Abstract
The goal of the study is to determine the extent of structural brain abnormalities in a multicenter sample of children and adolescents with a recent-onset first episode of psychosis (FEP), compared with a sample of healthy controls. Total brain and lobar volumes and those of gray matter (GM), white matter, and cerebrospinal fluid (CSF) were measured in 92 patients with a FEP and in 94 controls, matched for age, gender, and years of education. Male patients (n = 64) showed several significant differences when compared with controls (n = 61). GM volume in male patients was reduced in the whole brain and in frontal and parietal lobes compared with controls. Total CSF volume and frontal, temporal, and right parietal CSF volumes were also increased in male patients. Within patients, those with a further diagnosis of “schizophrenia” or “other psychosis” showed a pattern similar to the group of all patients relative to controls. However, bipolar patients showed fewer differences relative to controls. In female patients, only the schizophrenia group showed differences relative to controls, in frontal CSF. GM deficit in male patients with a first episode correlated with negative symptoms. Our study suggests that at least part of the GM deficit in children and adolescent-onset schizophrenia and in other psychosis occurs before onset of the first positive symptoms and that, contrary to what has been shown in children-onset schizophrenia, frontal GM deficits are probably present from the first appearance of positive symptoms in children and adolescents.
Keywords: MRI, first-episode psychosis, early-onset psychosis, brain morphometrics
Introduction
Patients with first-episode early-onset psychosis (first episode before 18 years of age with an onset of less than 6 months of positive symptoms before baseline assessment) may constitute a quite homogenous group to study, regarding the absence of course-related effects, such as duration and treatment. Cross-sectional magnetic resonance imaging (MRI) studies have demonstrated that childhood-onset schizophrenia is associated with smaller whole-brain and total gray matter (GM) volume, thinner cortical thickness, and larger ventricular volume than controls.1–4 Longitudinal studies have shown a back-to-front wave of GM loss in children with schizophrenia resembling the normal development pattern but in an exaggerated manner.5,6 In particular, parietal and motor cortex deficits have been shown at baseline while prefrontal, supplementary motor, sensorimotor, parietal, and temporal deficits appeared or increased the deficit over time.5 The few studies that have scanned adolescent-onset schizophrenia patients show decreased frontal (but not parietal or temporal) GM volumes and increased total and frontal cerebrospinal fluid (CSF) at onset.7–9
The specificity of these findings for schizophrenia has not been thoroughly investigated. Bipolar disorder patients frequently share many of the psychotic symptoms associated with schizophrenia. Thus, analysis of structural abnormalities in bipolar patients with psychotic symptoms and schizophrenia patients will shed light on the specificity of brain changes in the context of pathological processes related to psychosis.
The present study builds on earlier reports demonstrating brain abnormalities in adolescent-onset psychosis patients with a recent-onset first episode of psychosis (FEP).7,10 The objective was now to extend the study to a much larger, independent, and representative sample by recruiting subjects from 5 psychiatric units. Our sample includes a matched comparison group of healthy children and adolescents from each participating center to control for potential demographic factors known to affect brain morphometry. The main hypothesis of the study was that children and adolescents with first psychotic episodes will show volumetric differences in GM and CSF of the main brain lobes, particularly in the frontal. Furthermore, the goal of this article using a larger data set is to allow for subdivision of patients into diagnostic groups within psychosis.
Methods
Subjects
The sample used in this study consisted of 92 patients (28 females) and 94 controls (33 females), participating in the Child and Adolescent First-Episode Psychosis Study (CAFEPS).11 CAFEPS included patients from 6 different psychiatric units.
The inclusion criteria for patients were age between 7 and 17 years at the time of first evaluation and positive psychotic symptoms (within a psychotic episode) such as delusions or hallucinations of less than 6-month duration. Exclusion criteria were a concomitant Axis I disorder at the time of evaluation that might account for the psychotic symptoms, mental retardation as per the Diagnastic and Statistical Manual of Mental Disordes (Fourth Edition) (DSM-IV) criteria, pervasive developmental disorder, neurological disorders, history of head trauma with loss of consciousness, pregnancy, and drug-induced psychosis. When drugs were positive in the urine baseline assessment, we included the patient in the study if symptoms persisted 2 weeks after a negative urine test. Eight patients and 1 control fulfilled DSM-IV criteria for cannabis abuse. All the patients maintained psychotic symptoms 15 days after a negative urine test and did not fulfill criteria for drug-induced psychosis. Only 1 patient at recruitment abused cocaine, 1 abused alcohol, and 1 abused amphetamines. None of them fulfilled criteria for drug-induced psychosis. A detailed description of recruitment criteria, clinical and demographic characteristics, and methodology has been provided elsewhere.11
Diagnosis was confirmed according to the DSM-IV criteria using the Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime version (K-SADS-PL), Spanish adaptation.12 DSM-IV criteria were applied also at 1-year follow-up. Psychopathology was assessed with the Positive and Negative Syndrome Scale (PANSS), and duration of untreated psychosis was calculated at the first interview by asking about the first appearance ever of positive psychotic symptoms within a psychotic episode. At 1 year of follow up, 3 main diagnostic categories were established in the FEP group: schizophrenia, psychotic bipolar disorder, and other psychoses (including schizoaffective disorder: 7, 16.3%; psychotic disorder not otherwise specified: 15, 34.9%; depressive disorder with psychotic symptoms: 6, 14.0%; schizophreniform disorder: 6, 14.0%; brief psychotic disorder: 3, 7.0%; obsessive compulsive disorder with psychotic symptoms: 2, 4.7%; 4, 9.35% without specific diagnostic because the 1-year reassessment could not be performed.
