Abstract
Objective: Memory deficits have been shown in patients affected by schizophrenia (SZ) and bipolar (BP)/mood disorder. We recently reported that young high-risk offspring of an affected parent were impaired in both verbal episodic memory (VEM) and visual episodic memory (VisEM). Understanding better the trajectory of memory impairments from childhood to adult clinical status in risk populations is crucial for early detection and prevention. In multigenerational families densely affected by SZ or BP, our aim was to compare the memory impairments observed in young nonaffected offspring with memory functioning in nonaffected adult relatives and patients. Methods: For 20 years, we followed up numerous kindreds in the Eastern Québec population. After having characterized the Diagnostic and Statistical Manual of Mental Disorders phenotypes, we assessed cognition (N = 381) in 3 subsamples in these kindreds and in controls: 60 young offspring of a parent affected by SZ or BP, and in the adult generations, 92 nonaffected adult relatives and 40 patients affected by SZ or BP. VEM was assessed with the California Verbal Learning Test and VisEM with the Rey figures. Results: The VEM deficits observed in the offspring were also found in adult relatives and patients. In contrast, the VisEM impairments observed in the young offspring were present only in patients, not in the adult relatives. Conclusion: Implications for prevention and genetic mechanisms can be drawn from the observation that VEM and VisEM would show distinct generational trajectories and that the trajectory associated with VisEM may offer a better potential than VEM to predict future risk of developing the disease.
Keywords: schizophrenia, mood disorders-bipolar, child psychiatry, cognitive neuroscience
Introduction
Meta-analyses have shown that episodic memory is among the most impaired neurocognitive domains in schizophrenia (SZ) and in bipolar (BP) affective patients.1–4 Effect sizes (ESs) are large in SZ patients (>1.0) and moderate to large in BP patients (between 0.6 and 1.0) in the verbal episodic memory (VEM) and visual episodic memory (VisEM) domains,5 although VisEM has been less extensively investigated than VEM.6,7 Interestingly, in the study of Skelley et al,5 adult SZ patients displayed both VEM and VisEM deficits, whereas their adult nonaffected relatives had only VEM impairments. The mechanistic meaning of this differential pattern is unclear. Several studies suggest that memory deficits may be shared by SZ and BP, although BP patients are generally found less impaired than SZ.8,9
We recently reported in this Journal that the young high-risk (HR) offspring of SZ or BP parents shared important deficits in both VEM and VisEM,10 in fact the largest differences with normal controls were for these 2 cognitive domains (ESs of ∼0.8–0.9). This suggested that the memory impairments seen in patients would already exist in childhood/adolescence. However, little knowledge exists about the long-term predictive effect of memory impairments present early in life in populations at risk for SZ or BP.
A better understanding of the trajectory of such episodic memory dysfunctions from childhood to adulthood in risk populations is crucial for early detection, prevention, and comprehension of the genetic architecture of major psychoses. Longitudinal cohort studies starting in childhood take a long time to yield results, and the alternative strategy of accelerated longitudinal designs is also difficult to perform.11 We conceived an approach combining cross-sectional assessments in 3 complementary subsamples of different generations drawn from our Eastern Québec large multigenerational kindreds densely affected by SZ or BP that were characterized and followed up over 20 years.12,13 The 3 subsamples consisted of (1) young nonaffected offspring of a parent affected by SZ or BP, (2) nonaffected adult relatives from the older generations, and (3) the patients, ie, the adult family members affected by SZ or BP.
Using a cross-sectional generational stratification in our kindreds, the objective of the present study was to compare the VEM and VisEM impairments observed in the young offspring with the impairments in nonaffected adult relatives and patients. To our knowledge, this is the first study to investigate simultaneously, and with the same methodology, the neurocognitive impairments in episodic memory in young offspring and also in the affected and nonaffected adults from the same kindreds.
Methods
Sample
Ascertainment of Kindreds.
