Background
Proponents of early intervention have argued that outcomes might be improved if more therapeutic efforts were focused on the early stages of schizophrenia or on people with prodromal symptoms. Early intervention in schizophrenia has 2 elements that are distinct from standard care: early detection and phase-specific treatment (phase-specific treatment is a psychological, social, or physical treatment developed, or modified, specifically for use with people at an early stage of the illness).
Early detection and phase-specific treatment may both be offered as supplements to standard care or may be provided through a specialized early intervention team. Early intervention is now well established as a therapeutic approach in America, Europe, and Australasia.
Objectives
To evaluate the effects of: (a) early detection, (b) phase-specific treatments, and (c) specialized early intervention teams in the treatment of people with prodromal symptoms or first-episode psychosis.
Search Methods
We searched the Cochrane Schizophrenia Group Trials Register (March 2009), inspected reference lists of all identified trials and reviews, and contacted experts in the field.
Selection Criteria
We included all randomized controlled trials (RCTs) designed to prevent progression to psychosis in people showing prodromal symptoms or to improve outcome for people with first-episode psychosis. Eligible interventions, alone and in combination, included: early detection, phase-specific treatments, and care from specialized early intervention teams. We accepted cluster-randomized trials but excluded nonrandomized trials.
Data Collection and Analysis
We reliably selected studies, quality rated them, and extracted data. For dichotomous data, we estimated relative risks (RR), with the 95% CIs. Where possible, we calculated the number needed to treat/harm statistic (NNT/H) and used intention-to-treat analysis.
Results
Studies were diverse, mostly small, undertaken by pioneering researchers and with many methodological limitations (18 RCTs, total n = 1808). Mostly, meta-analyses were inappropriate. For the 6 studies addressing prevention of psychosis for people with prodromal symptoms, olanzapine seemed of little benefit (n = 60, 1 RCT, RR conversion to psychosis 0.58 CI 0.3–1.2) and cognitive behavioral therapy (CBT) equally so (n = 60, 1 RCT, RR conversion to psychosis 0.50 CI 0.2–1.7). A risperidone plus CBT plus specialized team did have benefit over specialist team alone at 6 months (n = 59, 1 RCT, RR conversion to psychosis 0.27 CI 0.1–0.9, NNT 4 CI 2–20), but this was not seen by 12 months (n = 59, 1 RCT, RR 0.54 CI 0.2–1.3). Omega 3 fatty acids (eicosapentaenoic acid; EPA) had advantage over placebo (n = 76, 1 RCT, RR transition to psychosis 0.13 CI 0.02–1.0, NNT 6 CI 5–96). We know of no replications of this finding.
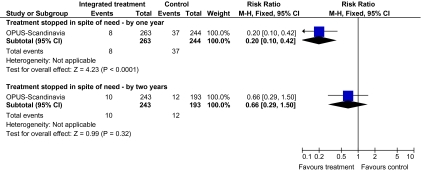
The remaining trials aimed to improve outcome in first-episode psychosis. Phase-specific CBT for suicidality seemed to have little effect, but the single study was small (n = 56, 1 RCT, RR suicide 0.81 CI 0.05–12.26). Family therapy plus a specialized team in the Netherlands did not clearly affect relapse (n = 76, RR 1.05 CI 0.4–3.0), but without the specialized team in China, it may (n = 83, 1 RCT, RR admitted to hospital 0.28 CI 0.1–0.6, NNT 3 CI 2–6). The largest and highest quality study compared specialized team with standard care (table 1). Leaving the study early was reduced (n = 547, 1 RCT, RR 0.59 CI 0.4–0.8, NNT 9 CI 6–18) and compliance with treatment improved (n = 507, RR stopped treatment 0.20 CI 0.1–0.4, NNT 9 CI 8–12, figure 1). The mean number of days spent in hospital at 1 year were not significantly different (n = 507, weighted mean difference, −1.39 CI −2.8–0.1), neither were data for “Not hospitalized” by 5 years (n = 547, RR 1.05 CI 0.90–1.2). There were no significant differences in numbers “not living independently” by 1 year (n = 507, RR 0.55 CI 0.3–1.2). At 5 years significantly fewer participants in the treatment group were not living independently (n = 547, RR 0.42 CI 0.21–0.8, NNT 19 CI 14–62). When phase-specific treatment (CBT) was compared with befriending no significant differences emerged in the number of participants being hospitalized over the 12 months (n = 62, 1 RCT, RR 1.08 CI 0.59–1.99).
Table 1.