Parental socioeconomic status was assessed with the Hollingshead-Redlich scale. The study was approved by the Ethics and Clinical Research Boards of all hospitals involved in the study. After complete description of the study to the subjects, written informed consent was obtained. All controls and patients met MRI safety criteria.
MRI Acquisition
Five different scanning facilities contributed data to the study: 2 Siemens Symphony, 2 General Electric Signa, and 1 Philips ACS Gyroscan, all 1.5-T scanners. One site (first site in table 2) received patients and controls from 2 Psychiatric Units that were located in the same city. Data were collected from each center and processed at one site.13 Two MRI sequences were acquired in axial orientation for each subject, a T1-weighted 3D gradient echo (voxel size 1 × 1 × 1.5 mm) and a T2-weighted Turbo-Spin-Echo (voxel size 1 × 1 × 3.5 mm). Full details about the acquisition parameters at each site, comparability between machines for this study, and the limitations involved in the analysis of multicenter data are provided in Reig et al.13 To minimize the effect of the multicenter design, the sample of patients and controls were matched within each of the 5 contributing institutions. The number of subjects scanned at each site are provided in table 1.
Table 2.
Volume Measurements (in cm3) of Controls and Patients
| Controls |
Patients |
Controls/Patients (Males)a | |||||||
| Females (n= 33) |
Males (n= 61) |
Females (n= 28) |
Males (n = 64) |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (y) | 15.4 | 1.8 | 15.4 | 1.9 | 15.3 | 1.9 | 15.8 | 1.9 | |
| Intracranial volume | 1432.8 | 103.0 | 1540.3 | 141 | 1366.8 | 114 | 1563.6 | 133 | |
| Total GM | 769.8 | 71.3 | 847.9 | 87.9 | 724.8 | 88.0 | 832.2 | 81.3 | *** |
| Total WM | 379.9 | 39.3 | 410.9 | 53.2 | 376.8 | 67.0 | 417.1 | 47.4 | |
| Total CSF | 283.0 | 45.4 | 281.4 | 50.3 | 264.5 | 63.3 | 314.1 | 60.2 | *** |
| GM | |||||||||
| Frontal, left | 70.8 | 8.9 | 80.2 | 10.3 | 67.9 | 8.1 | 75.8 | 9.2 | *** |
| Frontal, right | 73.6 | 9.0 | 83.7 | 10.8 | 70.9 | 9.8 | 77.9 | 9.9 | *** |
| Parietal, left | 58.5 | 7.9 | 64.2 | 8.5 | 54.8 | 8.8 | 62.4 | 8.7 | * |
| Parietal, right | 59.0 | 7.7 | 64.5 | 8.9 | 55.4 | 10.0 | 62.0 | 8.0 | *** |
| Temporal, left | 77.1 | 7.3 | 84.4 | 8.4 | 72.1 | 10.1 | 84.9 | 9.1 | |
| Temporal, right | 76.9 | 7.5 | 83.1 | 8.5 | 72.1 | 10.2 | 84.7 | 9.1 | |
| CSF | |||||||||
| Frontal, left | 28.2 | 5.9 | 27.5 | 7.0 | 26.6 | 9.0 | 31.9 | 8.3 | *** |
| Frontal, right | 29.9 | 6.2 | 28.5 | 6.8 | 28.0 | 9.3 | 33.6 | 8.6 | *** |
| Parietal, left | 24.9 | 6.3 | 23.4 | 6.7 | 22.4 | 7.2 | 26.0 | 6.1 | |
| Parietal, right | 22.7 | 5.5 | 22.3 | 6.5 | 21.0 | 8.2 | 25.5 | 6.5 | ** |
| Temporal, left | 23.2 | 3.9 | 23.0 | 4.0 | 21.7 | 5.1 | 25.8 | 5.2 | *** |
| Temporal, right | 21.9 | 3.8 | 21.4 | 4.4 | 20.1 | 4.5 | 24.3 | 5.7 | *** |
Note: GM, gray matter; WM, white matter; CSF, cerebrospinal fluid.
ANCOVA of male controls vs patients using residuals after correction for ICV, age, and scanner factors. No differences observed in females.
***P < .001; **P < .01; *P < .05.
Table 1.
Sociodemographic and Clinical Characteristics of Patients and Controls
| Controls (n = 94) | Patients (n = 92) | ||
| Age (y) (mean, range, SD) | 15.4, 19–9, 1.4 | 15.7, 18–9, 1.7 | NS |
| Gender (male/female) | 61/33 | 64/28 | NS |
| Education (y) (mean, SD) | 8.8, 1.4 | 8.3, 2.0 | NS |
| Parental socioeconomic status (1/2/3/4/5) | 10/23/24/11/26 | 19/29/21/11/12 | NS |
| Handedness: right/left/mixed | 87/5/2 | 85/7/0 | NS |
| Race/ethnicity (Caucasian/Hispanic/Black-African/Other) | 86/6/2/0 | 82/5/1/4 | NS |
| Duration of illness (mo) (mean, SD) | 2.1, 1.7 | ||
| Diagnosis (schizophrenia/bipolar/other psychoses) | 31/18/43 | ||
| Duration of treatment (wk) (mean, range, SD) | 4.8, 65.0–0, 9.5 | ||
| Treatment:quetiapine/olanzapine/risperidone(mg, mean, SD) | 21/23/48, 211.0/196.2/10.7, 5.0/3.4/1.6 | ||
| Subjects per site | 46/28/9/7/4 | 48/21/5/12/6 | NS |
Note: NS: nonsignificant differences.
