Abstract
Traumatic brain injury (TBI) is known to lead to a range of adverse psychiatric sequelae but the question of whether TBI is a risk factor for psychosis and, in particular, schizophrenia remains unclear. Studies examining this issue have yielded conflicting results. We carried out a systematic review of the literature on TBI and psychosis in order to identify all population-based controlled studies which provide estimates of risk for schizophrenia following TBI. Odds ratios (ORs) were combined using random effects meta-analysis. Our literature search yielded 172 studies which were considered to be potentially relevant. From these, we identified 9 studies that could provide estimates of risk in the form of ORs. The pooled analysis revealed a significant association between TBI and schizophrenia (OR = 1.65; 95% CI = 1.17–2.32), with significant heterogeneity between the studies. Estimates from the family studies (OR = 2.8: 95% CI =1.76–4.47) were higher than those from the cohort/nested case-control studies (OR = 1.42: 95% CI = 1.02–1.97) by a factor of almost 2. There did not appear to be a dose-response relationship between severity of head injury and subsequent risk of schizophrenia. This meta-analysis supports an increased risk of schizophrenia following TBI, with a larger effect in those with a genetic predisposition to psychosis. Further epidemiological and neuroscientific studies to elucidate the mechanisms underlying this association are warranted.
Keywords: traumatic brain injury, psychosis, systematic review, meta-analysis
Introduction
Traumatic brain injury (TBI) is associated with significant adverse mental health outcomes in up to one-third of survivors.1 It is well established that TBI increases the risk for a wide range of neuropsychiatric disturbances such as mood disorders, anxiety disorders, substance use disorders, personality change, and cognitive impairment,2 but the question of whether TBI is a risk factor for schizophrenia or psychosis remains somewhat controversial. A classic review published in 1969 by Davison and Bagley3 concluded that . . . among individuals who had experienced TBI, “the observed incidence (of psychosis) over 10- to 20-year periods is 2 to 3 times the expected incidence . . .” Three decades later, David and Prince4 reexamined this issue in a critical review and came to the conclusion that “the classical case-control studies report apparently irreconcilably different estimates for the association between head injury and schizophrenia” and that “. . . given the available published data, one must conclude that it is unlikely that head injury causes schizophrenia.” Kim5 published a narrative review of the literature which concluded that the evidence supported “a risk-modifying effect of TBI in individuals who are genetically at risk of schizophrenia” but did not support TBI as an independent risk factor for schizophrenia. A recent systematic review by Hesdorffer et al6 has concluded that “there is limited/suggestive evidence of an association between moderate or severe head injury and psychosis.” A comprehensive overview in a textbook chapter by Fleminger2 stated that “it is not possible to come to any definite conclusion about whether head injury can cause a chronic psychotic illness, schizophrenia, or schizophreniform psychosis,” although he goes on to say that “. . . a reasonable conclusion to draw is that head injury does increase the risk of psychosis, perhaps doubling it.” Therefore, it can be seen that there is little consensus between the previous reviewers of this topic. However, only one of the previous reviews6 had used systematic review methodology and none had used meta-analytic techniques to give pooled measures of risk. To help clarify this issue and move the debate forward, we conducted a systematic review and meta-analysis of the population-based literature to date on risk of schizophrenia among individuals who have suffered TBI compared with the risk of schizophrenia among a control group. To our knowledge, this is the first meta-analysis on this topic.
Methods
Literature Search
Standard methods for systematic review were used in this article. The following databases were searched from their inception to October 2010: PUBMED, OVID MEDLINE, PsychINFO, and EMBASE. We searched using the format “[psychosis OR schizophrenia OR psychotic disorder OR delusional disorder OR delusions OR nonaffective psychosis OR psychiatric illness OR psychiatric disorder] AND [TBI OR cerebral trauma OR head injury OR craniocerebral injury OR concussion OR open head injuries OR closed head injuries OR skull fractures]” using text words and indexing (MeSH) terms.
Inclusion Criteria
We included published articles that reported on
the risk of schizophrenia among individuals who have suffered TBI compared with the risk of schizophrenia in a nonbrain injured population-based control group and
allowed calculation of a risk estimate from data provided in the article.
TBI was not limited by severity.
Exclusion Criteria
Studies were excluded (1) if they were reviews, case reports, or case series, (2) if they comprised solely a follow-up study of a cohort of brain-injured patients with no comparison group, (3) if there was a nonpopulation-based control group (ie, a patient control group), or (4) if insufficient information was available to allow calculation of risk estimates.