We targeted all the multigenerational families densely affected by SZ or BP in the Eastern Québec (Canada) catchment area. Families “inclusion criteria” were (1) having at least one first-degree relative affected with the same disorder (SZ/BP) as the proband and (2) having at least 4 affected individuals sharing the same disorder. We gathered 48 SZ or BP kindreds over 20 years with an average of 26 members per kindred including an average of 6 affected by SZ or BP. The mean age of onset was 25.4 (SD 8.5) years for SZ and 28.8 (SD 10.3) years for BP. More details about the kindreds ascertainment have already been provided elsewhere.13–17
Young Offspring Subsample.
We enlarged the previously reported HR offspring sample drawn from the youngest generations of the kindreds.10 As previously described,10 the inclusion criteria were (1) having a parent with a definite Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) SZ or BP disorder and (2) having had a neuropsychological evaluation before being 23-year old. The offspring “exclusion criteria” were the presence of a diagnosis of DSM-IV psychotic disorder, BP or major depression, and brain and metabolic disorders known to cause neuropsychological impairments. Compared with our former report,10 the offspring sample was increased from 45 to 60 subjects (mean age = 17.4 years, SD 4.1, 51.7% males). Considering the exclusion criteria in the present sample, 37% of these 60 HR offsprings had an axis I nonpsychotic diagnoses distributed as follow: anxiety-like disorder 17%, substance abuse 5%, attention-deficit hyperactivity disorder 8%, and learning and language disorder 7%. Twenty-five of them had a parent affected by SZ, while 35 had a parent affected by BP. The offspring sample was composed of 40 sibships of which 23 comprised a single subject, 14 comprised 2, and 3 comprised 3.
Nonaffected Adult Relatives Subsample.
The inclusion criteria were (1) having a first-degree relative with a definite DSM-IV SZ or BP disorder and (2) having had a neuropsychological evaluation before being 55-year old. The exclusion criteria were the same as in offspring above. Ninety-two adult relatives were evaluated (mean age = 40.2 years, SD 10.5), 26 of them had a first-degree relative affected by SZ, 46 had a first-degree relative affected by BP, while the 20 remaining adult relatives had one first-degree relative affected by SZ and another by BP. Sixty-nine of the 92 adult relatives were the offspring of an affected parent, whereas 23 participants were either sibs or parents of a patient. Thirty percent of these adult relatives had an axis I nonpsychotic diagnoses: anxiety-like disorder 20%, substance abuse 8%, attention-deficit hyperactivity disorder 1%, and language disorder 1%. This nonaffected relatives sample spanned 48 sibships of which 36 comprised a single subject, 12 comprised 2, 8 comprised 3, and 2 comprised 4.
Patients Subsample.
The adult members affected by SZ or BP came from the same large densely affected multigenerational kindreds.13,14,18 The inclusion criteria were (1) a definite DSM-IV SZ or a BP diagnosis, (2) having undergone a neuropsychological evaluation before age 55, and (3) being in a clinical status allowing a reliable cognitive assessment. The exclusion criteria were a brain disorder, trauma, and metabolic disorder known to cause neuropsychological impairments. The present sample consisted of 40 patients (20 SZ and 20 BP, mean age = 43.7 years, SD 10.0, 40% men). The patient sample spanned 35 sibships of which 30 comprised a single patient and 5 comprised 2. Twenty-seven of the 40 patients had at least one first-degree relative among the 60 young offspring or the 92 nonaffected relatives, 12 of them (30%) had second- or third-degree relatives, and only 1 (2%) was not related to any of the subjects from the other 2 subsamples.
Healthy Control Subsample.
Two different unrelated control groups (n = 189) were used: an adult healthy control group (N = 113, mean age = 34.6 years, SD 9.9; 38% men) for both the nonaffected relatives and adult patients and a young healthy control group (N = 76, mean age = 16.9 years, SD 4.0; 50% males) for the offspring. The 2 study groups were balanced for age and gender with each of the 2 experimental subsamples. Participants were recruited in response to advertisements and were from the same population as the experimental subjects. The exclusion criteria were the same as those for offspring and nonaffected relatives with the addition of any lifetime axis I DSM diagnosis or a positive family history of SZ or BP spectrum disorders.
The study was personally explained, and a signed consent was obtained for all subjects, as reviewed by our University Ethics Committee.
Measurements
Psychiatric Ascertainment (in Offspring, Nonaffected Relatives, and Patients).