Segmentation and Region of Interest Definition
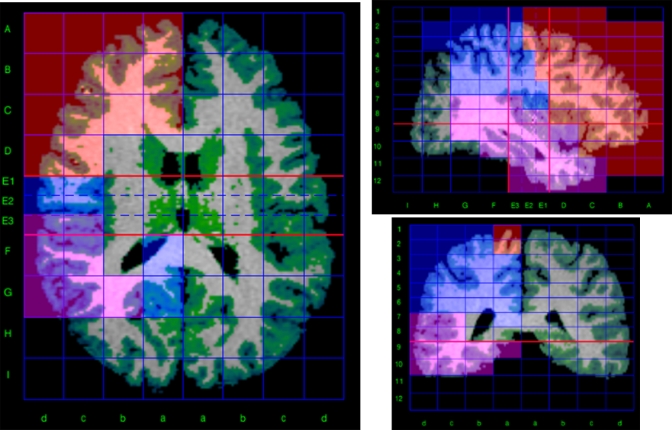
MRI images were processed using locally developed software incorporating a variety of image processing and quantification tools.13,14 To obtain volume measurements of the main brain lobes, we used a method for semiautomated segmentation of the brain based on the Talairach proportional grid system, performed in native space.15,16 Basically, a 2-step procedure was followed.14 First, an initial segmentation of cerebral tissues into GM, white matter (WM), and CSF was obtained using SPM2 (Statistical Parametric Mapping; Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) routines for multimodal (T1 and T2) segmentation. Because of the multicenter setup, multimodal tissue segmentation is more robust than single modality (T1 only) with regards to image contrast–related differences between sites. This assumption was corroborated in a previous study in which the repeatability of multimodal (T1 and T2) and T1-only segmentation was compared among the 5 scanners using the same methodology as in this study.13 The SPM algorithm for tissue segmentation includes a method to eliminate the effect of radio frequency field inhomogeneities.17 In a second step, the Talairach grid was built on the edited brain MRI by manually selecting the position of the anterior and posterior commissures (AC and PC) and a third point in the midsagittal plane. The coordinates of these points serve to calculate the transformation (rigid rotation) required to comply with the Talairach orientation: to set the AC-PC line in the axial horizontal plane and the interhemispheric plane in the vertical orientation.18 Then, our software application automatically finds the outer brain limits in Talairach orientation, and 3D grids are built for each brain. The Talairach grid obtained in this way represents a piecewise linear transformation and a tessellation of the brain into a 3D grid of 1056 cells representing homologous brain regions across subjects.18 The region of interest (ROI) measurements were obtained by superimposing the 3D tissue masks corresponding to GM, WM, and CSF onto each subject's Talairach grid, where the ROIs were defined as sets of Talairach grid cells (figure 1).13,15,16 Volume for each tissue type was measured on this MRI by summing up the data from the Talairach grid cells associated with each ROI.14 The validity of the Talairach-based procedure as an automated segmentation and quantification tool suitable for volumetric studies has already been proven,15,16 and it has been used in other multicenter studies.19 In our implementation, all manual procedures were performed by a single operator blind to the origin of each scan, thus avoiding any potential interrater variability. The error due to manual intervention in our segmentation procedures was highest for CSF measurements (around 4%), whereas for GM was less than 2% in all the ROIs studied.13
Fig. 1.
Segmentation and Quantification Method Based on the Talairach Proportional Grid System. The figure shows a triplanar view of the Talairach grid built in a particular brain. The colored set of grid cells define the frontal (red), parietal (pink), and temporal (blue) regions of interest of the right hemisphere. Green color overlaid on the magnetic resonance imaging shows the segmentation of gray matter tissue.
Measures
We chose to study the brain regions most likely to show volume changes, as previously observed in first-episode patients.7–9 The ROIs included in the analysis were the frontal, parietal, and temporal lobes, defined using the boundaries previously described for the Talairach method.15 Whole-brain GM and CSF were also measured. Intracranial volume (ICV) was obtained by adding the total GM, WM, and CSF volumes, including the cerebellum. For each ROI, we obtained volumes for each hemisphere and for GM and CSF. The occipital lobe was not included in the study because of its relatively high measurement error in multicenter data, which was 5.7% in GM and 17% in CSF values.13 Subcortical and cerebellar regions were not measured because in our multicenter setup, their segmentation using automated methods would require a thorough study of compatibility and validation. Multicentre reproducibility of WM was nearly twice lower than of GM,13 thus volume data for WM were not included in the study, also to reduce the dimensionality of the analysis. In addition to these technical problems, a structural study of WM would have been incomplete and inconclusive without data from other sequences such as diffusion-weighted imaging that provide functional information about connectivity.
Statistical Analysis
Differences between patients and controls in demographic data were assessed by Student's t and chi-square tests, according to the type of the variable.
Because age and total cranial size are known factors affecting regional cerebral volumes, their effect was removed using the residuals from the regression models obtained from the group of healthy controls, separately for each gender, following the procedure of Pfefferbaum et al.20 After this correction, volume variables were expressed as deviations (regression residuals) from the expected volumes in healthy individuals of the same age (in months) and brain size (ICV) as the patient. Thus, negative residuals could be seen as volume deficits relative to controls and vice versa. The comparative analysis of volume differences between groups was done using these regression residuals for each variable, which is equivalent to using brain size and age as covariates in an ANCOVA model.
