Abstract
Numerous researchers have pointed out over the last decades that there is a loss of the sense of the inner self in schizophrenia. In particular, the illuminating article of Sass et al. gives an underpinning explanation of the disease along these lines in each of its 3 manifestations, with positive, negative, or disordered symptoms. The crucial component of the analysis of these researchers is that of various disturbances in ipseity (the ongoing sense of “being there” accompanying all conscious experience) that can occur for a sufferer, giving a framework with which to understand the disease. Such analyses of schizophrenia in terms of distortions of the self go back much earlier. However, the more recent work has become more precise and embracing in terms of seeing most forms of schizophrenia as arising from such distortions. It also provides new ways of looking at and diagnosing the disease. In this article we propose to move the whole analysis closer into the brain itself by means of the CODAM neural network model of consciousness (where CODAM is an acronym for the “COrollary Discharge of Attention Movement” model of consciousness creation, to be described later). This allows both a mechanism to be formulated as to the basic brain-based cause of schizophrenia (with varieties of this cause correlated with the 3 main forms of it) as well as open up possible lines of research to be followed to help ameliorate the attention defects exposed in this approach.
Keywords: inner self, attention, creation of consciousness, attention copy signal, attentional blink, loss of gray matter, acetylcholine
The increased understanding of schizophrenia as arising from distortions of the self and stemming from earlier work61-64 have provided important insights into schizophrenia, as noted above. Thus, as stated in Sass et al.1 “…, schizophrenia is a disorder involving subtle but pervasive and persistent aspects of subjective experience.” Furthermore, these authors state that 1(p428) “Schizophrenia we propose, is a disorder or more specifically an ipseity disturbance, in which one finds certain characteristic distortions of the act of awareness.” Ipseity denotes the inner self, to be regarded as the prereflective self of Western phenomenology.1 In this article we identify ipseity with the inner self, and that with what has been termed the prereflective self,2 although there may be subtle differences between them according to different definitions in Western phenomonology2; these will be ignored here. We use the definition of ipseity of Sass et al.1(p428) “…the experiential sense of being a vital and self-coinciding subject of experience or first person perspective on the world.” It arises from a part of the self that is devoid of the components of the reflective self, which itself is composed of those characteristics of the self that can be obtain by reflecting, such as whether one has a beard or is impatient, and so forth. The prereflective self appears instead as content free, and its existence provides a center of gravity in which the ownership of ones experiences is gathered.
The more detailed analysis in Sass et al.,1 together with further studies of these authors and their colleagues on various aspects of possible breakdown of ipseity,3–6 give an impressive attack on the gross functionality of schizophrenia and allow it to be considered in a revealing light with respect to the nature of the sufferer's experience.
In spite of the progress made thereby, there is still the difficulty of a lack of fundamental understanding of the crucial component at issue: ipseity, or the inner self. Thus, the insights of the Western philosophers of the past and of the more recent present2 have not led to any understanding of the manner in which the brain can help create the inner self (but see Sass,6 chapter 7, appendix7; both being about the hippocampus-based “comparator” function) nor of the brain's role in mediating how that inner self interacts with the stimulus representations of the outside world.
This interaction of the inner self with the outside world, however, plays a crucial role in the new understanding gained for schizophrenia. The distortions observed in schizophrenic subjects are concerned in many cases with the manner in which the outside world becomes more difficult to be dealt with by the sufferers. Thus, in the case of Maria,8 she is reported as saying that “She was never able to […] immerse herself in the world because an invisible barrier prevented her full presence”8(p706). Similar accounts of this difficulty of dealing with the outside world are reported by many other schizophrenics. But how does the interaction between the inner self and the representations of the external world in the brain become distorted in schizophrenia? Such distortion plays a very important role in the disease, but it has not yet been possible to determine how they are caused. This is due to the absence of any neural model of how ipseity or the inner self is itself represented in the brain. The CODAM9–11 [COrollary Discharge of Attention Movement (neural network model of the creation of consciousness)] model presented shortly provides a possible brain-based neural network model.
In particular for schizophrenia, as pointed out in Sass et al.,1 the main problem in understanding schizophrenia is as to how the breakdown of inner consciousness leads, in a sufferer, to the two major components pinpointed in their study:
Hyperreflexivity, in which there is an exaggerated form of attention being paid to self features as if they were external objects. As specified in Sass et al.,1(p428) hyperreflexivity denotes “…forms of exaggerated self-consciousness in which a subject or agent experiences itself, or what would be normally inhibited as an aspect or feature of itself, as a kind of external object.” It seems likely that this may result from a breakdown of some form of inhibition to prevent the unexpected awareness of these self features from breaking through into consciousness.
Reduction of what is termed “self-affection,” in which there is a diminished implicit sense of “being there,” so of self-presence. The term “self-affection” is not related to liking or other emotion, but refers to the manner in which the sense of subjectivity can affect itself and acknowledge its own presence. This, it was claimed in Sass et al.1 was reduced as an important basic component of experience in schizophrenics.
The first of these components can be recognized as part of the larger spectrum of “loss of common sense” and will be treated as such later in the article in terms of the neural model, CODAM, of consciousness. This allows building a bridge from the brain of the schizophrenic to their inner experience. In particular, this bridge will help pinpoint how the damage to the schizophrenic's experience can be seen to arise from a concomitant damage to their attention control system in the brain, as a part of damage to the CODAM model itself.
The second component will also be considered in terms of damages to CODAM leading to the resultant altered experience of the schizophrenic. I propose therefore to use this model because it helps understand the nature of the inner self and thereby the two important components recognized in the study of Sass et al.1 as basic to the schizophrenic experience.
In the article by Sass et al.,1 it was pointed out that these two distortions—of hyperreflexivity and of self-affection—are in fact complementary aspects of a single underlying component, that of the inner self or ipseity. This component (ipseity) and the possible damages to its functionality are also the center of our approach to schizophrenia through the CODAM model. In our view, CODAM implements a source of the experience of ipseity itself.
It should be added that there are two forms of hyperreflexivity noted in the work by Sass and Parnas,5 termed “reflective” and “operative”; the former is of a largely willed or voluntary kind, the latter is unwilled or involuntary. It is this latter more basic form of hyperreflexivity that is the primary focus in Sass et al.,1 and with which we are concerned in this article. We note, then, that the ipseity component recognized in the CODAM model to be discussed shortly is not a willful or volitional process and that distortions of such a neural system will be of the operative hyperreflexive type.
One important problem presently faced about schizophrenia, as seen from this vantage point of a breakdown of common sense about the world and of one's presence in it, is that of comprehending how to move this understanding further into the brain and thereby how the disease could be ameliorated. Much success has been gained recently by new drugs for the disease as well as by new behavioral treatments (such as cognitive brain therapy and attention/cognitive training schemes). However, such drug-based or cognitive behavior therapy-based advances do not seem to be much related to the deeper understanding arising from the appreciation that ipseity distortion plays a crucial role across the whole range of schizophrenic symptoms.
One of the threads of this article is that we can begin to bridge this gap between these two approaches—that by drug and behavioral treatments and that of ipseity distortion—by creating a specific brain-based model. This model would take account of the former treatment and activity-based approaches by including a neural network model of attention and its related cognitive processing powers (with an associated biochemical basis). At the same time, its neural activity would also be interpretable in terms of various stages that arise in the creation of conscious experience. Such a model of consciousness is obviously controversial, but needs to be attempted to make progress toward a more unified view of schizophrenia.
Over the last decade, a model of consciousness as based on the brain has been developed.9,10 This model uses attention as its basis and leads to a description of consciousness fully consistent with that arising from the basic analyses of Western phenomenology.12 In particular, the “protention–primal impression–retention” main sequence of events for consciousness discovered through phenomenology is explained in the attention-based CODAM model in terms of the necessary dynamics of the processing of attention to support object-based neural activity accessing buffer working memories for report.11 By way of further explanation, we add that these 3 fundamental components of experience are defined as follows: Protention is equal to the preparatory activity leading to consciousness, the primal impression is defined as the actual first moment of conscious experience of the external stimulus, retention is the subsequent decaying memory of this conscious experience that may last some seconds after the primal impression occurred.
The purpose of this article is firstly to explain this attention-based CODAM model of the creation of consciousness, with particular reference to the details of the creation of the inner self or ipseity through the dynamics of the CODAM model. Such a neural explanation of the inner self is crucial for any brain-based model of consciousness. But it is even more so if there is to be exploration of possible distortions in that process of creation of the inner self. It is then shown how such distortions as are provided by the descriptions of their inner experience of schizophrenics can be explained by certain distortions specifically in that process of inner self creation in the brain-based CODAM model, and hence in the brain.
Attention is well known to be disturbed in schizophrenia. The attentional blink (AB) is a paradigm in which a rapid sequence of stimuli, occurring at say 10 Hz, is presented to a subject who has to detect first one target and then a second. The subject has the greatest difficulty if the time lag between the two stimuli is about 270 ms, the so-called “attentional blink” (to be discussed in more detail later in the article). The paradigm is well known to be sensitive to various components of attention and has been shown to be increased in schizophrenics as compared with controls.13 This implies that schizophrenics have a lower level of attention control of stimuli in the rapid serial visual presentation (RSVP) task to which they are exposed than do controls.13 Other paradigms also indicate the lower level of attention control (and related cognitive control) experienced by schizophrenics.14,15
Attention is also a component of information processing for which there exists some evidence that its strengthening (by suitable exercises) can go toward ameliorating the symptoms of schizophrenia. This may help explain the successes gained by schizophrenics involved in the various cognitive training programs.14,16
The approach being taken in this article is that attention is a control system somewhat similar to that of motor control in the brain. This has been advocated by the premotor theory of attention17 and allows the application to attention of motor control ideas18 (for which there is increasing experimental support). Disturbances in motor control and especially in agency (where agency is the knowledge of who is performing an action on one's body: oneself or another) have been put forward as basic to the difficulties of schizophrenics.19 The extension of the analysis of similar defects to the attention control system thereby allows investigation of the conscious experience of the schizophrenic if one accepts that attention functions as the gateway to consciousness and the inner self (as strongly supported by the neuroscience community). Such a shift also allows avoidance of the strictures in Taylor10 and Gallagher20,21 against the motor control approach to schizophrenia (and especially that of distorted agency). For attention and motor control are seen to be somewhat divorced in the brain.22 More importantly, the CODAM attention control model of Taylor9,10 avoids the difficulty of having to have the intention of a thought before experiencing it; this avoidance is obtained through the attention circuitry employed in the CODAM model. This will be investigated shortly in more detail.
