obsessive-Compulsive Inventory-Revised. J Psychopathol Behav Assess. 2005;27:317–324. [Google Scholar]
- 32.Abramowitz JS, Deacon BJ. Psychometric properties and construct validity of the Obsessive-Compulsive Inventory-Revised: replication and extension with a clinical sample. J Anxiety Disord. 2006;20:1016–1035. doi: 10.1016/j.janxdis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Gönner S, Leonhart R, Ecker W. The Obsessive-Compulsive Inventory-Revised (OCI-R): validation of the German version in a sample of patients with OCD, anxiety disorders, and depressive disorders. J Anxiety Disord. 2008;22:734–749. doi: 10.1016/j.janxdis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Hautzinger M, Brähler M. Allgemeine Depressionsskala (ADS) [General Depression Scale] Göttingen, Germany: Hogrefe; 1993. [Google Scholar]
- 35.Woodward TS, Munz M, LeClerc C, Lecomte T. Change in delusions is associated with change in ‘‘jumping to conclusions”. Psychiatry Res. 2009;170:124–127. doi: 10.1016/j.psychres.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Woodward TS, Buchy L, Moritz S, Liotti M. A bias against disconfirmatory evidence is associated with delusion proneness in a nonclinical sample. Schizophr Bull. 2007;33:1023–1028. doi: 10.1093/schbul/sbm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodward TS, Moritz S, Menon M, Klinge R. Belief inflexibility in schizophrenia. Cogn Neuropsychiatry. 2008;13:267–277. doi: 10.1080/13546800802099033. [DOI] [PubMed] [Google Scholar]
- 38.Kinderman P, Bentall RP. Self-discrepancies and persecutory delusions: evidence for a model of paranoid ideation. J Abnorm Psychol. 1996;105:106–113. doi: 10.1037//0021-843x.105.1.106. [DOI] [PubMed] [Google Scholar]
- 39.Osterrieth PA. Le test de copie d'une figure complexe; contribution a l'etude de la perception et de la memoire (Test of copying a complex figure; contribution to the study of perception and memory) Arch Psychol. 1944;30:206–356. [Google Scholar]
- 40.Moritz S, Peters MJV, Karow A, Deljkovic A, Naber D. Cure or curse? Ambivalent attitudes towards neuroleptic medication in schizophrenia and non-schizophrenia patients. Mental Illness. 2009;1:4–9. doi: 10.4081/mi.2009.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine C, Gardner M, Craigie J, Gold I. Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn Neuropsychiatry. 2007;12:46–77. doi: 10.1080/13546800600750597. [DOI] [PubMed] [Google Scholar]
- 42.Keefe K, Warman D. Reasoning, delusion-proneness, and anxiety: an experimental investigation. Clin Psychol Psychother. doi: 10.1002/cpp.683. [DOI] [PubMed] [Google Scholar]
- 43.Agelink MW, Majewski T, Wurthmann C, et al. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. J Clin Psychopharmacol. 2001;21:8–13. doi: 10.1097/00004714-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Moritz S, Vitzthum F, Veckenstedt R, Randjbar S, Woodward TS. Detecting and defusing cognitive traps: metacognitive intervention in schizophrenia. Current Opinion in Psychiatry. doi: 10.1097/YCO.0b013e32833d16a8. In press. [DOI] [PubMed] [Google Scholar]