The age and ICV regression residuals obtained in the previous step were used to test for volume differences between patients and controls by an ANCOVA model using scanner site as a factor of no interest in the analysis.13 Because of known differences in brain morphometrics between males and females21 and also because we have found significant group × gender interactions for some variables, we did the comparison between patients and controls separately for each gender. We discarded using a full factorial design including the interaction group × gender on the model because of the different sample size for males and females, which makes an unbalanced model that compromises the interpretation of factor effects, specially for the interaction term. Another comparative analysis between patients and controls was also done by subdividing the patient sample into 3 diagnostic groups: schizophrenia, bipolar, and other psychosis. Differences between these groups were tested using post hoc procedures (Dunnett's test) whenever the ANCOVA model revealed significant differences between groups. In variables showing significant differences between group means, Cohen’s effect size (ES) were calculated to provide an index of the magnitude of differences. The association between symptom scores (PANSS positive, negative, and general scales) and volumetric variables (age and ICV residuals) was studied using Pearson's correlation. Normality of the distributions and homoscedasticity of variance among groups was checked prior to the analysis. Statistical analyses were done using SAS 9.0 (Statistical Analysis System, Cary, North Carolina), and a 2-tailed P-value lower than .05 was considered statistically significant.
Results
Sociodemographic and Clinical Characteristics
Patients and controls were not significantly different in terms of age, sex, education, years of education, race, or handedness (table 1). Duration of illness prior to the MRI scan was very short (mean 2 months; table 1). No differences were observed between male and female patients regarding PANSS scores in total positive (mean, SD: 14.0, 6.2 and 15.8, 6.5, respectively, for females and males) or negative (mean, SD: 17.5, 6.9 and 17.4, 6.3, respectively, for females and males) subscales.
Differences Between Patients and Controls
Results of the ANCOVA of age and ICV residuals using scanner as factor of no interest suggest that only male patients showed significant differences relative to controls (table 2). Male patients showed smaller whole-brain GM volume (F1,123 = 16.3; P < .0001; ES = 0.77) and larger CSF volume (F1,123 = 20.3; P < .0001; ES = 0.81). GM volumes of male patients were smaller than controls in the frontal lobe in both right (F1,123 = 28.2; P < .0001; ES = 0.98) and left hemispheres (F1,123 = 17.5; P < .0001; ES= 0.79) and in the parietal lobe (right: F1,123 = 10.9, P = .0013, ES = 0.61; left: F1,123 = 5.3, P = .0230, ES = 0.42). Male patients also showed larger CSF volumes in the frontal (right: F1,123 = 17.6, P < .0001, ES = 0.63; left: F1,123 = 11.4, P = .0012, ES = 0.84), temporal (right: F1,123 = 20.3, P < .0001, ES = 0.79; left: F1,123 = 21.8, P < .0001, ES = 0.77), and right parietal (F1,123 = 8.3; P = .0047; ES = 0.56) lobes (table 2). The statistical significance of most of these differences persist even after a stringent Bonferroni correction considering a total of 30 tests (P = .0017).
Differences Between Patients Subgroups and Controls
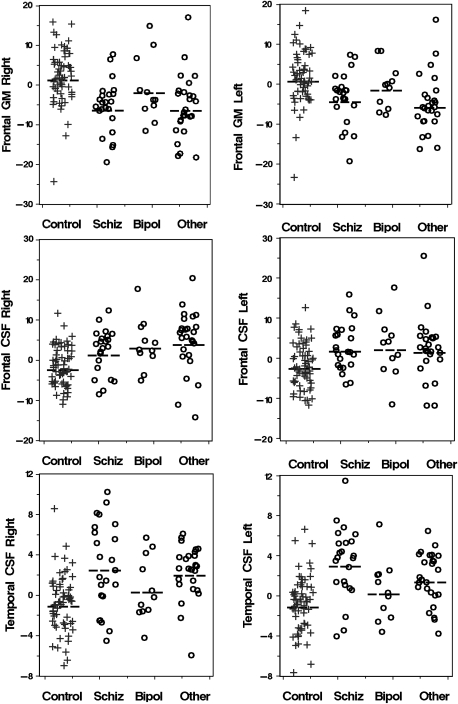
Results of the post hoc Dunnett's test revealed that male schizophrenia patients showed significantly smaller whole-brain GM (P = .0008) and larger CSF volumes (P = .0027) (table 3; figure 2) than controls. Male schizophrenia patients also showed smaller GM volumes in the frontal (right: P < .0001; left: P = .0009) and right parietal lobe (P = .0061) and larger CSF volumes in the temporal (right: P = .0005; left: P = .0001) and frontal lobes (right: P = .0261; left: P = .00147). The males in the other psychosis group showed differences relative to controls in the same ROIs as male schizophrenia patients (table 3; figure 2), smaller whole-brain GM (P = .0008), larger CSF volume (P = .0001), smaller GM volume in the frontal (right and left: P < .0001) and parietal (right: P = .0045; left: P = .0187) lobes, and larger CSF volumes in the frontal (right: P < .0001; left: P = .0047), right parietal (P = .0111), and temporal lobes (right and left: P < .0001) (table 3; figure 2). Bipolar male patients showed significant differences from controls only for CSF values; whole-brain (P = .0131), frontal (right P = .0017; left P = .0134), and right parietal (P = .0110) (table 3; figure 2) lobes. The same ANCOVA model testing for differences between the 3 clinical subtypes revealed no significant differences.
Table 3.