Other approaches to a brain-based understanding of schizophrenia have been attempted. Thus, a recent article in this journal23 proposes that distorted synaptic learning processes are at the root of schizophrenic symptoms, leading to poor monitoring of actions. Our approach is more specific than this as to brain circuitry involved, but can accommodate the modifications of online learning suggested by these authors. However, our proposal attempts to relate more closely to experiential modifications that changes in brain circuitry bring about than does the model in the study by Frith19. Moreover, the alterations of the reported inner experience of the schizophrenics are regarded here as crucial clues as to what modifications of underlying brain circuitry need occur in order to bring about the various reported schizophrenic experiences.
A brain-based approach must also take proper account of modifications in brain connectivity and gray cell count as observed experimentally in schizophrenics. An article by Thompson and colleagues24 has looked carefully at the developmental changes in the brains of early-onset schizophrenics as compared with normal adolescents. Their discovery of a wave of excessive gray matter loss as compared with controls, starting in the parietal lobes, is an important guide to any relation to overall circuitry assumed to be damaged or otherwise modified in such schizophrenics. Because much of the underlying CODAM brain circuitry is now understood,25 it is possible to use such graded loss observed24 to relate and understand experiential changes endured by the young developing schizophrenics.
Steps 1 and 2: Modeling Attention
Presently, a consensus of neuroscientists agrees that consciousness is attention based. Thus, we need to start by exploring attention as thoroughly as possible and hope that in the process we will discover neural activity that can be seen, from its character, as supporting conscious experience. In particular, there needs to be activity supporting the experience of the inner self. There are numerous models of consciousness that have been proposed (see, eg, articles cited in Taylor et al.26), but so far only CODAM9,10 has a basis in attention and is seen to have room for the inner self.
In order to give a simple introduction to CODAM, we propose to analyze how we might best model attention and consciousness in 4 steps, the first 2 in this section being about attention proper, and the further 2 steps in the following section as to how consciousness might be created by extending our model of attention. In such a manner, it is hoped to make the basis of CODAM clearer than approaching it in only one big step.
Step 1
In step 1, we specify attention as a control system. In other words, there are considered to be 2 parts of the brain that can be differentiated from each other:
I. The Controlled portion of the brain
II. The Controller portion of the brain.
The attention control system works in the obvious manner of sending control signals from the controller to the controlled parts of the brain to achieve a desired end or goal:
Controller → Controlled
This bipartite division of the brain has been justified by many brain experiments, on a range of mammals from humans to rats; in the human case, see, eg, the studies by Corbetta and Shulman27 and Bressler et al.28 The controlled portion of the brain consists mainly of the lower level sensory areas involved in processing input features, like the visual or auditory cortices. In the former, eg, there is a breakdown of an input stimulus into feature components of ever-increasing complexity along the hierarchy of V1, V2, V3, V4, and so on, concluding with object representations in the temporal lobe. Each of these modules can be attended to, in a correlated fashion, when attention is paid to a visual stimulus. On the other hand, the controller consists of parietal and prefrontal regions involved in setting and holding goals (of where and/or what to attend to) and in sending an attention signal back to the controlled regions in order to amplify the representation of the attended stimulus in the visual (or other sensory) field.
Again, much experimental data support this notion that the attention signal from the controller to the controlled region amplifies the activity of those neurons involved in representing the attended stimulus while at the same time inhibiting those neurons involved in distracter representations. It is in this way that the brain representation of the attended stimulus becomes dominant in the sensory cortices and can be used for higher level processing, such as thinking, reasoning, and so on.
The above controller/controlled division of the brain is not all that attention does, because it is even possible that it can fold back and “attend to itself,” but such further extensions do not change the main work that attention does for manipulating inputs in the brain from the outside world.
Step 2
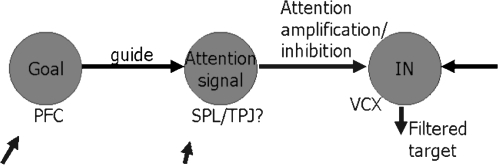
Step 2 can now be taken, by separating the controller brain region into a biasing or Goal module and a module for creating the attention signal to move the focus of attention under the guidance of the Goal module. These two modules are shown in the Ballistic Control model of attention movement in figure 1, with the goal module (denoted “Goal” in figure 1) being in the prefrontal cortex (PFC) and the generator of the signal causing movement of the focus of attention (denoted “Attention signal” in figure 1) being in the superior parietal lobe (SPL) or in the tempero-parietal junction (TPJ). The third module in figure 1 is denoted “IN,” being the visual cortex (VCX), where object and spatial features of the input stimulus are extracted in the cortex. Various inputs to the Goals and Attention Signal modules are shown, corresponding to emotional and long-term memory influences. There is also the important output from the IN module, being the attention-amplified target representation and denoted “Filtered Target” in the figure. It is this latter signal that the faculty of attention delivers to the rest of the brain.
Fig. 1.
The Ballistic Control Model of Attention. PFC, prefrontal cortex; SPL, superior parietal lobe; TPJ, tempero-parietal junction; VCX, visual cortex.
In the figure, the sites observed by brain imaging techniques activated by attention tasks are written beneath each of the functional modules.
Steps 3 and 4: Extending Attention to Consciousness
Step 3
Ballistic control occurs in the simplest example of firing a gun—it is aimed and then fired. Once fired, it is not possible, by feedback, to modify where the bullet is going. A step toward improving such control, being step 3, is to introduce a site where the attended stimulus can be stored for a short time (such as over a few seconds). Usually called a buffer or working memory site, such sites have also been observed, being able to hold activity for a few seconds.29–31 This buffered activity could be used to correct the mechanism to produce it if the buffered activity were in error, as compared with the goal stimulus to be attended to. We will come back to the error-correcting process shortly.
It is now accepted that buffer sites (there are more than one, these being different for different modalities) in the brain play an important role in consciousness. If they are destroyed by stroke, then the associated conscious experience is annihilated. That experience will return if there is recovery of these buffer sites. It has been accepted in neuroscience that these buffer sites provide the content of the conscious experience of the particular attended stimulus (in association with lower level stimulus activity in the various feature modules). It is thought that a given attended stimulus attains awareness due to its activity being augmented by attention, with distracters being rejected (by inhibition from the attention feedback signal). Such an acceptance/rejection process could occur by access to the buffer site being through incoming activity, amplified by attention, attaining a threshold of activity, and thereby activating the relevant buffer code. This helps explain why attention is needed for consciousness of a stimulus.
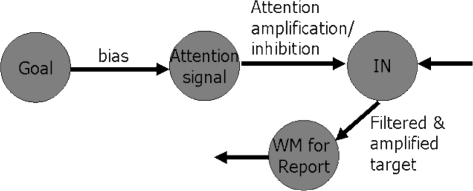
The extended ballistic model of attention control is shown in figure 2. The working memory module (denoted “WM for Report” in figure 2) is used to store the attended stimulus representation for report to other such sites, so leading to thinking or reasoning, or just reporting.
Fig. 2.
The Extended Ballistic Attention Control model, with activity on the extra working memory buffer (beyond the modules in figure 1) allowing for further use of the representation of the attended stimulus, for reasoning, thinking, and so on, by the subject. WM, working memory.
The extended ballistic control model, from what was said above, would appear to possess room for explaining consciousness, at least of the content of stimuli in the environment being attended to. But there is the important question: Who has the experience of that consciousness? There would appear to be no inner self that can have such an experience. Just holding activity in a brain site does not mean that activity becomes part of the conscious experience of the owner of that brain. Continued activity can occur in various sites, such as hippocampus, but that organ can be lost without loss of consciousness, as in the well-known case of the subject HM (whose hippocampus and middle temporal lobes were removed to reduce his terrible epileptic seizures, but who remained conscious till the recent end of his life). Something is still missing in the attention model created so far. Who is having the conscious experience?
Step 4
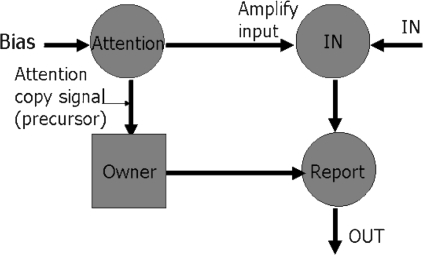
The fourth and final step to a model of consciousness is that taken in CODAM: This involves the presence and use of a corollary discharge (a copy) of the attention movement signal, so as to provide greater efficiency in moving the focus of attention. It is this final step that is more conjectural but needs to be made to bring attention control into the modern age. The resulting architecture is shown in figure 3. Here the goal module, providing the bias to where the attention focus must move, is just indicated by the bias input on the left. This causes the “Attention” module to generate the required movement of the focus of attention to the relevant biased position or object and so to amplify the input activity representing the attended stimulus (spatially or as a specific object). The IN module denotes the VCX for visual attention, as before.
Fig. 3.
The CODAM (COrollary Discharge of Attention Movement) Model.
The Attention copy signal is shown proceeding from the “Attention” module to a module denoted “Owner” in figure 3. This acts as a buffer to hold the content of this attention copy signal for a short time. The buffered signal will thereby be available for a number of things: to speed up the access of the attended stimulus activity into the working memory module and also to correct any errors that might be made (such as allowing distracters to creep into the working memory module, so into the content of consciousness). The copy signal can also be used to increase the posterior-going attention signal if there is a possible problem with a distracter. This extension thereby allows corrections to be made to attention movement, in comparison with the rather inflexible ballistic control. It is important to notice that these corrections can be made early in the creation of consciousness of a given stimulus, because the corollary discharge is available immediately after the attention movement signal has been produced, and does not have to wait on the amplification of posterior neural activity (which may take some 100–200 ms).