Study Selection and Data Extraction
We examined all titles and abstracts and obtained full texts of potentially relevant studies. We read each article to determine whether it met inclusion criteria for the review. We searched reference lists of included studies. We extracted data relating to risk for schizophrenia following TBI from each article or calculated risk estimates from data available in the article. In extraction of data from the articles, we took a conservative approach and used the risk estimates pertaining to narrow definition of schizophrenia and the longest follow-rate reported in the articles for the main analysis. We contacted authors where necessary to obtain extra information to calculate risk estimates. We extracted information on source of information about TBI, severity of head injury, age at onset of head injury, and source of information on psychotic outcomes.
Data Analysis
Estimates of risk of schizophrenia associated with TBI from different studies were combined using random-effects meta-analysis, with heterogeneity among studies estimated using Cochran Q and the I2 statistic.7 The I2 statistic describes the percentage of variation among studies that is due to heterogeneity rather than chance, and I2 values of 25%, 50%, and 75% can be taken to indicate low, moderate, and high levels of heterogeneity, respectively. We carried out subgroup analyses on mild vs severe TBI, childhood TBI and on broadly defined psychosis because there was felt to be sufficient numbers of studies in each group to allow these analyses. Meta-regression was undertaken to examine the influence of study design (family vs nested case-control/cohort). All of the analyses were undertaken with Stata statistical software package,8 using the “metan” package.9
Results
Our literature search and search of reference lists yielded 9131 references and, after perusing the titles, 172 were considered to be potentially relevant. From examination of the abstracts and, where indicated, full texts of the articles, we identified 9 studies (see table 1) which met our inclusion criteria, of which 2 were nested case-control studies,12,17 5 were cohort studies,10,13,15,16,18 and 2 were family studies.11,14 Two studies15,16 reported from the same dataset but for different age groups and therefore, both were included in this analysis. A summary of the 9 studies included in the analysis is presented in table 1.
Table 1.
Details of Studies Included in the Meta-Analysis of Risk of Psychosis Following Traumatic Brain Injury (TBI)
| Authors | Type of Study | Total N | Sample Description | Source of Information About TBI | Source of Information About Psychosis | Risk Estimate for Psychosis Following TBI, OR (95% CI) |
| Silver10 | Cohort | 5034 | Probability sample of adults from New Haven portion of the NIMH Epidemiologic Catchment Area programme | Patient report of history of severe TBIa (Have you ever had a severe head injury that was associated with loss of consciousness?) Lifetime | NIMH Diagnostic Interview Schedule (diagnosis of schizophrenia) | 1.8 (1.0–3.3)b |
| Malaspina11 | Family | 1931 | National Institute of Mental Genetics Initiative for Schizophrenia and Bipolar Disorders | Patient report- Diagnostic Interview for Genetic Studies question on Head Injurya (combined mild, moderate, and severe) | Diagnostic Interview for Genetic Studies | 3.32 (1.77–6.22)c |
| Nielsen12 | Nested Case-Control | 91 168 | Record linkage sample | Hospital admission register ICD-8 Admission for head injury (concussion and severe head injury) | Danish National Patient Register —diagnosis of schizophrenia | 0.91 (0.84–1.008)d; 0.94 (0.84–1.05) Mild TBI; 0.89 (0.76–1.04) Severe |
| Timonen13 | Cohort | 10 934 | Northern Finland 1966 Birth Cohort Study | Hospital discharge registers and case notes of outpatient clinics (ICD definition) Up to age 15 | Finnish Hospital Discharge Register | 1.1 (0.41–2.96)e |