Summary of Findings Table
| SPECIALIZED TEAM compared with STANDARD CARE for psychosis | ||||||
| Patient or population: patients with psychosisSettings: ScandinaviaIntervention: SPECIALIZED TEAMComparison: STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) | Number of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| STANDARD CARE | SPECIALIZED TEAM | |||||
| Compliance with treatment—treatment stopped in spite of need—by 1 y Follow-up: 12 mo | Low-risk populationa | RR 0.2 (0.1–0.42) | 507 (1 study) |
 moderateb moderateb
|
||
| 100 per 1000 | 20 per 1000 (10–42) | |||||
| Medium-risk populationa | ||||||
| 150 per 1000 | 30 per 1000 (15–63) | |||||
| High-risk populationa | ||||||
| 200 per 1000 | 40 per 1000 (20–84) | |||||
| Compliance with treatment—treatment stopped in spite of need—by 2 y Follow-up: 24 mo | Low-risk populationa | RR 0.66 (0.29–1.5) | 436 (1 study) |
 moderateb moderateb
|
||
| 20 per 1000 | 13 per 1000 (6–30) | |||||
| Medium-risk populationa | ||||||
| 60 per 1000 | 40 per 1000 (17–90) | |||||
| High-risk populationa | ||||||
| 100 per 1000 | 66 per 1000 (29–150) | |||||
| Service use: 1. Average mean number of days per month in hospital—by 5 y Follow-up: 5 y | The mean Service use: 1. Average mean number of days per month in hospital—by 5 y in the intervention groups was 1.11 lower (3.21 lower to 0.99 higher) | 547 (1 study) |
 moderateb moderateb
|
|||
| Service use: 2. Not hospitalized—by 5 y Follow-up: 5 y | Low-risk populationa | RR 1.05 (0.9–1.22) | 547 (1 study) |
 moderateb moderateb
|
||
| 300 per 1000 | 315 per 1000 (270–366) | |||||
| Medium-risk populationa | ||||||
| 500 per 1000 | 525 per 1000 (450–610) | |||||
| High-risk populationa | ||||||
| 700 per 1000 | 735 per 1000 (630–854) | |||||
| Social outcomes: 1. Not living independently—by 5 yFollow-up: 5 y | Low-risk populationa | RR 0.42 (0.21–0.83) | 547 (1 study) |
 moderateb moderateb
|
||
| 50 per 1000 | 21 per 1000 (10–41) | |||||
| Medium-risk populationa | ||||||
| 100 per 1000 | 42 per 1000 (21–83) | |||||
| High-risk populationa | ||||||
| 150 per 1000 | 63 per 1000 (31–124) | |||||
| Social outcomes: 2. Not working or in education—by 5 yFollow-up: 5 y | Low-risk populationa | RR 1.06 (0.92–1.23) | 547 (1 study) |
 moderateb moderateb
|
||
| 200 per 1000 | 212 per 1000 (184–246) | |||||
| Medium-risk populationa | ||||||
| 500 per 1000 | 530 per 1000 (460–615) | |||||
| High-risk populationa | ||||||
| 800 per 1000 | 848 per 1000 (736–984) | |||||
*The basis for the “assumed risk” (eg, the median control group risk across studies) is provided in footnotes. The “corresponding risk” (and its 95% CI) is based on the assumed risk in the comparison group and the “relative effect” of the intervention (and its 95% CI).RR: Risk ratio
GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect.Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.Very low quality: We are very uncertain about the estimate.
Medium control risk is that of the control group of the trial.
Limitations in design: rated “Serious.” There were limitations but rating serious may be harsh (but limited choice). Attempts were made to address these all though the study. Blinding not clear. Selective reporting possible.
Fig. 1.
Comparison—specialized team vs standard care outcome—compliance with treatment
Phase-specific treatment EPA oils suggested no benefit (n = 80, 1 RCT, RR no response 0.90 CI 0.6–1.4) as did phase-specific treatment brief intervention (n = 106, 1 RCT, RR admission 0.86 CI 0.4–1.7). Phase-specific active cognitive therapy for early psychosis found no benefit but participants given vocational intervention were more likely to be employed (n = 41, 1 RCT, RR 0.39 CI 0.21–0.7, NNT 2 CI 2–4). Phase-specific cannabis and psychosis therapy did not show benefit (n 47, RR cannabis use 1.30 CI 0.8–2.2), and crisis assessment did not reduce hospitalization (n = 98, RR 0.85 CI 0.6–1.3). Weight was unaffected by early behavioral intervention.
Authors' Conclusions
There is emerging, but as yet inconclusive evidence, to suggest that people in the prodrome of psychosis can be helped by some interventions. There is some support for specialized early intervention services, but further trials would be desirable, and there is a question of whether gains are maintained. There is some support for phase-specific treatment focused on employment and family therapy, but again, this needs replicating with larger and longer trials. Full details are published in the Cochrane Review.1
Funding
The original version of the review was funded by a grant in 2000 from the NHS Nationally Commissioned R&D Programme.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011, doi: 10.1002/14651858.CD004718.pub3. Issue 6. Art. No.: CD004718. DOI: 10.1002/14651858.CD004718.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]