It is the attention copy signal that, it is claimed in CODAM, provides the “owner” content of the relevant neural activity. It generates the experience of the “inner self,” that of ipseity. As such, the CODAM extension of figure 3 allows for the inclusion of the necessary complexity of consciousness creation by attention to begin to tackle, eg, the experiences of schizophrenics.
The various modules are explained in the text by the figure. The only new module is the “Owner” module not present in figures 1 or 2.
Evidence for CODAM
What is the evidence for the existence of such an attention copy signal in the brain, and hence of a CODAM style of attention control architecture? There are several such lines of evidence, of which the three main ones are (1) the proposed premotor model of attention17; (2) the improvement of control achieved by the use of an attention copy signal; (3) experimental results coming from the AB,32 trying to model these results through CODAM,33 and how the AB is increased in schizophrenics, with again modeling of experimental results by CODAM.13
The premotor model of attention17 is based on the idea that attention and motor control share a certain amount of brain circuitry at the higher levels in the brain. A copy or corollary discharge model has been used by a number of researchers to better understand features of motor control in the brain,18 and a motor control copy or corollary discharge signal has even been directly observed there.34,35 The attention copy model of CODAM is a natural extension of the motor control processes in the brain to its companion faculty of attention. The considerable overlap of brain sites, such as in eye movement control vs covert (eyes fixed) attention control would lead one to expect a similar corollary discharge to exist for attention control as it does for motor control. CODAM fulfills that expectation.
The range of models of engineering control was enormously enlarged over the past few decades by the development of models employing a corollary discharge of the control signal to make the control more efficient. This was achieved by inserting into the control structure a predictor or forward model of the expected state of the controlled system (where, eg, the state of a steel-making plant would at its simplest be the temperature of the steel, and its forward model would predict the change in temperature of the steel when a certain amount of coal was used; for attention in the brain the state of the controlled system is that for the neuron activity of the “Report” buffer of figure 3, not for the whole environment in lower sensory brain areas, while the predictor would be the corollary discharge buffer, so the activity on the “Owner” module of figure 3). Such a predictor uses the corollary discharge signal to make a prediction of what the next state of the system would be if the control action were to be used on the system (such as feeding heat to the steel-making plant). Such a prediction would then be used to check for (and thereby correct) errors about to be made by the original control action, as described earlier.
The process of filtering out the distracters, leaving solely the attended stimulus activity for further manipulation, as in reasoning, thinking, and so on, is an important component of attention. With the extended attention copy model of figure 3, the owner module is the predictor, and its output will thereby be the predicted state of the attended stimulus (ahead of its arrival). This predictor activity can be used to compare with that on the goal module. The difference between these two neural activities will be an error that can be used to modify the attention control signal accordingly (to remove the error) even before arrival of the attended stimulus to the working memory buffer. The output of the owner module can also be used to speed up access to the working memory report module of figure 3. In both ways (error correction and speed-up of attention amplification), greater efficiency will thereby be obtained in attention control. Evolutionary theory would lead us to expect such mechanisms therefore to be used in the brain. This leads to a clear function for consciousness (as seen in the CODAM model): to make the bringing of stimuli into the focus of attention more efficient (faster and more error free). This negates the idea that consciousness is a pure epiphenomenon.
3. The AB has been much studied over the last few decades in normal people due to the paradigm being able to explore how attention can be broken down by a very difficult task and thereby several of its components exposed. The AB involves a rapid serial presentation of visual stimuli (RSVP), such as digits and letters, at a rate of about 10 Hz. In the paradigm used recently13 to compare the AB between schizophrenics and normal controls, the RSVP stream involved the digits 2, 3, 4, 5, 6, 7, 8, and 9 as distracters and the capital letters A, C, E, J, K, R, T, and Y as targets. The task for both classes of subjects (37 schizophrenics and 26 normal controls) was to correctly identify a first target (T1) (at the fixed focus of attention) when it appeared, and then to identify a second target (T2) (also at the same attention focus) after the appearance of the first. The second target could appear at one of several lags after the first, thus testing the difficulty of such identification by determining the probability of determining T2 given T1 was correctly detected, denoted by Pr(T2|T1), for various lags. The resulting values of Pr(T2|T1) were then plotted against the time lag of the detection of T2 after T1 (from lags 1 to 5 in the first instance).
In normal people, the AB arises as follows. Some time is expected to be taken by a subject attending to processing the first target T1. If the second target T2 is shown too soon after the first, then T2 may not be allowed to be processed by the events involved in the processing of T1. Thus, if T2 is presented within a few lags after T1, there is observed a dip in the plot of Pr(T2|T1) against lag number for control subjects, with the least value of Pr(T2|T1) being at about 3 lags (or 270 ms) after T1. That time delay is thought to occur when attention is being focused maximally on T1, it has been suggested, so preventing attention being spread further to T236: It is when “attention blinks shut.”
It was found furthermore13 that the curve for the values of Pr(T2|T1) for schizophrenic patients over the set of 5 lags was very similar to that for the normal controls but was lowered by a constant value of about 0.2 at each lag. The minimum of the U-shape of the two curves was similar (this being the AB itself) lasting from lag 2 to lag 4, and a minimum at lag 3. A check was made that neither of the results was due to visual masking by increasing the duration of the distracter stimuli after T1 or T2. This modification did not increase the difference between the normal controls and the schizophrenics, so implying that there was little effect of visual masking and the main effect was due to the difficulty that T2 experienced in trying to gain access to an appropriate visual short-term site (called the “WM for Report” site in figure 2) while T1 was still being processed. Thus, the AB is larger for schizophrenics as compared with normal controls, due, it is expected, to schizophrenics having less control over their movement of attention focus (from T1 to T2 in this case).
A mathematical formulation of the CODAM model, with the more complete architecture of figure 4 (to be discussed shortly), was used to simulate the AB33 and showed results consistent with numerous publications of the U-shape of the curve of Pr(T2|T1) against the time lag of T2 after T1. The basic mechanism of the CODAM model leading to this result on the AB in the RSVP case was due to the inhibition present in the detailed CODAM model being used. This arose from the activity of the first target achieving its access to the “WM for Report” module of figure 3 also inhibiting distracter-based activity trying also to gain access to that module. Because T2 will be regarded by a subject's brain as a distracter compared with T1 (at least if T1 is still being processed), there will be inhibition of T2, thereby bringing about the damage to its detection as shown by the AB (especially damage to the corollary discharge signal associated with T2 on its “Owner” buffer37).
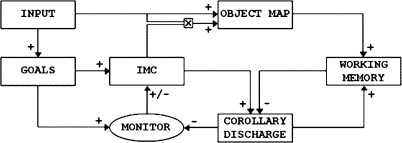
Fig. 4.
The Full CODAM Model of the Control of Attention. The action of the various modules of this figure and the relation to those in figure 3, are explained in the text.9,10 CODAM, COrollary Discharge of Attention Movement; IMC, inverse model controller.
Furthermore, the model results were supported by more recent data of32 on the presence of inhibition of T1 on its Report module caused by the activity of the attention copy model for moving the focus of attention to T2. The resulting alterations of the usual sequence of brain waves observed during conscious report (so access to the “WM for Report” module of figure 3) fitted very well with the simulation results of these inhibitory processes obtained from an extended version of CODAM with inhibitory feedback from the “Owner” module of figure 3 to distracter nodes on the “WM for Report” module of figure 3.38
Finally, simulations were also made of how damage to the input of the WM Report module of figures 3 or 4 (by reducing the strength of the response for each neuron in the modules feeding to it, especially the corollary discharge buffer or “Owner” module of figure 3) can affect the AB curve (of Pr(T2|T1) against the time lag between T1 and T2) so as to mimic what might be occurring in the case of a schizophrenic. The simulation results (N. Fragopanagos, PhD, and J. G. Taylor, PhD, personal communication, 2006) fitted closely the modification of the AB curve for schizophrenics as compared with that for controls in the study by Wynn et al.,13 in that there was a reduction of the ability to observe T2, due to the reduced T2 input to its buffer, the WM Report module of figure 3, given that T1 was observed. Reduction of such input could be due to the reduction of output from the “Owner” module of figure 3, consistent with a reduction of the sense of ipseity in schizophrenics.
Overall Conclusions and the Full CODAM
The basic postulate of the attention copy approach to consciousness is that the experience of ownership of a phenomenological experience is generated by the activity of this attention copy signal residing briefly on its component of the relevant working memory site. Such a site is shown in figure 4 as the “Corollary Discharge” module (and equal to the “Owner” module of figure 3). The architecture of figure 4 is a more complete (and complex) extension of that of figure 3 and was that used in the simulation of the AB and other attention-based paradigms (N. Fragopanagos, PhD, and J. G. Taylor, PhD, personal communication, 2006).33,38,39 There is now included in figure 4 the Goals module (biasing the movement of the focus of attention, as in figure 2), the corollary discharge module (the ‘owner’ module of figure 3), and the new “Monitor” module (used to generate the error signal between the predicted future attended state of the VCX, as contained in the corollary discharge module, and the Goals module). The module generating the signal to move the focus of attention, so at the center of attention control, is denoted IMC (for inverse model controller) and is identical to the “attention” module of figure 3. Finally, the input module IN of figure 3 has been extended to both an “input” module and an “object map” module in figure 4. The arrows indicate the direction of flow of information, with the positive or negative signs indicating if the input to a given module is excitatory or inhibitory. The extra cross symbol in figure 4 on the input from the IMC to the object map indicates that the attention feedback from the IMC is suitably fused with the input to the object map (in the original simulation, a multiplicative fusion was used).
We note that there is a crucial difference between the CODAM attention control architecture of figure 3 (or its more complete form in figure 4) and that for standard control theory or that suggested as arising for motor control in the brain18: The attended state estimator (denoted “working memory” in figure 4) and the predictor module (denoted “corollary discharge” in figure 4) only concern themselves with the attended target stimulus and not with distracters (except to inhibit them). Thus, there is not an estimate of the whole environment as contained in the posterior cortex (a role played by posterior VCX in the case of vision), but only of the attended state of the environment, where the environment is represented in the brain by the activity across lower level posterior cortex. This is the basis of the filter process of attention and produces a working memory module activation in which distracters are not represented; this is a crucial step in a complex environment to reduce its complexity for efficient higher level processing.