| Abdel Malik14 | Family | 169 | Ongoing study of familial schizophrenia-genetic linkage of narrowly defined schizophrenia | From SCID-1 and supplemented by collateral information from family and medical records. consesnus rating | Structured Clinical Interview for DSM-III-R (diagnosis of schizophrenia and schizophrenia spectrum disorders) | 2.27 (1.08–4.38)f; 1.91 (0.84–4.38), (broad diagnosis) |
| Massagli15 | Cohort | 1960 | Adult Health Maintenance Organization—Group Health Co-operative of Puget Sound children 14-year old or less | Computerized databases on all inpatient and outpatient visits and diagnoses—info on Mild TBI onlya | Computerized databases on all inpatient and outpatient visits and diagnoses ICD-9 codes | 3.01 (0.9–10.2)g |
| Fann16 | Cohort | 939 | Adult Health Maintenance Organization—Group Health Co-operative of Puget Sound (members 15 years or older) | Computerized databases on all inpatient and outpatient visits and diagnoses (mild, moderate and severe TBIa) | Computerized databases on all inpatient and outpatient visits and diagnoses | 1.8 (1.22–2.77)h; 1.1. (0.4–3.1) mild TBI; 3.6 (1.0–12.3) severe TBI |
| Harrison17 | Nested Case-Control | 47 754 | Record linkage sample of a cohort of Swedish men and women born between January 1973 and December 1980 | Swedish Inpatient Discharge Register (hospital admission for concussion and skull or intracranial injuries) | Swedish Inpatient Discharge Register (schizophrenia and nonaffective psychosis) | 1.10 (0.82–1.47) (narrow definition); 1.27 (1.1–1.47) (broad definition |
| Chen18 | Cohort | 20 970 | Record linkage of 2 datasets in Taiwan (adults aged >18) | TBI Registry (Head and Spinal Cord Injury Research Group of the Taiwan Neurosurgical Society) 2001–2002 | Taiwan National Health Insurance Research Dataset (2001–2002) | 1.99 (1.28–3.08)i |
Note:DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; NIMH, National Institute of Mental Health; ICD, International Classification of Diseases; SCID, Structured Clinical Interview for DSM Disorders.
Centres for Disease Control and Prevention classification: Mild, <1 h or no concussion and no documented lesions; Moderate, prolonged loss of consciousness or documented intracranial or brain lesion.
OR not controlling for alcohol abuse.
OR for schizophrenia subjects vs never mentally ill subjects in combined pedigrees.
Risk for combined mild (concussion) and severe head injury calculated from data provided in article.
ORs for schizophrenia calculated from supplemental data provided by authors.
OR for TBI ≤ 15 y + narrow diagnosis.
The 3-y follow-up rate was used in analysis.
Diagnosis of psychotic disorders includes schizophrenia, hallucinations or paranoia, or organic psychotic disorder diagnosis: antipsychotic prescription. Combined risk for both mild and moderate/severe head injury calculated from data in article (3-y follow-up rates used).
OR adjusted for income and geographical location. Narrow definition ICD-9 295.
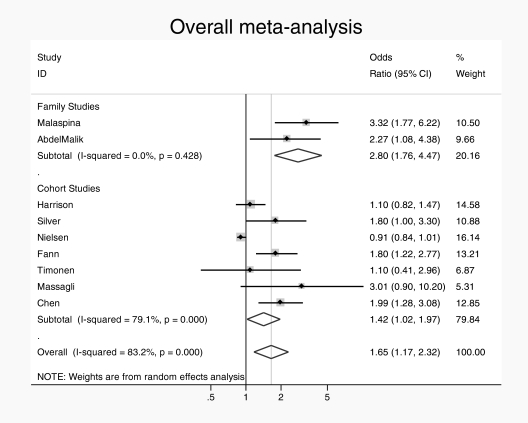
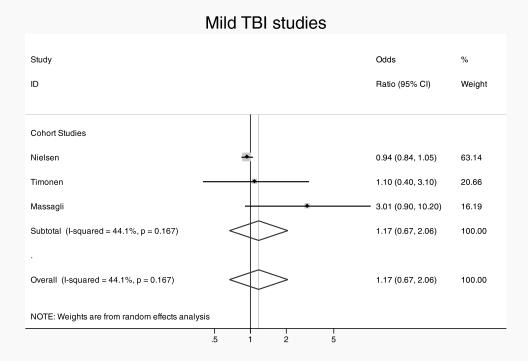
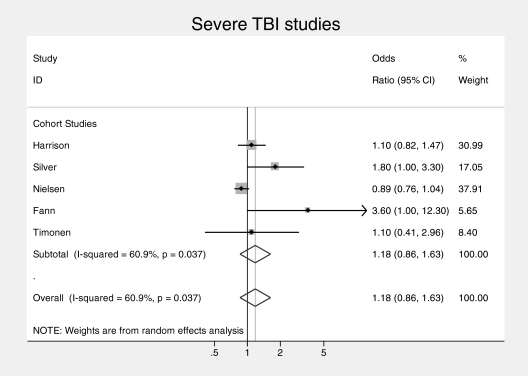
The overall pooled analysis revealed significant heterogeneity (in the high range) between studies (I2 = 83.2%, P < .001). Accordingly, a random effects model was used. There was an overall association between TBI and subsequent schizophrenia (pooled OR = 1.65, 95% CI = 1.17–2.32) (see figure 1). When studies were subdivided by study design, the 2 family studies11,14 yielded a pooled OR of 2.8 (95% CI = 1.7–4.5) with no heterogeneity (I2 = 0.0%, P = .43) and the 7 cohort/nested case-control studies yielded a pooled OR of 1.42 (95% CI = 1.0–1.9) with a high degree of heterogeneity (I2 = 79%, P < .002). Family studies found larger effect than cohort/case-control studies, by a factor of almost 2, with trend-level significance on meta-regression (OR = 1.94 [95% CI = 0.9–4.3]; P = .08). When subdivided by severity