In offspring, the method consisted in a direct interview with the parents and the children (K-SADS19 for subjects under 18 or the Structured Clinical Interview for DSM-IV20 for those over 18). A lifetime best estimate DSM-IV diagnosis used all available information concerning the offspring,21 just like for the parents and relatives.15,17 A detailed description of best estimate diagnosis and distribution of diagnoses in these offspring has already been reported.21
The adult relatives and the patients had the same lifetime best estimate procedure, based on information from a direct structured interview with the subjects (Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorder-III-R) and with their relatives as well as on all available medical records across lifetime. Based on this information, a consensus diagnosis was derived by a panel of 4 research psychiatrists who were blind to diagnoses in relatives.15–17
Neuropsychological Assessments.
All the cognitive domains assessed by means of the full neuropsychological battery are provided in the table 1 and Supplementary table S1 of the supplement. In this study, we focused on the free recall measures of VEM and VisEM tests because they showed the largest ESs in the comparison of offspring with controls.10 VEM was assessed with the California Verbal Learning Test (CVLT)22 “total and delayed recalls” in which subjects had to learn a series of words presented orally over 5 trials, and to immediately recall them after each presentation (total recall of 5 trials), or with a 20-min delay (delayed recall). VisEM was assessed with the Rey Complex Figure Test (RCF)23 immediate and delayed recalls. Subjects had to copy a figure and then recall it after 3 min (immediate recall) and 30 min (delayed recall). The tests were administered in the same order in all subjects, with child, adolescent, or adult versions, depending on the age of the subject. Subjects were individually assessed in a quiet room for a period of 3–4 h (for the full battery; see supplement) by certified psychologists or PhD students who were blind to diagnoses and supervised by a senior neuropsychologist (N.R.). Pauses were offered when needed.
Table 1.
Comparisons in Patients, Nonaffected Relatives, and Controls on the Full Neuropsychological Battery
| Multilevel Modela Adjusted for Age and Gender |
||||||||
| Post Hoc Analyses |
||||||||
| Adjusted Mean (SE) |
Patients vs Controls |
NAARs vs Controls |
||||||
| Cognitive Functioning | Patients (N = 40) | Nonaffected Relatives (N = 92) | Controls (N = 113) | Global P Valueb | P Valuec | ESd (95% CIe) | P Valuec | ESd (95% CIe) |
| Intelligence | ||||||||
| Global IQf | 86.57 (2.01) | 98.17 (1.35) | 107.4 (1.24) | <.0001 | <.0001 | −1.49 (−1.89, −1.09) | <.0001 | −0.72 (−1.01, −0.44) |
| Sustained attention | ||||||||
| CPT-hit reaction time block changeg | −0.003 (0.004) | −0.007 (0.003) | −0.003 (0.002) | .7991 | −0.0005 (−0.39, 0.39) | 0.16 (−0.13, 0.44) | ||
| CPT-hit standard error block changeg | −0.02 (0.01) | −0.02 (0.01) | −0.02 (0.01) | .7991 | −0.04 (−0.43, 0.36) | 0.05 (−0.23, 0.33) | ||
| Selective attention | ||||||||