To repeat, improvement in attention processing (so going from step 3 to step 4 above) is achieved by the use of the attention copy signal buffered for a short time on the corollary discharge module. As noted in the study by Sergent et al.32 for the AB, there is inhibition of the access to the “working memory” module in figure 4 for the first target by the access of the second to its “corollary discharge” module of figure 4, as they report in their experiments.32 It is that sharpening-up process which has been suggested to be carried out by the attention copy signal, as a process of filtering out distracters (and is described by inhibition in the input from the corollary discharge module of figure 4 to the working memory site, needing to be added to the excitation sign of figure 4). At the same time, the monitor can be used to reactivate the attention signal (generated by the attention controller) by a rehearsal goal.39 Thus, the various components of the extended architecture of CODAM can achieve considerable processing efficiency by means of the several features of attention control the model contains. As such, the CODAM architecture is an attention analog to the motor control models proposed as making motor control more efficient than by use of a purely ballistic control system, although with added inhibition (from the corollary discharge signal) to prevent distracter access to the input buffer, and the filtering process to leave only the attended stimulus activity as the attended state representation on its working memory buffer.
The “immunity to error by misidentification of the first person pronoun” feature40 is an important component of experience. It arises from the fact that one cannot ask a friend, when they say they are in pain: “Are you sure it is you who are in pain—could it be someone else?” They are sure it is they who are in pain. This feature of experience can be explained as part of CODAM by the lack of error in the boosting process of the attention feedback signal (together with the inhibition of distracters). If an incorrect stimulus (a distracter) is trying to enter the visual working memory, then it will be inhibited by the ownership signal (from activity on the “corollary discharge” module of figure 4), which will only let through to awareness of content that target stimulus representation which is desired by the original goal (for endogenous attention) or has grabbed attention control (for exogenous attention).
We note that originally this feature of experience (immunity to error about “I”) was claimed to have logical status,40 but more recently, experiments have shown that the feature has only empirical grounds for validity. Thus, in the study by Mizumoto and Ishikawa,41 it was shown by the use of a head-mounted display (showing the user an externally based view of his body) that such immunity could be lost. A similar situation could arise in a CODAM-based version of the inner self, where breakdown of parts of the system could destroy the properties required to give a unique attribution to oneself of incoming stimuli onto the relevant working memory buffer. We will discuss an example of that in the case of hallucinations in schizophrenia later.
The copy of the attention movement signal held on the corollary discharge buffer of figure 4 is proposed as the source of the inner self. The related neural activity will function, it has been proposed,9–11 so as to represent the owner of the about-to-be-expected attention-amplified activity due to arrive at the sensory working memory module of figure 4. Such an interpretation of the source of the ownership of conscious content is consistent with the interpretation of the inner self as being content free.2 This is due to the high-level coding of activity in the corollary discharge module of figure 4 and that in the attention controller or IMC of figure 4 (from which it receives its activity). There is other neural activity to which the corollary discharge module has access, such as that of the Monitor and the attention controller, besides that on the sensory working memory buffer, all providing further expansion of the ownership activity on the corollary discharge buffer. These other activities involve crucial processing stages, so that the corollary discharge module functions as being at the center of a network of highest level processing sites in the attention network. This module therefore has high-level content that preserves its character of “nothingness”42 but expands its processing powers.
We add that nothingness was an important concept in Sartre's philosophy,42 and as noted in Sokolowski12(p220), in a comment on Sartre's philosophy: “; negation is not merely a feature of our judgments, but is given in the intuitive experience that precedes judgment.” In CODAM, this intuitive experience of negation preceding judgment is created by the activity of the corollary discharge just before the access of the content of consciousness, describing a particular attended stimulus, to attain its buffer working memory, as explained earlier.
We can relate to the results of Western phenomenology mentioned earlier (the protention–primal impression–retention sequence of the creation of consciousness) by means of the identification of “protention” with the early stages of manipulation by the corollary discharge signal, especially on its buffer. Second, we can identify the “primal impression” with the accessing of the activated and attention-amplified lower level stimulus activity onto its working memory buffer. Finally, we identify “retention” with the decaying activity on this working memory buffer.
The attention copy model is related to the higher order thought (HOT) approach to consciousness.43,44 This popular approach to consciousness supposes that there are several levels of thoughts in the brain. Consciousness is supposed to arise by a higher level thought “thinking” about a lower level one. There are numerous problems about this approach, especially why it is that consciousness is conferred on a thought by having a higher level thought focus on it. However, we may link up the CODAM model presented above to the HOT idea by regarding the second-order “thought” of the HOT approach as the attention copy signal or corollary discharge of CODAM. There are no further even higher order “thoughts” in the CODAM approach beyond the possible second level, so avoiding the infinite regress well known to occur in the standard HOT approach.2 In CODAM, the attended stimulus signal, once on its working memory buffer, is assumed to be able to access other high-level sites for report, thinking, and so on, and hence becoming conscious. The critical question of for whom the buffered signal is conscious is answered by the corollary discharge signal: that provides an owner of the experience, but no content itself, so not actually being a second-level module “thinking” of the lower level sensory buffer module.
Another popular model of consciousness is that of the Global Workspace (denoted GW45,46). Here, it is assumed there to be a globally connected set of modules (the GW) for which once a stimulus gains access to it the stimulus becomes conscious. It can be seen that this complements nicely the CODAM model, which involves the details of the process of access of the activity denoting an attended stimulus to the GW (identifying the GW as the set of working memory buffers in the brain). How this activity is then handled is then part of the GW model. However, we see that CODAM puts the emergence of consciousness into the dynamical interactions involved in the amplification of an input stimulus to gain access to its part of the GW, especially aided by the associated corollary discharge mechanism. Thus, the GW approach needs the further dynamical mechanism of CODAM to endow the activity entering the GW with consciousness (otherwise, the GW model is just a collection of working memory buffers, which we have already noted has no experience of consciousness).
We now turn to the question raised earlier: How does the CODAM model of consciousness face up to the criticisms of the control model approach in Cermolacce et al.8 and Gallagher20; we do not have the intention to think and then do the thinking (or have a conscious experience): We just think. But Cermolacce et al.8(p710) even claim that “In fact, the major problem of the neuro-cognitive models for us […] is that they conceive the self as a detached initiator of action or as an independent owner of experience […] distinct and distant from the self.”
This is not a correct criticism of the attention copy CODAM model of figure 4. For the self, as inner self identified as the activation of the corollary discharge buffer is explicitly not an initiator of any action, be it of motor or attention movement form. Its purposes, as already described above, and more fully in numerous articles as referenced in the articles by Taylor10,11 (and citations therein), are to speed up the access to the sensory buffer by the attended stimulus representation and to ensure freedom of such access from distracters causing errors of access to consciousness. In CODAM, all initiation is done by goal modules in frontal cortex and motivation sites in orbitofrontal cortex (along with subcortical motivational sites, as in hypothalamus, nucleus accumbens and brain stem sites). The dreaded homunculus has been completely deconstructed in the process. At the same time, the attention copy signal is not an independent owner of experience, as Cermolacce et al.8 and Gallagher20 might claim. Its functioning is tightly bound together with the incoming stimulus representation to achieve access by the latter to its buffer.
Finally, we summarize the various brain sites in table 1, now recognized, by many brain imaging experiments, as employed by most of the components of CODAM (so as to draw together the various remarks already made on relevant brain sites in the article); the various sites are taken mainly from the recent review of Corbetta et al.25
Table 1.
Brain sites for the components of CODAM
| Module | Function | Suggested Site |
| GOALS endogenous | Maintain task goals | DLPFC/FEF |
| GOALS exogenous | Attentional boosting of input | MFG |
| IMC (endogenous) | Attention control | SPL/IPS |
| IMC (exogenous) | Attention redirection | TPJ |
| WM | Stimulus maintenance during delay | SPL/IPS/IPL |
| OBJECT MAP | Perceptual processing of input | DOC/IT |
| MONITOR maintain | Monitor WM activations | FEF |
| MONITOR compare | Compare WM and probe | Cb/pulv/ACC |
Note: ACC, anterior cingulate cortex; Cb, cerebellum; CODAM, COrollary Discharge of Attention Movement; DLPFC, dorsolateral prefrontal cortex; DOC, dorsal occipital cortex; FEF, frontal eye field; IMC, inverse model controller; IPL, inferior parietal lobe; IPS, intraparietal sulcus; IT, inferior temporal lobe; MFG, medial frontal gyrus; pulv, pulvinar nucleus of the thalamus; SPL, superior parietal lobe; TPJ, tempero-parietal junction; WM, working memory.
The sites specified in table 1 are as observed by the use of functional magnetic resonance imaging (fMRI) and indicate the presence of two coupled circuits for the movement of attention:
The dorsal route for endogenous (internally held) attention, involving the dorsolateral prefrontal cortex and frontal eye field as sites of goal activation, the SPL as the site of the attention control signal generator, and the VCX (dorsal occipital cortex and infero-temporal lobe) as the area under the control of the attention feedback signal;
The ventral route for exogenous (externally based) attention redirection, with the medial frontal gyrus as involving exogenous goal activities, TPJ as a component of attention redirection to modify the dorsal attention controller guiding visual attention feedback, and various sites in SPL/inferoparietal sulcus/inferior parietal lobe as buffer working memory sites.
We will not use in this article these details of the difference between exogenous and endogenous attention control, though we felt they should be noted; CODAM will here be taken to apply to both circuits. Moreover, we will assume the existence of attention circuits in other modalities with a control structure similar to that of figure 1; there is some experimental support for such an assumption, although that goes beyond the remit of this article.
The above description and discussions of the CODAM model for consciousness indicate that there is strong support for the validity of CODAM up to and including step 3, the extended ballistic model. The corollary discharge signal required for validation of step 4 has theoretical and some experimental support but requires much more. Thus, CODAM is still only tentative. Its ability to explain the rich phenomena of schizophrenia would undoubtedly provide it with further support.
Schizophrenia Explained by CODAM?