of TBI, the pooled ORs were similar with studies providing rates for mild TBI specifically,12,13,15 yielding a pooled OR of 1.17 (95% CI = 0.7–2.1) with low heterogeneity (I2 = 44%, P < .17), (see figure 2) and the studies giving estimates for severe TBI specifically,10,12,13,16 yielding a pooled OR of 1.18 (95% CI = 0.9–1.6) with moderate heterogeneity (I2 = 61%, P < .04) (see figure 3). Subgroup analysis of the risk of TBI associated with a broadly defined psychosis14,17 yielded a pooled OR of 1.3 (95% CI = 1.1–1.48) with no heterogeneity. Subgroup analysis of the risk associated with Childhood TBI (less than 15 y)13–15 yielded a pooled OR of 1.6 (95% CI = 0.6–2.6) with no heterogeneity.
Fig. 1.
Overall pooled risk estimate for risk of psychosis following traumatic brain injury.
Fig. 2.
Pooled risk estimate for psychosis following mild traumatic brain injury.
Fig. 3.
Pooled risk estimate for psychosis following severe traumatic brain injury.
Discussion
This article adds to the literature on the association between TBI and subsequent schizophrenia. Following a systematic review and meta-analysis, we report an increased risk of schizophrenia following TBI of about 60%. However, finding an association does not mean that causality is definitively established. As discussed in detail by David and Prince,4 it is difficult to tease apart whether the TBI caused the psychosis or whether a particular individual was already on the trajectory toward psychosis before the injury occurred. The association could also be due to confounds such as substance abuse19 or the existence of premorbid factors including motor and attentional problems, which are known to be associated with later schizophrenia.20
The contribution of TBI seems to be greater among those with an inherited vulnerablity to schizophrenia.11,14. Malaspina11 found that TBI doubled the risk for schizophrenia in family members of probands with schizophrenia but also noted that TBI was more frequent among relatives of schizophrenia probands than among relatives of control probands suggesting that genes for schizophrenia may influence the exposure to TBI, as well as the consequences. Fann and colleagues16 also found an increased rate of preexisting psychosis among individuals with head injury and speculated that psychosis increases the risk for TBI. The apparent complexity of the causal pathway between TBI and schizophrenia adds to the difficulty in investigating this relationship.
The apparent lack of a dose-response relationship between severity of head injury and risk for psychosis is intriguing, particularly in view of the recent interest in outcomes of mild head injury among athletes and soldiers.1 This lack of effect of severity of TBI has been noted previously in studies comparing characteristics of cases with head injury and psychosis with matched head injury controls with no psychosis.21,22 On the one hand, a strong dose-response relationship between exposure and outcome would provide some reassurance that an association may be causal. On the other hand, it is possible that some other aspect of the head injury, such as location of trauma, or psychosocial stress associated with the trauma, may be more relevant than the clinical measure of severity in increasing risk of psychosis.
Our analysis did not support the hypothesis that childhood or adolescent head injury is more likely to be associated with later schizophrenia.23,24 However, this subgroup analysis was based on just 3 studies.13–15 Two of these studies14,15 did find significant associations between childhood or adolescent onset TBI and later schizophrenia but the largest study did not find an association.13 Previously, Wilcox and Nasrallah24 reported a highly significant 10-fold increase in risk for schizophrenia following head injury before the age of 10 years, but this study was not included in the meta-analysis because it used surgical controls.