Volume Measurements (in cm3) of Patients in Each Diagnostic Group, by Gender
| Schizophrenia |
Bipolar |
Other Psychosis |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Schizophreniaa | Bipolara | Other Psychosisa | |
| Males (n) | 24 | 12 | 28 | ||||||
| Age (y) | 15.8 | 2.1 | 16.2 | 2.0 | 15.7 | 1.6 | |||
| Intracranial volume | 1577.8 | 151.6 | 1489.1 | 63.4 | 1582.0 | 128.0 | |||
| Total GM | 835.3 | 88.5 | 800.5 | 63.7 | 842.7 | 80.6 | *** | *** | |
| Total WM | 422.3 | 51.7 | 395.5 | 40.9 | 421.4 | 44.8 | |||
| Total CSF | 319.9 | 78.7 | 293.0 | 46.2 | 317.7 | 43.6 | ** | * | *** |
| GM | |||||||||
| Frontal, left | 76.1 | 10.5 | 74.7 | 8.7 | 76.0 | 8.5 | *** | *** | |
| Frontal, right | 77.4 | 10.7 | 77.4 | 11.3 | 78.6 | 8.8 | *** | *** | |
| Parietal, left | 62.9 | 8.7 | 60.2 | 8.7 | 62.9 | 8.9 | |||
| Parietal, right | 62.2 | 8.1 | 60.1 | 8.5 | 62.6 | 8.0 | ** | ** | |
| Temporal, left | 86.4 | 10.0 | 79.5 | 6.2 | 85.7 | 8.6 | |||
| Temporal, right | 85.3 | 9.6 | 79.8 | 6.7 | 86.1 | 9.1 | |||
| CSF | |||||||||
| Frontal, left | 32.2 | 9.6 | 30.5 | 7.9 | 32.3 | 7.5 | * | * | ** |
| Frontal, right | 32.6 | 9.5 | 31.9 | 7.0 | 35.3 | 8.3 | * | ** | *** |
| Parietal, left | 27.2 | 7.8 | 24.3 | 5.4 | 25.8 | 4.5 | |||
| Parietal, right | 25.4 | 8.1 | 24.9 | 5.5 | 25.9 | 5.1 | * | * | |
| Temporal, left | 26.9 | 6.7 | 23.0 | 3.9 | 26.0 | 3.7 | *** | *** | |
| Temporal, right | 24.9 | 7.4 | 21.6 | 4.0 | 24.8 | 4.0 | *** | *** | |
| Females (n) | 7 | 6 | 15 | ||||||
| Age (y) | 15.2 | 1.9 | 15.2 | 2.4 | 15.3 | 1.9 | |||
| Intracranial volume | 1437.1 | 144.0 | 1303.3 | 133.9 | 1358.9 | 84.3 | |||
| Total GM | 766.0 | 105.6 | 687.3 | 111.2 | 720.9 | 71.2 | |||
| Total WM | 377.8 | 41.2 | 365.6 | 77.7 | 380.1 | 75.1 | |||
| Total CSF | 293.2 | 53.1 | 250.3 | 72.3 | 257.7 | 64.7 | |||
| GM | |||||||||
| Frontal, left | 69.9 | 7.5 | 64.7 | 10.0 | 68.2 | 7.9 | |||
| Frontal, right | 74.5 | 9.5 | 69.2 | 13.3 | 70.1 | 9.0 | |||
| Parietal, left | 56.1 | 10.8 | 50.6 | 11.3 | 55.6 | 7.3 | |||
| Parietal, right | 56.3 | 9.1 | 52.6 | 15.9 | 56.0 | 8.5 | |||
| Temporal, left | 77.8 | 10.9 | 66.3 | 11.3 | 71.7 | 8.7 | |||
| Temporal, right | 77.4 | 13.2 | 68.5 | 10.4 | 71.1 | 8.6 | |||
| CSF | |||||||||
| Frontal, left | 33.8 | 11.0 | 21.4 | 7.2 | 25.5 | 7.2 | * | ||
| Frontal, right | 35.7 | 11.4 | 25.4 | 7.9 | 25.8 | 7.6 | ** | ||
| Parietal, left | 25.9 | 6.5 | 22.2 | 7.8 | 21.1 | 7.3 | |||
| Parietal, right | 22.3 | 7.1 | 23.3 | 13.6 | 19.7 | 6.8 | |||
| Temporal, left | 23.9 | 2.9 | 18.2 | 4.0 | 22.0 | 5.7 | |||
| Temporal, right | 20.9 | 2.0 | 18.9 | 5.4 | 20.2 | 5.0 | |||
Abbreviations are explained in the first footnote to table 2.
Post hoc Dunnett's tests comparing each patient group vs controls using residuals after correction for intracranial volume, age, and scanner factors.
***P < .001; **P < .01; *P < .05.
Fig. 2.
Scatter Plots Showing Individual Data Points Illustrating Distribution of Gray Matter (GM) and Cerebrospinal Fluid (CSF) Variables in Male Subjects Showing the Most Significant Differences Relative to Controls (table 3). Volume data are expressed as residuals after correction for intracranial volume, age, and scanner factors (see “Methods” for details). Dashed lines mark the mean for each group (+, controls; O, patients).
In female patients, only the schizophrenia group showed significantly larger CSF volumes in the frontal lobe (right: P = .0085; left: P = .0207), relative to controls (table 3). However, these differences should be taken with caution because of the small sample size of the patient group (n = 7).