We now attack the problem of explaining some of the symptoms of schizophrenia through the attention copy CODAM model of the previous section. We consider each of the 3 kinds of symptoms sequentially, using the discussion of Sass et al.1 We also include in our considerations the prodromal symptoms considered in Sass et al.,1 regarding this as indexing a precursor stage of the disease.
Prodromal Symptoms
Such symptoms have been carefully discussed in Sass et al.,1 where the paucity of data was noted but also that there were results from follow-back studies of the early experiences of schizophrenics. In particular the study of Klosterkotter et al.,47 confirming earlier studies, showed that in the prodromal stage there are a multitude of anomalous experiences such as varieties of depersonalization, disturbances of the stream of consciousness, and distorted bodily experiences. As noted in Sass et al.,1 the patient complains of a profound change but cannot easily describe it; complaints can be from “I don't feel myself” or “I am not myself” to “I am losing contact with myself” or “I am becoming a monster.” A patient will even say that “My I-feeling is diminished” or “My I is disappearing for me”.1(p438)
Such symptoms could arise from a CODAM type of attention control by a reduction of the activity on the corollary discharge (attention copy) signal buffer posited in CODAM to be the source of the experience of the “inner self” or “owner.” It is this component in CODAM that is supposed to provide the sense of ownership of the forthcoming phenomenological experience of content—the red of the rose, the bouquet of the wine, the flavor of the steak. If there is reduced activity on the corollary discharge buffer then, by CODAM this ownership will itself be reduced, and the experience of “losing contact with myself” would have a physiological basis. Thus, the hypothesis fitting many of the prodromal symptoms is that they are caused by incipient reduction in the activity of the corollary discharge buffer.
Positive Symptoms
These symptoms, especially the first-rank symptoms, are, as noted in Sass et al.,1 defined by a “kind of diminished self-affection.” This is described by a reduction of the sense of inhabiting one's own thoughts, feelings, actions, and so on. All have become alien, to some degree, even to the point of regarding them as under the control of an alien force or other person. A sufferer may comment that they “only feel half there.”
Such a sense of loss can be ascribed, by the CODAM model, to the reduction of the normal effect of the corollary discharge/attention copy signal in aiding the brain's attended stimulus representation attain awareness. In the positive symptom schizophrenic case, then, the attended stimulus is thereby reduced in its activity when it finally achieves consciousness. There will therefore be expected to be a lowering in this process of the involvement of the inner self with the resulting sensory buffer activity. Extending this to all lower level brain activity to which attention can be paid leads to the diminished self-affection cited by various authors.48,49
We note that reduced activity on the corollary discharge buffer has already been introduced for the prodromal symptoms. Here, we go beyond the lowered value of corollary discharge activity on its buffer by considering in more detail the lower value of the degree of inhibition produced on the sensory input buffer by the output of this corollary discharge. This reduction will successively let in more and more distracters as the level of inhibition is reduced. But then inputs of which the subject is not normally conscious will arise in the subject's experience. This process may not be trivial in the case of inner speech causing hallucinations, as we now consider.
A great deal of discussion has been given over the last 2 decades about the thesis that damage to the motor control corollary discharge or some related motor control comparator system using such a signal in the schizophrenic brain is at the root cause of their hallucinations associated with inner speech, regarded as one of the important positive symptoms.19 The agent (the subject) misattributes the origin of their inner speech to that of an alien. However, the thesis has numerous problems, some noted earlier.8,20 In the CODAM approach, the inner speech is still in the subject's stream of consciousness. The content of the inner speech will be caused by subconscious thought processes emerging into the subject's consciousness due to emotional salience or some other value they possess (such as being unpleasant about themselves). This inner speech will thereby attain the sensory buffer for report to other brain sites. However, the subject does not own such inner speech (this not being a question of agency but ownership). This paradoxical situation (“it's in my consciousness as being reportable but I don't own it”) is one explicable by the general feature of reduction of the power of the corollary discharge activity in controlling access to consciousness of input to the sensory buffer. If there were no strong corollary discharge signal at the emergence of the inner speech onto the sensory buffer, then there would be no ownership tag associated with it. Hallucinatory phenomena as part of the positive symptoms would thus be interpreted by the subject as involving loss of ownership. There is still the question as to how the hallucinations or the inner speech can enter into consciousness if not boosted by the corollary discharge mechanism of CODAM described earlier. This must involve a complex dynamical situation, with, say, emotionally valued inner speech signals bringing attention to focus on itself as signals at both unconscious and working memory level. In other words, attention would be drawn not only to lower level but even working memory–level brain activity. Thus, fully owned consciousness would then arise, with the associated ownership experience (from the corollary discharge), soon after the inner speech attains the auditory buffer. Thus, the hallucinatory signals, on the CODAM approach, would enter fully into the subject's consciousness by a two-stage process:
(a) initial access of the inner speech onto the auditory buffer (due to its valence and unaided and uninhibited by the corollary discharge system);
(b) the focus of attention then being drawn to this salient inner speech at various levels in the brain, thereby inserting it into the subject's “owned” stream of consciousness.
Undoubtedly, the experience of having a signal being reportable across one's brain (the component (a) above) followed shortly thereafter by its being acknowledged as in one's own consciousness, would be disturbing, and could well be ascribed as being caused by an outside agent. We note finally that this is the example mentioned earlier as to an empirical breakdown of the “immunity to error of misidentification of the first person pronoun.”
Negative Symptoms
These include poverty of speech, affective flattening, avolition, apathy, and a general lack of notice of the outer world. However, as importantly noted in Sass et al.,1 some subjects report no deficit in affect or thinking, but did report being lost in their own thoughts and reducing their actions. Following Blankenburg,50 it would appear that for the central defect (more generally in schizophrenia but most specifically for the negative symptoms), there is a “loss of natural self evidence—of common sense orientation to the world.”
We can begin to see in general how such a loss could occur in the case of damage to the attention copy model of figures 3 or 4. If the attention copy signal were especially reduced in its distracter inhibition component, then otherwise prevented inputs would be allowed into consciousness. This is especially noted in schizophrenic cases of bodily processes becoming available to consciousness.
The poet and essayist Antonin Artaud is a famous case of this. He describes his consciousness as being invaded by bodily functions, which would normally be outside consciousness. Thus, he writes of “the limbo of a nightmare of bone and muscles, with the sensation of stomach functions snapping like a flag in the phosphorescence of the storm” and “images of bloody old cottons pulled out in the shape of arms and legs, images of distant and dislocated members.”51–53. In particular, in the study by Sass53 there is a lengthy and deep description of the writings of Artaud and how his experiences are explicable in the framework of loss of ipseity.
More generally, the common sense schema (concerning use of a familiar external object often met with) that normally operates outside awareness would now intrude on conscious processing. As a result, the originally automatic schemata would be coded in such a manner they could not be carried out under conscious control. Thus, the subject would have to “start all over again” to learn how to handle these distracters and so overlearn them to regain their being automatic. So the subject would feel uncertain as to how to proceed, and certainly could not do so automatically and smoothly. This would therefore explain the “loss of natural self-evidence” in many cases of schizophrenia. Attention would have to be brought to bear on the schemata involved (if they involve responses) or the sensations (if they are only at the level of perceptions). Altogether, the patient would be expected to be considerably slowed down, if not completely flummoxed, by such apparitions in their consciousness.
It is necessary to take careful note of how what would otherwise be automatic schemata can enter consciousness. These schemata would normally have no connection to the sites of consciousness creation, here being taken as the buffer working memory sites dotted about various higher order sites in the brain. However, to become an automatic schema a sequence of actions and visual states, eg, must be overlearnt. Initially, there will have been effortful, attended processing of this sequence, so of connections to the relevant buffer sites (hence, the schema is in consciousness). As learning proceeds to an overlearnt situation, the possible internal models trained to hold any such schema become outside attention (which is not now needed to be applied to run off the schemata in an error-free manner). In other words, the internal models can function outside consciousness. The manner this occurs may be, eg, by inhibition of connections to the relevant buffers otherwise granting consciousness to the states of the internal model. But the problem in schizophrenia is that such inhibition may fail, as we are positing it does for the inhibition (of distracters) arising from the attention copy signal. There will then no longer be efficient prevention of such distracters from impinging on the visual buffer. This leads to a loss of “common sense” about the world.
Disorganization Symptoms
These occur along the lines expressed by their title: incoherence and pressure of speech, poverty of speech content, distractibility, tangentiality, and derailment. It is clear that these would occur if the overall attention control system were itself breaking down, although this may occur in either a patchy or a sequential manner. If the latter were so, then there will be a whole spectrum of increasingly disorganized symptoms. The ones mentioned above could finally conclude in an almost total breakdown in the overall control guidance by the attention control signal generator. On the other hand a patchy process of breakdown of the attention control circuitry will not lead to such an ordered process of breakdown of behavior but be closer to the variation of symptoms in many reported cases. In either situation, the nature of the temporality of the overall loss of attention control is of importance in understanding the mechanisms involved in causing the overall control breakdown.
The writer Artaud described his experiences of this state graphically33(p294):
This slackening, this confusion, this fragility … correspond to an infinite number of new impressions and sensations, the most characteristic of which is a kind of disappearance or disintegration or collapse of first assumptions which even causes me to wonder why, for example, red (the color) is considered red and affects me as red, why a judgment affects me as a judgment and not as a pain, why I feel pain, and why this particular pain, which I feel without understanding it.
Yet again the loss of common sense about the world is evident not only in Artaud's experience but also the plethora of impressions rushing in on him, so that he finds it difficult to concentrate on a single one. However, he is driven to do so and thence to lose a common sense approach to the world, with reduced ability to inhibit the components normally processed automatically in association with the various stimuli in his sensory field, such as the affordances they provide and the processing schemata they automatically generate in relevant internal models.
That there is a loss of distracter inhibition in schizophrenia is supported by the observed increased AB in schizophrenics, as we discussed earlier in the article.
Relating CODAM to Brain Sites Involved in Schizophrenia.
The simulation of the behavioral results described above in terms of the effects on the AB of schizophrenia give support to the CODAM model of consciousness in its application to schizophrenia. There is further support arising from a more detail approach using results on changes in the structure of the brain as schizophrenia progresses. We take here the sites observed by Thompson and colleagues,24 which were mentioned earlier as revealing most dramatically an excessive loss of gray matter compared with controls in the brains of early-onset schizophrenics (followed by yearly MRI brain scans from age 13 to 18 years).