Limitations
Methodological heterogeneity: As discussed in previous reviews of this topic,4–7 there are many methodological differences between the studies examining TBI and psychosis such as: (1) different sources of information about the head injury—self-report10,11,14 vs hospital admission data; (2) different degrees of severity of head injury studied; (3) different definitions of psychotic illness—narrow schizophrenia outcomes,10–13 vs broader definitions,14,17 and (4) variations in length of follow-up post TBI ranging from 3 years13–16 to 35 years.14 Nevertheless, we felt that there was sufficient similarity between the exposure and outcome variables in these case-controlled population-based studies to allow us to proceed to meta-analysis. Although there was a high degree of statistical heterogeneity in the data for the cohort and nested case-control studies, there was no heterogeneity for the family studies. We used a random effects meta-analysis and meta-regression to take account of heterogeneity.
Location of TBI: We were not able to examine the effect of location of the TBI in this analysis. One of the limitations of the use of hospital discharge registers as a source of exposure information is that exact location of the brain injury cannot be determined. It has been proposed by some investigators that temporal and frontal lobe lesions are more likely to be associated with an increased risk of later psychosis compared with lesions in other brain regions.3,21,22 However, the classic 22-year follow-up study of 3532 Finnish soldiers by Achte and colleagues25 found no association between location of head injury and the subsequent development of psychosis
Another limitation is that none of the studies included in this review provided information on epilepsy. This could be a potential confounder of the association as head injury can cause epilepsy2 and epilepsy is associated with an increased risk of psychosis.26,27
In conclusion, our systematic review and meta-analysis has found that there is an increased risk of schizophrenia following TBI. The increase in risk associated with TBI is not large, in the order of about 60%, but this is not unusual for environmental risk factors for schizophrenia.28 In particular, the risk appears higher in those who have a family history of schizophrenia suggesting a gene-environment interaction29 or an epigenetic mechanism.30 Investigation of the molecular or epigenetic consequences of TBI in relation to psychosis risk, or genetic investigation of families in which TBI and psychosis cluster, may be fruitful lines of enquiry.
Funding
European Community's Seventh Framework Program (HEALTH-F2-2009-241909; Project EU-GEI). M.C. is supported by a Clinician Scientist Award from the Health Research Board Ireland (CSA/2004/1) and also recieves support from a 2009 NARSAD Essel Foundation Independent Investigator Award and The Stanley Medical Research Institute. D.C. is supported by the Health Research Board Ireland, NARSAD, Science Foundation Ireland, and the Stanley Medical Research Institute.
Acknowledgments
We thank Dearbhla Connor for help with the systematic review. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Bryant RA, O’Donnell ML, Creamer M, McFarlane AC, Clark CR, Silove D. The psychiatric consequences of traumatic injury. Am J Psychiatry. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- 2.Fleminger S. Head injury. In: David A, Fleminger S, Kopelman MD, Lovestone S, Mellers JDC, editors. Lishman’s Organic Psychiatry: A Textbook of Neuropsychiatry. 4th ed. Chichester, UK: Blackwell Publishing; 2009. pp. 167–279. [Google Scholar]
- 3.Davison K, Bagley CR. Schizophrenia-like psychoses associated with organic disorders of the central nervous system: a review of the literature. Current problems of neuropsychiatry. Br J Psychiatry. 1969;(Special Publication 4):S113–S184. [Google Scholar]
- 4.David A, Prince M. Psychosis following head injury: a critical review. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i53–i60. doi: 10.1136/jnnp.2004.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim E. Does traumatic brain injury predispose individuals to develop schizophrenia? Curr Opin Psychiatry. 2009;21:286–289. doi: 10.1097/YCO.0b013e3282fbcd21. [DOI] [PubMed] [Google Scholar]
- 6.Hesdorffer DC, Rauch SL, Tamminga CA. Long-term psychiatric outcomes following traumatic brain injury: a review of the literature. J Head Trauma Rehabil. 2009;24:452–459. doi: 10.1097/HTR.0b013e3181c133fd. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Thompson SG. Quantifying heterogeneity in a Meta- Analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 8.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 9.Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 10.Silver JM, Kramer R, Greenwald S, Weissman M. The association between head injuries and psychiatric disorders: findings from the New Haven NIMH Epidemiologic Catchment Area Study. Brain Inj. 2001;15:935–945. doi: 10.1080/02699050110065295. [DOI] [PubMed] [Google Scholar]