Association Between Volume and Symptom Scores
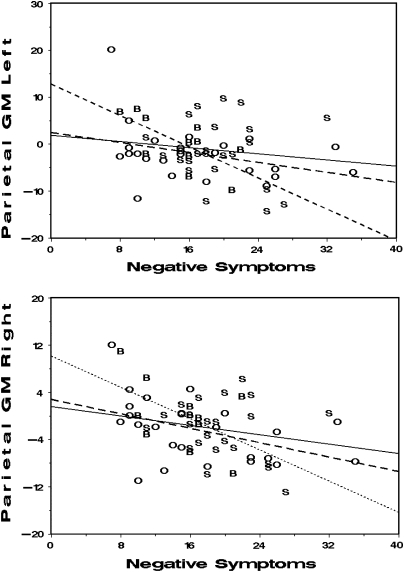
In male patients, the correlation analysis revealed a significant negative relationship between negative symptoms and whole-brain GM volume (r = −.292, P = .025). In the ROI analysis of GM volumes, this correlation was significant only in the parietal lobe (right: r = −.399, P = .002; left: r = −.296, P = .023). This correlation seems to be of the same nature for the 3 diagnostic groups (figure 3). No correlation was observed between duration of illness and volume measurements. In female patients, no correlation between volume and symptoms nor with duration of illness were observed.
Fig. 3.
Plots showing significant relationships between negative symptoms and GM volumes of parietal lobes in male patients (right: r = −.399, P = .002; left: r = −.296, P = .023). Data points show volume data expressed as residuals after correction for intracranial volume, age, and scanner factors (see “Methods” for details). Patients are labeled by clinical subtypes (S: schizophrenia [continuous line]; B: bipolar disorder [dotted line]; O: others [dashed line]).
Discussion
Several main findings emerge from this study. We found that total, frontal, and parietal GM volumes are reduced in males of first- and recent-onset episodes of psychosis compared with controls and that total CSF volume, and frontal, temporal, and right parietal CSF volumes are increased. We also found that nonschizophrenia nonaffective psychosis group behaves similarly to schizophrenia in terms of observed structural brain changes. Finally, we have shown that in male patients, negative symptoms have a negative correlation with total GM volume from beginning phases of early-onset psychotic disorders.
Volumetric Findings
In this study, we observed volume differences in structures that have been associated with psychosis in different stages of the disorder. Decreased GM and increased total intracranial CSF have been repeatedly shown in cross-sectional studies of adult samples, both in first episodes and in chronic patients.22 Our sample contains, to our knowledge, the largest recent and early-onset psychosis sample reported in the literature. The results add to our previous findings in a smaller sample, showing that recent-onset schizophrenia adolescents had decreased frontal GM and increased total intracranial CSF and left frontal CSF.7,10 The results also partially replicate the few other early-onset studies available. In a sample of chronic child-onset schizophrenia patients, Rapoport and her team showed reduced GM and increased CSF volume in the brains of those patients.3,6,23 This group showed only parietal deficits at baseline. Frontal deficits were only significant at follow-up. This frontal deficit could be an age effect, meaning that it is plausible that only at the developmental time when frontal GM reduction occurs physiologically during adolescence,21 does the excessive loss associated with schizophrenia take place. The mean age of the National Institute of Mental Health sample was 13 years at baseline and 17 years at follow-up, while the mean age at baseline in our sample was around 15 years. The former sample had a duration of illness before baseline of around 3 years, and ours was a very recent-onset sample, with less than 3-month duration of illness, which argues against frontal deficit being just an effect of chronicity. The Oxford group reporting on a sample of 16 adolescents with first episodes of schizophrenia, with a duration of illness of around 18 months, showed only larger ventricular volumes and changes in left temporal lobe that were present from the beginning of the disorder.8,9 Relative to these other studies, our sample of patients is of particular interest because of the large sample size and the short period between the appearance of positive symptoms and MRI scanning.
Timing of GM Reduction and CSF Increase
Results from the literature regarding CSF enlargement are conflicting in their interpretation. CSF enlargement has been shown from the beginning of illness and at all ages of onset. It has usually been considered suggestive of degeneration.4 However, some authors argue that the differences between the brains of schizophrenia patients at the beginning of the illness and those of controls reflect abnormal neurodevelopment.24 Trying to determine when the brain reduction occurs, Woods developed a series of formulas that are based on the assumptions that maximum cranial volume is driven by brain growth and that, once the cranium reaches its peak size, this is not reduced. Using this approach, the authors found a quantitatively similar reduction of volume before and after the brain reaches its maximum capacity.25 The timing of the GM reduction cannot be concluded from a cross-sectional study such as ours, but we hereby show that the GM reduction is present from the first stages of the disorder, which is in agreement with other studies.7,10 Longitudinal studies suggest that the rate of cortical loss seen in child-onset schizophrenia during adolescence reaches a plateau during early adulthood.26 In conclusion, several studies, including ours, concur that it is during preadolescence or adolescence when exaggerated GM loss takes place in the frontal lobe.
The consideration of GM loss as degenerative or developmental is to some extent conceptual. Some maturational processes continue well after infancy or adolescence. Early insults (including prenatal) can have a pruning or regressive effect, as in late maturational processes. Some authors explicitly call neurodegenerative those changes occurring in adolescence in which a greater than expected disruption in the process of synaptic loss is found,27 while others usually call developmental any event occurring before maturation is completed.24
Diagnostic-Related Differences
In our sample, both reduced GM and enlarged sulcal CSF were found in schizophrenia and “other psychosis” patients, but these differences were smaller in bipolar patients. This finding supports previous findings showing the relative specificity of these 2 markers to schizophrenia28 in comparison with bipolar disorders. Two studies have shown that in severe bipolar patients ventricular enlargement may also be present.29 In fact, an association between number of affective episodes and ventricular volume has been shown in 2 different studies30,31 and may underlie the ventricular enlargement shown in those studies.