In particular, the earliest loss was of parietal brain tissue that preceded puberty. It was found that “Second, parietal and motor cortices showed a severe early deficit with diffuse loss in other (but not temporal) cortical regions.”24(p11653) Subsequent loss of gray matter was then observed to occur in a “dynamic wave of progression” from parietal cortices into superior frontal, dorsolateral prefrontal and temporal cortices. Moreover, this dynamic wave was conjectured as being triggered partly by genetic and partly by environmental influences, from the known deficit in parietal lobes of adult patients relative to their genetically identical controls (for monozygotic discordant twins).
Important further features were also reported in the study in Thompson et al.24: Faster loss in temporal cortices was correlated, across patients, with a more severe level of positive schizophrenic symptoms, whereas faster loss rates in frontal cortex was correlated with patients with more severe negative symptoms.
We can attempt to relate these loss rates and symptoms with defects in the CODAM model. This had already been discussed in the previous section, where we considered the four classes of symptoms, being prodromal, positive, negative, and disorganization symptoms. These could be considered as arising from successively enlarged defects in the processing by the various modules in the CODAM model of figure 1. Such enlargement can be seen to correspond to the dynamic wave of degradation of gray matter in various parts of the brain noted above and described more fully in Thompson et al.24
In more detail, we see that the prodromal symptoms can be ascribed to the parietal gray matter degradation in the earliest prepubertal stage in the adolescents in that report, where we site the attention copy buffers as part of the parietally placed working memory buffer sites. These are especially important in the CODAM model as supporting both the attention control signal generator and the sensory input and corollary discharge buffers. Critically, degradation in the last of these modules will be expected to cause reduced output from that site, so the reduced level of inhibition of distracters; this would lead to the loss of common sense as well as reduction in the sense of “I” as noted earlier as occurring in prodromal symptoms. Associated motor problems also fit into these results.
The later degradations in the temporal lobes are correlated with positive schizophrenic symptoms.24 In CODAM, such degradations are expected to produce degrading effects on object representations (in the temporal lobe) as well as those on the TPJ, a crucial part of an extended attention controller (on inclusion of the SPL) in providing exogenous attention control.25 The first of these degradations (those in the temporal lobe) would be expected to cause unexpected and noisy object activations and lead to unexpected hallucinations not ascribed to oneself, both in audition as well as in vision, as already described in more detail above. There may also be loss of affordance codes (in superior temporal sulcus, eg), thus explaining some of the motor control deficits in schizophrenia. The second (associated with loss of TPJ) would lead to loss of attention to the unexpected appearance of stimuli, so the slowing of responses. This may contribute to the sense of loss of the self, as noted earlier under positive symptoms, as well as to a slight delay in the experiencing of consciousness reported by several schizophrenics.
Later loss of gray matter occurring in the PFC will be correlated with the development of negative symptoms.24 This would correspond to loss of goals and the resulting attention guidance and fits well with the related negative symptoms of avolition and apathy; for the reduction of goal activation would lead to less overall involvement of attention to the outside world.
Not only are goals held in PFC but also decision making occurs as guided by activities there, especially in cingulate cortex, but also supported by activity in dorsolateral PFC. Thus, if these areas are damaged (due to excessive loss of gray matter), then not only are goals more difficult to store but also decisions are difficult to make, even if goals are available. Prefrontal symptoms are well known to involve loss of decision-making ability, so this is also expected to occur during disorganization symptoms.
Finally, the consolidation of degradation across these areas of the cortex would be expected to lead to increased breakdown of increasing many of the control circuits of CODAM in the brain and so to less overall control of attention. This was noted earlier as basic to the disorganization symptoms. Thus, these symptoms are to be regarded as arising from the general breakdown of the CODAM attention control model.
Thus, there would be expected a gradual progression of damage in some schizophrenics of their attention control system and related experiences of consciousness. It would be important to explore this aspect further in order to determine if such progression is observable across patients, or if, instead, schizophrenic breakdown of the attention control system is patchy. Both possibilities should be looked out for: some patients having patchy attention control system degeneration, some having a continuous and ever-more embracing loss of attention control. These differences between gradual degeneration and patchy loss of control structures in the brain might be due to differences of damage to the neuromodulatory systems underpinning the overall attention control system.
Biochemical Underpinning
An excess or deficit of various neuromodulators has been suggested as a causative factor in schizophrenia: too low a level of dopamine (as part of the hypofrontality observed by numbers of researchers in schizophrenia) or excessive dopamine sensitivity (as in the striatum54), serotonin, noradrenaline (conjectured as crucial in the exogenous attention circuitry in the brain25), excessive or reduced acetylcholine (the basis of normal attention control). Any or all of these breakdowns in correct levels of normal function may occur. Here, we consider the proposition that acetylcholine may be of crucial importance in schizophrenia, because of its known essential character in attention control (as discussed more fully shortly). Other neuromodulators may ultimately need to be considered (as in the case of noradrenaline mentioned above), but there is considerable controversy about how this neuromodulator acts in a global manner, while the understanding of acetylcholine is understood somewhat better. We will also seriously consider dopamine, following N. Fragopanagos, PhD, and J. G. Taylor, PhD (personal communication).
Attention is often regarded as a mechanism by which attended objects become perceptually more salient, akin to increasing their contrast. It was demonstrated by means of human psychophysics that attention can also be described as a mechanism that reduces contextual integration, thereby ensuring that task-irrelevant information is prevented from influencing the processing of task-relevant information.55 It has been suggested above that in the CODAM model this reduction can occur by inhibition. This was partially verified by the results of Shoemaker40 on the AB, a result already referred to.
To investigate possible neuronal bases of this phenomenon, there have been various studies of the effects of attention on spatial integration by cells in V1 of the macaque monkey. In line with their psychophysical results, attention directed to parafoveal locations reduced spatial integration by reducing the summation area of V1 neurons. Effects of acetylcholine application and attention were largely similar, with acetylcholine reducing spatial integration by reducing the neuron's summation area. These data demonstrated that attention can alter perceptual and neuronal spatial integration and that acetylcholine contributes to task-dependent receptive field dynamics. It can also provide a basis for the possible inhibitory effects observed previously. These effects were supported further by results of Herrero et al.,56 in which single-cell recordings were taken from the monkey's visual area V1 while the monkey was performing an endogenously controlled attention task. In such situations, attended visual cells (one for which its receptive field is being attended to) increased their firing rate. Importantly, a similar increase of the cell's firing rate was observed by application of a low dose of acetylcholine to the cell.
However, these results have only explored how attention affects low-level cells by feedback amplification that could be supported by further acetylcholine input. It is known that cell amplification under attention occurs in higher levels of cortex, from many experiments (see, eg, Sarter et al.57). Moreover, neurophysiological studies have demonstrated that increases in cholinergic transmission in sensory areas enhance the cortical processing of thalamic inputs. Cholinergic activity also suppresses the retrieval of internal associations, thereby further promoting sensory input processing. Behavioral studies document the role of cortical cholinergic inputs in attentional functions and capacities by demonstrating, eg, that the integrity of the cortical cholinergic input system is necessary for attentional performance and that the activity of cortical cholinergic inputs is selectively enhanced during attentional performance (as already discussed for V1 neurons above). Other neuromodulators (specifically noradrenaline, serotonin, and dopamine) do not possess this property.
It has been hypothesized58 that the cortical cholinergic input system generally acts to optimize the processing of signals in attention-demanding contexts. Such signals “recruit,” via activation of basal forebrain corticopetal cholinergic projections, the cortical attention systems and thereby amplify the processing of attention-demanding signals (termed “signal-driven cholinergic modulation of detection”). The activity of corticopetal cholinergic projections is also modulated by direct prefrontal projections to the basal forebrain and, indirectly, to cholinergic terminals elsewhere in the cortex; thus, cortical cholinergic inputs are also involved in the mediation of top-down effects, such as the knowledge-based augmentation of detection of signals and the filtering of irrelevant information. Thus, depending on the quality of signals and task characteristics, cortical cholinergic activity reflects the combined effects of signal-driven and cognitive modulation of detection. This hypothesis begins to explain signal intensity or duration-dependent performance in attention tasks, the distinct effects of cortex-wide vs prefrontal cholinergic deafferentation on attention performance, and it generates specific predictions concerning cortical acetylcholine release in attention task-performing animals.
This general approach58 has also been applied to obtain a better understanding of schizophrenia. In particular, it has been proposed in Sarter et al.57 that there is a correlation between the increased sensitization in schizophrenic patients to dopamine, especially in the striatum (see the latest experimental support for this in Howes et al.54) and that for acetylcholine in the cortex. The reasoning behind this causal chain from DA sensitivity to acetylcholine dysregulation (and excessive input to the cortex) has been suggested in Sarter et al.57 as being due to the following causal chain: increased dopamine sensitivity in the nucleus accumbens, the resulting increased GABAergic signal from there to the basal forebrain, resulting in an excessive cholinergic output from there to the cortex. The resulting excess of acetylcholine in cortex causes a severe disruption to the attention control system as well as lower level sites where attention is targeted.
We thus arrive at the Sarter-Bruno model of cholinergic disregulation of cortex as the main form of neuromodulatory disturbance leading to schizophrenia, misplacing that of the dopamine-based hypoactivity of PFC. As the authors state “In general this scenario extends traditional models of schizophrenia that have focused on the DA system and represents the hypothesis that dysregulation of the cortical cholinergic input system is the primary mediator in the information processing impairments in schizophrenia, specifically of the attentional abnormalities that contribute to the manifestation of psychotic symptoms.”57(p119)
More specifically, the features of attention deficit in schizophrenia, such as observed quantitatively in the AB13 would appear to arise from reduced inhibition; earlier we argued that the important schizophrenic symptom of the loss of common sense arose directly from the reduced ability to prevent distracters. The authors state that “the performance of animals with an abnormally reactive cortical cholinergic input system is characterized by increases in the number of false alarms, given by the number of claims for targets present in non-signal trials.”58 They conclude that in such animals, “attentional significance would be attributed to irrelevant or normally unattended stimuli.”58(p106) Furthermore, they suggest that antipsychotic drugs work so as to reduce the dopamine load and hence, by a suitable biochemical causal chain, that of oversupply of acetylcholine. It is in that manner that an initially counterintuitive modulation (excess acetylcholine leads to attention dysfunction, whereas previously we noted how acetylcholine added to neurons leads to increased neural response) can be understood: in such a situation, thalamic (externally based) inputs are emphasized, with correlated reduction of associative or top-down control.