- 11.Malaspina D, Goetz RR, Friedman JH, Blehar MC, et al. Traumatic brain injury and schizophrenia in members of schizophrenia and bipolar disorder pedigrees. Am J Psychiatry. 2001;158:440–446. doi: 10.1176/appi.ajp.158.3.440. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen AS, Mortensen PB, O’ Callaghan E, Mors O, Ewald H. Is head injury a risk factor for schizophrenia? Schizophr Res. 2002;55:93–98. doi: 10.1016/s0920-9964(01)00205-5. [DOI] [PubMed] [Google Scholar]
- 13.Timonen M, Miettunen J, Hakko H, et al. The association of preceding traumatic brain injury with mental disorders, alcoholism and criminality: the Northern Finland 1966 Birth Cohort Study. Psychiatry Res. 2002;113:217–226. doi: 10.1016/s0165-1781(02)00269-x. [DOI] [PubMed] [Google Scholar]
- 14.Abdel Malik P, Husted J, Chow EW, Bassett AS. Childhood head injury and expression of schizophrenia in multiply affected families. Arch Gen Psychiatry. 2003;60:231–236. doi: 10.1001/archpsyc.60.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massagli TL, Fann JR, Burington BE, Jaffe KM, Katon WJ, Thompson RS. Psychiatric illness after mild traumatic brain injury in children. Arch Phys Med Rehabil. 2004;85:1428–1434. doi: 10.1016/j.apmr.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Fann JR, Burington MS, Leonetti A, Jaffe K, Katon WJ, Thompson RS. Psychiatric illness following traumatic brain injury in an adult health maintenance organisation population. Arch Gen Psychiatry. 2004;61:53–61. doi: 10.1001/archpsyc.61.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Harrison G, Whitley E, Rasmussen F, Lewis G, Dalman C, Gunnell D. Risk of schizophrenia and other non-affective psychosis among individuals exposed to head injury: case control study. Schizophr Res. 2006;88:119–126. doi: 10.1016/j.schres.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y-H, Chui W-T, Chu S-F, Lin H- C. Increased risk of schizophrenia following traumatic brain injury: a 5-year follow-up study in Taiwan. Psychol Med. September 22, 2010 doi: 10.1017/S0033291710001819. doi:10.1017/S00332917. [DOI] [PubMed] [Google Scholar]
- 19.Arseneault L, Cannon M, Witton J, Murray R. The causal association between cannabis and psychosis: an examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 20.Cannon M, Caspi A, Moffitt TE, et al. Evidence for early childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–457. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 21.Sachdev P, Smith JS, Cathcart S. Schizophrenia-like psychosis following traumatic injury: a chart-based descriptive and case-control study. Psychol Med. 2001;31:231–239. doi: 10.1017/s0033291701003336. [DOI] [PubMed] [Google Scholar]
- 22.Fujii D, Ahmed I. Characteristics of psychotic disorder due to traumatic brain injury: an analysis of case studies in the literature. J Neuropsychiatry Clin Neurosci. 2002;14:130–140. doi: 10.1176/jnp.14.2.130. [DOI] [PubMed] [Google Scholar]
- 23.O’Callaghan E, Larkin C, Redmond O, Stack J, Ennis JT, Waddington JW. “Early-onset schizophrenia” after teenage head injury. A case-report with magnetic resonance imaging. Br J Psychiatry. 1988;153:394–396. doi: 10.1192/bjp.153.3.394. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox JA, Nasrallah HA. Childhood head trauma and psychosis. Psychiatry Res. 1987;21:303–306. doi: 10.1016/0165-1781(87)90013-8. [DOI] [PubMed] [Google Scholar]
- 25.Achte KA, Hillbom E, Aalberg V. Psychoses following war brain injuries. Acta Psychiatr Scand. 1969;45:1–18. doi: 10.1111/j.1600-0447.1969.tb06197.x. [DOI] [PubMed] [Google Scholar]
- 26.Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy. Br Med J. 2005;331:23. doi: 10.1136/bmj.38488.462037.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundram F, Cannon M, Doherty CP, et al. Neuroanatomical correlates of psychosis in epilepsy: a voxel-based morphometry study. Br J Psychiatry. 2010;197:482–92. doi: 10.1192/bjp.bp.110.080218. [DOI] [PubMed] [Google Scholar]
- 28.Cannon M, Clarke MC. Risk factors for schizophrenia: broadening the concept, pushing back the boundaries. Schizophr Res. 2005;79:5–13. doi: 10.1016/j.schres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rutten BPF, Mill J. Epigenetic mediation of environmental influences in major psychotic disorders. Schizophr Bull. 2009;35:1045–1056. doi: 10.1093/schbul/sbp104. [DOI] [PMC free article] [PubMed] [Google Scholar]