The fact that the “other psychosis” group shows findings similar to the schizophrenia group probably reflects the heterogeneity of this subgroup, comprising 6 different diagnoses (see “Methods”). In addition, the stability of the nonaffective and nonschizophrenia psychotic disorders in adolescence, contrary to the schizophrenia and bipolar disorder diagnosis, is in fact very low.32,33 We were very restrictive in delimiting the schizophrenia and bipolar groups, and it is expected that some psychotic depressions in the “others” group will subsequently change to the bipolar group and that schizophreniform and schizoaffective patients will change to the schizophrenia group.
Early age of onset could also underlie and be responsible for the similarities in our findings between different diagnostic groups. Decreased GM can be an indicator of unspecific developmental deviations, which could cause any genetically driven disorder to be expressed earlier in life. Vulnerable brains may express psychotic disorders earlier.
Gender Issues
We found no differences in female patients in general compared with female controls. Within females, only the schizophrenia group showed minor differences in CSF of the frontal lobe relative to controls. Studies have systematically found more anomalies in males than in females. This could be because the male brain is more vulnerable than the female brain or it may just reflect false negatives in the analysis of females related to sample size. However, in this study, our sample should be large enough to show differences, if they were in the same range as those found in males. Although the literature shows gender differences in clinical features such as earlier age at onset, poorer premorbid adjustment, more brain abnormalities, and poorer outcome in males, the findings have been considered consistent with normal sexual dimorphism in brain development and gender-assigned social roles and do not invoke the need for a sex-specific etiological factor.34 Factors related to gender and pubertal status in the evolution of the illness and hormonal status at the time of MRI evaluation should be considered. In fact, physiological changes might explain differences as large as 10% in certain CSF volumes in females.35 This, however, needs further research.
The assumption that brain or brain region size or volume is associated with integrity, function, or level of maturation is not to be taken for granted. Many examples undermine this assumption. In fact, longitudinal studies have been contradictory in correlating GM loss with prognosis.5,6,36,37
Psychopathology and Brain Volumes
The finding that negative symptoms negatively correlate with total GM and CSF volume is consistent with previous findings in early-onset3,6,38 and adult samples.39 However, this association between morphometric and clinical variables should be taken with caution because the overall strength of these correlations was low. Only the right parietal lobe would persist after a Bonferroni correction for multiple comparisons. This result converges with the literature findings that associate parietal deficits with the deficit syndrome in schizophrenia studies.40,41
Limitations
This study has several limitations. The first is the multicenter nature of study. Although this made it possible to obtain data from a large number of patients, the use of different MRI systems adds an additional source of error in the analysis. This error due to scanner effect was assessed in a prior study13 and has been explicitly addressed by including scanner site as a covariate in the models tested. Also due to this limitation, data from the occipital lobes were excluded from the analysis because of the high error observed in our multicenter reliability study.13 The second main limitation is the small sample size of clinical subtypes within patients for each gender, which limited the results of the post hoc analyses. This limitation had a stronger effect in the case of female sample. However, the statistical power of the female sample in the regional GM values was higher than 80% (mean and SD data from table 2, assuming an alpha error level of 20%). Finally, the findings on the group of bipolars included may not be generalized to nonpsychotic bipolars.
Despite these limitations, within the neuroimaging literature, our study includes a relatively large sample of first episodes of early-onset psychosis and provides further data supporting frontal and parietal GM deficits within the FEP.
Funding
Ministerio de Sanidad y Consumo; Instituto de Salud Carlos III (Red de Investigación G03/032, RETICS RD06/0011 [REM-TAP Network], FIS PI052271); Consorcio Para El Desarrollo De Tecnologías Avanzadas De Imagen En Medicina Programa Consorcios Estratégicos Nacionales en Investigación Técnica, Ministerio de Industria; Fundación Alicia Koplowitz; Centro de Investigación Biomédica en Red de Salud Mental, Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación. Support also given by the Generalitat de Catalunya to the Child Psychiatry and Psychology Group (2009 SGR 1119).