An important aspect of the above explanation of the action of excessive acetylcholine in more detail is that of the separation between the action of acetylcholine on thalamic and on associative inputs. The former, it has been found experimentally, are differentially amplified, the latter suppressed by acetylcholine inputs. Moreover, the former of these occurs through nicotinic receptors, the latter through muscarinic ones. This helps explain the effects observed in V1 mentioned above through a reduction of the lateral (associative) inputs to a cell as compared with the direct thalamic inputs. But then these latter would be increased in number, and hence, there would be reduced prevention of them entering cortex.
It is in this way that it is possible to reconcile the effects of increased cortical acetylcholine with the “loss of common sense” in schizophrenia. Thus, lateral connections (regarded as inhibitory) in cortex would in general be decreased in effectiveness by the excess acetylcholine; the direct thalamic inputs would include distracters as well as targets. All of these latter inputs would be amplified by the excess acetylcholine, so leading to loss of common sense due to excess input information and increased difficulty of filtering out only the target stimulus input. That itself would be defined by some top-down signal (guided by a prefrontal goal state, eg). Such feedback would itself be regarded as associative, and hence reduced in effect on input signal filtering.
We conclude that the Sarter-Bruno model of acetylcholine excess in cortex in schizophrenics allows for an explanation of the important reduction of control by top-down feedback information in attention-based processing in cortex. In the CODAM model, we see that an important component of such feedback involves the effect of signals from the corollary discharge buffer site onto the sensory buffer site. If that feedback has its associative effect reduced due to excessive acetylcholine, then the sense of “I am losing I” expressed by schizophrenics will be explained. The first-person pronoun will not be misidentified but will find it increasingly difficult, as the acetylcholine level increases, to consider itself in control of the attended information processing. Ever more distracters will be attempting to attain, and ultimately gain access to the visual working memory buffer. Hence, the impression of loss of control over what is in consciousness will occur, and first-rank symptoms will arise. As the acetylcholine level rises even further, one can conjecture that the loss of effect of prefrontal goals increases to such an extent that firstly negative symptoms arise (due to inability to preserve a reasonable level of attention control) and finally disorganization symptoms will develop, with the attention control system almost completely out of control. However, a linear progression from positive to negative to disorganization symptoms need not be present due to variations in levels of acetylcholine in different cortical sites as time develops, leading to the possibly patchy development of brain degradation mentioned earlier.
Implications for Diagnosis and Treatment of Schizophrenia
The main result of the above discussion is to give a brain and neuromodulatory basis for the deficits of schizophrenia in its various symptoms across the range of prodromal, positive, negative, and disorganization symptoms in terms of various breakdowns of the attention control system of the brain. In particular, the CODAM model was employed to indicate how the several alterations in the experience of the inner self in schizophrenia could be understood in terms of specific degradations of control between modules in the CODAM model. Furthermore, an explanation of how cortical acetylcholine levels can cause the known range of prodromal, positive, negative, and disorganization symptoms was given on the basis of the Sarter-Bruno hypothesis, when employed in a general analysis of acetylcholine effects on experience as given through the CODAM model.
A method being used in more detail to catch distortions of internal experience is, eg, the EASE (examination of anomalous self-experience) questionnaire.59 This arose from experiential studies of several hundred schizophrenic patients, especially those in first admission. The EASE questionnaire was set up not only for the treating physicians but also to help the patient begin to describe what may be very strange and frightening experiences to them. In the process of making these experiences explicit, it may be that patients begin to come to terms with them.
At the same time, physicians can, aided by the responses to the questionnaire, begin to develop both drug and exercise treatments. Such exercise treatments are being used in terms especially of exercises for improving cognitive and attention-based processing.16 This speaks directly to the CODAM model and the discussion given earlier as to how various breakdowns in the modules of CODAM help explain the various components of prodromal, positive, negative, or disorganization symptoms. Further results coming from EASE responses to an ever-increasing number of schizophrenics would be important to follow the inner experiences under the disease more closely.
Conclusions
Through the CODAM model we have presented an initial bridge between experience and the brain activities that produce that experience. CODAM is based on a model of attention control over the stimulus-driven activity in the brain. It is natural to point out that attention-controlled activity is not the only activity that the brain supports; automatic or subliminal activity is also of crucial importance in brain processing. Thus, subliminal processing is recognized as the basis of creative processing. But also attention control is still important during the period of subliminal processing—such as in the period of creative thinking—due to the importance of such creative processing leading to an important subliminal thought and then attention is directed back to the train of thought. Thus, attention is still seen to play a crucial role throughout the various stages of brain processing. How schizophrenics can handle creativity tasks is unclear, although they would be expected to be worse than normals at such tasks due to their lower levels of control of attention they possess than normals. This could lead to a valuable research project to explore creative defects in schizophrenics as compared with normal subjects.
Attention is also brought into play when automatic motor control fails; we have not discussed here schizophrenics’ difficulties with motor control at any length, considering it outside the remit of this article. However, we note that motor attention (intention) has been modeled along similar control lines to CODAM (which has been extended thereby to visuomotor control systems60). Thus, motor control difficulties could also be pursued on a brain basis following that or similar models.
We then described in some detail how the inner self of consciousness could arise from a suitably precisely defined model of attention processing. In particular, the corollary discharge of the attention movement control signal—the attention copy signal or CODAM model—was argued as providing a basis for the inner self. The CODAM model is crucially based on a component attention copy signal used to speed up access to consciousness. Moreover, this copy signal can inhibit distracters, which are important for each of us to keep out of consciousness, these distracters being normally processed at a subliminal level. Identification of the various components of the overall CODAM circuitry activity was also made with the protention–primal impression–retention sequence of Western phenomenology.
Turning to schizophrenia, this last aspect is basic to a possible understanding of the symptoms of loss of common sense in the world. We considered each of the four symptom classes of schizophrenia in turn: prodromal, positive, negative, and finally disorganized symptoms. Each of these was explained in general terms through defects in the CODAM model brought about by damages to the variety of CODAM model modules. In general, we found that initially defects were expected to arise in the attention copy buffer module and then spread successively across the CODAM model modules (although more patchy changes may also occur).
We then considered how dysfunction in the acetylcholine system could lead to the observed symptoms. The Sarter-Bruno hypothesis (that there is an increase in cortical acetylcholine above normal, driven originally by increased striatal dopamine) was shown to support the mechanisms considered briefly in the CODAM model. In particular, the excess level of cortical acetylcholine was argued to be at the basis, through a CODAM model of top-down attention control, of modification of experiences as indicated by the prodromal, positive, negative, and disorganization symptoms.
Finally, we underlined the importance of attention-based exercise for possible amelioration of schizophrenic symptoms, in conjunction with medication. The CODAM model hypothesis implies that such amelioration would be mediated by reduction of cortical acetylcholine levels. Such reduction may also be attempted directly by suitably developed drugs.
We thus can summarize what has been presented as new in this article as follows:
A brain-based attention control architecture has been presented, through CODAM, to give an underlying framework by which the various components of consciousness can be decomposed into that of ipseity and the experience of content of attended stimuli.
From the above model, various symptoms of schizophrenia were explained as arising initially by damage to the corollary discharge component and ultimately by overall degradation of the functioning of the various modules of CODAM.
The manner in which an excess of dopamine in the basal ganglia54 can be seen as a causative feature in the breakdown of the functioning of the modules of CODAM due to excess of cortical acetylcholine.
The crucial role played by the malfunction of the overall attention control system in schizophrenia (especially in disorganization symptoms), and the need for further exploration of how that might be reversed, say by drug-induced reduction of acetylcholine.
A direct explanation of the positive symptoms of thought and speech insertion, by means of the breakdown of the ability of the attention corollary discharge signal to be involved in the accessing of inner speech and thought to the relevant sensory buffer (thereby avoiding the numerous difficulties of the explanation in19 in terms of their claimed damage to the motor control corollary discharge system).
At least four research avenues can be seen to arise from the brain-based model of schizophrenia presented in this article:
(I) A more careful study of the manner in which attention is controlled in the normal brain, especially using brain imaging machines. Thus, by fMRI and transcranial magnetic stimulation (and also allied to electro-encephalography and magneto-encephalography analyses) the flow of activation through the various modules of the control circuitry proposed under CODAM needs to be followed in more precision, especially pinpointing the attention copy signal components that are being used to provide the initial control aspects of buffer working memory access of attended stimuli.
(II) An extension of this study to schizophrenics with a variety of symptoms, so as to clarify the degradation of the control circuitry as expected to occur from the results described in Thompson et al.24: A particular approach to this would use the EASE questionnaire in order to clarify the inner experiences as completely as possible.
(III) A more detailed study of the hypothesis introduced in Sarter et al.,57 as described earlier in the article, that an excess of acetylcholine is at the root of schizophrenia. This covers a broad range of components when applied to a model such as that of CODAM for consciousness, especially a detailed computational and experimental analysis of the amplification/inhibition brought about by a suitable level of acetylcholine into the attention control modules and the manner in which excess of acetylcholine causes attention control breakdown.
(IV) The need for a detailed study of exercises that could be developed to ameliorate the deficits arising in the corollary discharge component of the attention control system. This part of CODAM is particularly subtle, and its more complete understanding may be helped by specific attention exercises designed specifically to target it (say based on aspects of the AB paradigm, or similar paradigms needing fast attention responses).
Funding
European Union (EU Cognitive Systems MATHESIS project, grant 027674).
Acknowledgments
The author would like Dr N Fragopanagos, who performed the simulations of the damaged AB to model schizophrenic deficits in the AB.