Acknowledgments
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Greenstein D, Lerch J, Shaw P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 2.Sowell ER, Toga AW, Asarnow R. Brain abnormalities observed in childhood-onset schizophrenia: a review of the structural magnetic resonance imaging literature. Ment Retard Dev Disabil Res Rev. 2000;6:180–185. doi: 10.1002/1098-2779(2000)6:3<180::AID-MRDD5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport JL, Giedd J, Kumra S, et al. Childhood-onset schizophrenia. Progressive ventricular change during adolescence. Arch Gen Psychiatry. 1997;54:897–903. doi: 10.1001/archpsyc.1997.01830220013002. [DOI] [PubMed] [Google Scholar]
- 4.Arango C, McMahon RP, Lefkowitz DM, Pearlson G, Kirkpatrick B, Buchanan RW. Patterns of cranial, brain and sulcal CSF volumes in male and female deficit and nondeficit patients with schizophrenia. Psychiatry Res. 2008;162:91–100. doi: 10.1016/j.pscychresns.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sporn AL, Greenstein DK, Gogtay N, et al. Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am J Psychiatry. 2003;160:2181–2189. doi: 10.1176/appi.ajp.160.12.2181. [DOI] [PubMed] [Google Scholar]
- 7.Moreno D, Burdalo M, Reig S, et al. Structural neuroimaging in adolescents with a first psychotic episode. J Am Acad Child Adolesc Psychiatry. 2005;44:1151–1157. doi: 10.1097/01.chi.0000179055.46795.3f. [DOI] [PubMed] [Google Scholar]
- 8.James AC, Javaloyes A, James S, Smith DM. Evidence for non-progressive changes in adolescent-onset schizophrenia: follow-up magnetic resonance imaging study. Br J Psychiatry. 2002;180:339–344. doi: 10.1192/bjp.180.4.339. [DOI] [PubMed] [Google Scholar]
- 9.James AC, James S, Smith DM, Javaloyes A. Cerebellar, prefrontal cortex, and thalamic volumes over two time points in adolescent-onset schizophrenia. Am J Psychiatry. 2004;161:1023–1029. doi: 10.1176/appi.ajp.161.6.1023. [DOI] [PubMed] [Google Scholar]
- 10.Reig S, Moreno C, Moreno D, et al. Progression of brain volume changes in adolescent-onset psychosis. Schizophr Bull. 2009;35:233–243. doi: 10.1093/schbul/sbm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Fornieles J, Parellada M, Gonzalez-Pinto A, et al. The child and adolescent first-episode psychosis study (CAFEPS): design and baseline results. Schizophr Res. 2007;91:226–237. doi: 10.1016/j.schres.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Soutullo CA. Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS), adapted to Spanish from Spain. http://www.cun.es/la-clinica/departamentos-y-servicios-medicos/psiquiatria-y-psicologia-medica/mas-sobre-el-departamento/unidades/psiquiatria-infantil-y-adolescente. Accessed July 2008. [Google Scholar]
- 13.Reig S, Sanchez-Gonzalez J, Arango C, et al. Assessment of the increase in variability when combining volumetric data from different scanners. Hum Brain Mapp. 2009;30:355–368. doi: 10.1002/hbm.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desco M, Pascau J, Reig S, et al. Multimodality image quantification using Talairach grid. Paper presented at: Proceedings of SPIE Medical Imaging. February 2001; San Diego, CA. [Google Scholar]
- 15.Andreasen NC, Rajarethinam R, Cizadlo T, et al. Automatic atlas-based volume estimation of human brain regions from MR images. J Comput Assist Tomogr. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Kates WR, Warsofsky IS, Patwardhan A, et al. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Res. 1999;91:11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 17.Ashburner J, Friston KJ. Multimodal image coregistration and partitioning—a unified framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 18.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical; 1988. [Google Scholar]
- 19.Patwardhan AJ, Eliez S, Warsofsky IS, et al. Effects of image orientation on the comparability of pediatric brain volumes using three-dimensional MR data. J Comput Assist Tomogr. 2001;25:452–457. doi: 10.1097/00004728-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 21.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gogtay N, Sporn A, Clasen LS, et al. Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry. 2004;61:17–22. doi: 10.1001/archpsyc.61.1.17. [DOI] [PubMed] [Google Scholar]
- 24.Weinberger DR, Marenco S. Schizophrenia as a neurodevelopmental disorder. In: Weinberger D, editor. Schizophrenia. 2nd ed. Malden, MA: Blackwell Publishing; 2003. pp. 326–348. [Google Scholar]
- 25.Narr KL, Toga AW, Szeszko P, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Arango C, Kahn R. Progressive brain changes in schizophrenia. Schizophr Bull. 2008;34:310–311. doi: 10.1093/schbul/sbm166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 28.McDonald C, Marshall N, Sham PC, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163:478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- 29.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 30.Brambilla, Harenski K, Nicoletti M, et al. MRI study of posterior fossa structures and brain ventricles in bipolar patients. J Psychiatr Res. 2001;35:313–322. doi: 10.1016/s0022-3956(01)00036-x. [DOI] [PubMed] [Google Scholar]
- 31.Strakowski SM, DelBello MP, Zimmerman ME, et al. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am J Psychiatry. 2002;159:1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- 32.Fraguas D, de Castro MJ, Medina O, et al. Does diagnostic classification of early-onset psychosis change over follow-up? Child Psychiatry Hum Dev. 2008;39:137–145. doi: 10.1007/s10578-007-0076-3. [DOI] [PubMed] [Google Scholar]
- 33.Hollis C. Adult outcomes of child- and adolescent-onset schizophrenia: diagnostic stability and predictive validity. Am J Psychiatry. 2000;157:1652–1659. doi: 10.1176/appi.ajp.157.10.1652. [DOI] [PubMed] [Google Scholar]
- 34.Todd J, Michie PT, Jablensky AV. Association between reduced duration mismatch negativity (MMN) and raised temporal discrimination thresholds in schizophrenia. Clin Neurophysiol. 2003;114:2061–2070. doi: 10.1016/s1388-2457(03)00246-3. [DOI] [PubMed] [Google Scholar]
- 35.Teasdale GM, Grant R, Condon B, et al. Intracranial CSF volumes: natural variations and physiological changes measured by MRI. Acta Neurochir Suppl (Wien) 1988;42:230–235. doi: 10.1007/978-3-7091-8975-7_45. [DOI] [PubMed] [Google Scholar]
- 36.van Haren NE, Pol HE, Schnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 38.Alaghband Rad J, McKenna K, Gordon CT, et al. Childhood-onset schizophrenia: the severity of premorbid course. J Am Acad Child Adolesc Psychiatry. 1995;34:1273–1283. doi: 10.1097/00004583-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Gur RE, Mozley PD, Shtasel DL, et al. Clinical subtypes of schizophrenia: differences in brain and CSF volume. Am J Psychiatry. 1994;151:343–350. doi: 10.1176/ajp.151.3.343. [DOI] [PubMed] [Google Scholar]
- 40.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 41.Tamminga CA, Thaker GK, Buchanan R, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49:522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]