References
- 1.Sass LA, Parnas J. Schizophrenia, consciousness and the self. Schizophr Bull. 2003;29:427–444. doi: 10.1093/oxfordjournals.schbul.a007017. [DOI] [PubMed] [Google Scholar]
- 2.Zahavi D. Subjectivity & Selfhood. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 3.Parnas J, Handest P, Jannsson L, Saebye D. Anomalous subjective experiences among first admitted schizophrenia spectrum patients: empirical investigation. Psychopathology. 2005;38:259–267. doi: 10.1159/000088442. [DOI] [PubMed] [Google Scholar]
- 4.Parnas J, Handest P, Saebye D, Jansson L. Anomalies of subjective experience in schizophrenia and psychotic bipolar illness. Acta Psychiatr Scand. 2003;108:126–133. doi: 10.1034/j.1600-0447.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 5.Sass L, Parnas J. Explaining schizophrenia: the relevance of phenomenology. In: Chung MC, Fulford KMW, Graham G, editors. Reconceiving Schizophrenia. Oxford, UK: Oxford University Press; 2007. pp. 63–95. [Google Scholar]
- 6.Sass L. Madness and Modernism. Cambridge, MA: Harvard University Press; 1992. [Google Scholar]
- 7.Hemsley DR. Disruption of the “sense of self” in schizophrenia: potential links to disturbances of information processing. Br J Med Psychol. 1998;71:115–124. doi: 10.1111/j.2044-8341.1998.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 8.Cermolacce M, Naudin J, Parnas J. The “minimal self” in psychopathology: re-examining the self-disorders in the schizophrenia spectrum. Conscious Cogn. 2007;16:703–714. doi: 10.1016/j.concog.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JG. A control model for attention and consciousness [abstract 839.3] Soc Neurosci. 2000;26:2231. [Google Scholar]
- 10.Taylor JG. CODAM: a model of attention leading to the creation of consciousness. Scholarpedia. 2007;2:1508. [Google Scholar]
- 11.Taylor JG. The I's eye view of consciousness. J Conscious Stud. In press. [Google Scholar]
- 12.Sokolowski R. Introduction to Phenomenology. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 13.Wynn JK, Breitmeyer B, Nuechterlein KH, Green MF. Exploring the short term visual store in schizophrenia using the attentional blink. J Psychiatric Res. 2006;40:599–605. doi: 10.1016/j.jpsychires.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Kopp B. Mnemonic intrusions into working memory in psychometrically identified schizotypal individuals. J Behav Ther Exp Psychiatry. 2007;38:56–74. doi: 10.1016/j.jbtep.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Uhlhaas PJ, Mishara AL. Perceptual anomalies in schizophrenia: integrating phenomenology and cognitive neuroscience. Schizophr Bull. 2007;33:142–156. doi: 10.1093/schbul/sbl047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velligan DI, Kern RS, Gold JM. Cognitive rehabilitation for schizophrenia and the putative role of motivation and expectancies. Schizophr Bull. 2006;32:474–485. doi: 10.1093/schbul/sbj071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizolatti G, Riggio L, Sheliga BM. Space and selective attention. In: Umiltà C, Moscovitch M, editors. Attention and Performance XV. Conscious and Unconscious Information Processing. Cambridge, MA: MIT Press; 1994. pp. 231–265. [Google Scholar]
- 18.Desmurget M, Grafton S. Forward modelling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- 19.Frith CD. The Cognitive Neuropsychology of Schizophrenia. Hove, UK: Erlbaum; 1992. [Google Scholar]
- 20.Gallagher S. Neurocognitive models of schizophrenia: a cognitive model of immunity to error through misidentification. Psychopathology. 2004;37:8–19. doi: 10.1159/000077014. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher S. Self-reference and schizophrenia: a cognitive model of immunity to error through misidentification. In: Zahavi D, editor. Exploring the Self: Philosophical and Psychopathological Perspectives on Self-Experience. Philadelphia, PA: John Benjamin; 2000. pp. 203–239. [Google Scholar]
- 22.Rushworth MS, Johansen-Berg H, Gobel SMG, Devlan JT. The left parietal lobe and premotor cortices: motor attention and selection. Neuroimage. 2001;20:S89–S100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson PM, Vidal C, Gledd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor JG, Cleeremans A, Freeman WJ. eds. Brain and consciousness Neural Netw. 2007; 20:929–1060. [DOI] [PubMed]
- 27.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 28.Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28:10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonides J, Schumacher EH, Smith EE, et al. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravizza SM, Behrmann M, Fiez JA. “Right parietal contributions to verbal working memory: spatial or executive?”. Neuropsychologia. 2005;43:2057–2067. doi: 10.1016/j.neuropsychologia.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Husain M, Mannan S, Hodgson T, Wojciulik E, Driver J, Kennard C. Impaired spatial working memory across saccades contributes to abnormal search in parietal neglect. Brain. 2001;124:941–952. doi: 10.1093/brain/124.5.941. [DOI] [PubMed] [Google Scholar]
- 32.Sergent C, Baillet S, Dehaene S. Timing of the brain events underlying access to consciousness during the attentional blink. Nat Neurosci. 2005;8:1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 33.Fragopanagos N, Kockelkoren S, Taylor JG. A neurodynamic model of the attentional blink. Cogn Brain Res. 2005;24:568–586. doi: 10.1016/j.cogbrainres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Diamond MR, Ross J, Morrone MC. Extraretinal control of saccadic suppression. J Neurosci. 2000;20:3449–3455. doi: 10.1523/JNEUROSCI.20-09-03449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro KL, Raymond JE, Ansell KM. Attention to visual pattern information produces the attentional blink in rapid serial visual presentation. J Exp Psychol Hum Percept Perform. 1994;20:357–371. doi: 10.1037//0096-1523.20.2.357. [DOI] [PubMed] [Google Scholar]
- 37.Vogel EK, Luck SJ, Shapiro K. Electrophysiological evidence for a post-perceptual locus of suppression during the attentional blink. J Exp Psychol. 1998;241:1656–1674. doi: 10.1037//0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- 38.Taylor JG, Fragopanagos N. Resolving some confusions over attention and consciousness. Neural Netw. 2007;20:993–1003. doi: 10.1016/j.neunet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Korsten NJH, Fragopanagos N, Hartley M, Taylor NR, Taylor JG. Attention as a controller. Neural Netw. 2006;19:1408–1421. doi: 10.1016/j.neunet.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Shoemaker S. Self-reference and self-awareness. J Philos. 1968;65:556–570. [Google Scholar]
- 41.Mizumoto M, Ishikawa M. Immunity to error through misidentification and the bodily illusion experiment. J Conscious Stud. 2005;12:3–19. [Google Scholar]
- 42.Sartre J-P. Being and Nothingness. London: Routledge; 1943. [Google Scholar]
- 43.Rosenthal DM. Two concepts of consciousness. Philos Stud. 1986;49:329–359. [Google Scholar]
- 44.Rosenthal DM. Thinking that one thinks. In: Humphreys G, Davies M, editors. Consciousness. Oxford: Blackwell; 1993. [Google Scholar]
- 45.Baars BJ. Cambridge: Cambridge University Press; 1998. A cognitive theory of consciousness. [Google Scholar]
- 46.Baars BJ. The conscious access hypothesis: origins and recent evidence. Trends Cogn Sci. 2002;6:47–51. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- 47.Klosterkotter J, Hellmich M, Steinmeyer EM, Schulze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 48.Schnieder K. Clinical Psychopathology. Translated by MW Hamilton. New York, NY: Grune and Stratton; 1959. [Google Scholar]
- 49.Mellor CS. First rank symptoms of schizophrenia. Br J Psychiatry. 1970;117:15–23. [PubMed] [Google Scholar]
- 50.Blankenburg W. First steps toward a psychopathology of “common sense” [trans A Mishara] Philos Psychiatry Psychol. 2001;8:303–315. [Google Scholar]
- 51.Artaud A. In: Antonin Artaud Anthology. Hirschman J, editor. San Francisco, CA: City Lights Books; 1965. [Google Scholar]
- 52.Artaud A. In: Antonin Artaud: Selected Writings. Sontag S, editor. Trans H Weaver. New York, NY: Farrar, Straus & Giroux; 1976. [Google Scholar]
- 53.Sass L. Negative symptoms, schizophrenia, and the self. Int J Psychol Psychologic Ther. 2003;3:153–180. [Google Scholar]
- 54.Howes OD, Montgomery AJ, Asselin M-C, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 55.Roberts MJ, Thiele A. Spatial integration and its moderation by attention and acetylcholine. Front Biosci. 2008;13:37–42. doi: 10.2741/2963. [DOI] [PubMed] [Google Scholar]
- 56.Herrero JL, Roberts MJ, Delicato LS, Gieselman MA, Dayan P, Their A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophr Bull. 2005;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- 58.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unravelling the attentional function of cortical cholinergic inputs: interactions between signal driven and cognitive modulation of signal detection. Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Parnas J, Moller P, Thalbitzer J, Jansson L, Handest P, Zahavi D. EASE: examination of anomalous self-experience. Psychopathology. 2005;38:236–258. doi: 10.1159/000088441. [DOI] [PubMed] [Google Scholar]
- 60.Hartley M, Fagard J, Essaily R, Taylor JG. Observational versus trial and error effects in a model of infant learning paradigms. Proc ICANN2008. Artificial Neural Networks. V Kurkova, V Kurkova-Pohlov & J Kotnik eds. LNCS #5164. Berlin: Springer. 2008;Part II: 277–289. [Google Scholar]
- 61.Berze J. Die Primare Insuffizienz der Psychishcen Aktivitat: Ihr Wesen, ihre Erscheinungen and ihre Bedeutung als Grundstorungen der Dementia Praecox und des hypophrenen Uberhaupt. Leipzig, Germany: F Deutke; 1914. [Google Scholar]
- 62.Minkowski E. La schizophrenia. Psychopathologie des shizoides et des schizophrenes. Paris, France: Payot; 1927. [Google Scholar]
- 63.Kimura B. Ecrits de Psychopathologie Phenomenologique. Trans Boderlique. Paris, France: P.U.F; 1992. [Google Scholar]
- 64.Sass LA. Self and world in schizophrenia: three classic approaches in phenomenological psychiatry. Philos Psychiatry Psychol. 2001;8:251–270. [Google Scholar]