| CPT omissionsg | 3.94 (0.67) | 2.26 (0.43) | 1.03 (0.37) | .0090 | .0002 | −0.94 (−1.35, −0.53) | .0869 | −0.40 (−0.68, −0.11) |
| CPT commissionsg | 13.69 (1.27) | 12.01 (0.80) | 10.36 (0.70) | .2510 | −0.56 (−0.96, −0.16) | −0.22 (−0.50, 0.07) | ||
| CPT detectability d’ | 0.68 (0.08) | 0.79 (0.05) | 0.89 (0.05) | .2510 | −0.59 (−0.99, −0.19) | −0.21 (−0.50, 0.07) | ||
| Stroop interference score | −0.49 (1.93) | 0.14 (1.30) | 5.27 (1.19) | .0411 | .5521 | −0.33 (−0.69, 0.04) | .1079 | −0.38 (−0.67, −0.10) |
| Verbal episodic memory | ||||||||
| CVLT total recall (VEM) | 48.52 (1.45) | 54.06 (0.97) | 59.15 (0.89) | <.0001 | <.0001 | −1.08 (−1.46, −0.70) | .0013 | −0.59 (−0.87, −0.31) |
| CVLT delayed recall | 10.19 (0.45) | 11.84 (0.30) | 12.98 (0.28) | <.0001 | <.0001 | −0.99 (−1.37, −0.61) | .0422 | −0.43 (−0.71, −0.15) |
| CVLT recognition | 14.33 (0.22) | 14.80 (0.15) | 15.24 (0.13) | .0190 | .0032 | −0.73 (−1.09, −0.36) | .1856 | −0.33 (−0.60, −0.05) |
| Visual episodic memory | ||||||||
| Rey immediate recall | 15.31 (0.97) | 20.29 (0.65) | 21.27 (0.59) | <.0001 | .0001 | −0.90 (−1.28, −0.52) | .6778 | −0.15 (−0.43, 0.12) |
| Rey delayed recall (VisEM) | 15.25 (0.94) | 20.10 (0.63) | 21.48 (0.57) | <.0001 | <.0001 | −1.01 (−1.39, −0.62) | .6073 | −0.22 (−0.50, 0.06) |
| Rey recognition | 18.85 (0.37) | 20.22 (0.25) | 20.36 (0.22) | .0205 | .0282 | −0.61 (−0.99, −0.24) | .6778 | −0.06 (−0.34, 0.22) |
| Working memory | ||||||||
| Total spatial span | 14.31 (0.52) | 15.77 (0.35) | 16.94 (0.32) | .0017 | .0140 | −0.65 (−1.01, −0.28) | .1146 | −0.37 (−0.64, −0.09) |
| Total digit span | 14.93 (0.68) | 16.14 (0.46) | 18.46 (0.43) | .0002 | .0020 | −0.76 (−1.13, −0.38) | .0058 | −0.53 (−0.82, −0.25) |
| Executive function/problem solving | ||||||||
| WCST total errorsg | 38.63 (3.19) | 26.42 (2.13) | 19.60 (1.91) | .0001 | .0001 | −0.94 (−1.32, −0.55) | .0708 | −0.41 (−0.70, −0.13) |
| WCST number of categories completed | 4.13 (0.26) | 5.18 (0.17) | 5.51 (0.15) | .0008 | .0004 | −0.86 (−1.25, −0.47) | .4094 | −0.28 (−0.57, 0) |
| WCST trials 1st categoryg | 33.55 (3.57) | 17.33 (2.38) | 17.44 (2.13) | .0039 | .0412 | −0.59 (−0.97, −0.21) | .6778 | −0.12 (−0.40, 0.16) |
| WCST failure to maintain setg | 1.03 (0.20) | 0.70 (0.13) | 0.79 (0.12) | .7991 | −0.16 (−0.54, 0.21) | 0.06 (−0.22, 0.34) | ||
| WCST learning to learn | −2.41 (1.04) | −1.04 (0.62) | 0.26 (0.54) | .2510 | −0.63 (−1.06, −0.19) | −0.29 (−0.58, 0.01) | ||
| Executive function/initiation | ||||||||
| Letter fluency test | 9.99 (0.67) | 10.73 (0.45) | 12.44 (0.41) | .0205 | .0837 | −0.51 (−0.88, −0.15) | .0575 | −0.42 (−0.69, −0.14) |
| Category fluency test | 17.47 (0.73) | 19.39 (0.49) | 21.37 (0.45) | .0005 | .0056 | −0.70 (−1.07, −0.33) | .0219 | −0.47 (−0.75, −0.19) |
| Executive function/planning | ||||||||
| Total number of problems solved in minute | 3.55 (0.42) | 5.54 (0.28) | 5.22 (0.26) | .0043 | .0205 | −0.63 (−1.00, −0.26) | .6778 | 0.11 (−0.17, 0.39) |
| Total time violationsg | 1.62 (0.24) | 0.93 (0.16) | 0.77 (0.14) | .0739 | −0.48 (−0.86, −0.11) | −0.17 (−0.45, 0.11) | ||
| Total rule violationsg | 1.05 (0.20) | 0.44 (0.14) | 0.28 (0.12) | .0480 | .0675 | −0.55 (−0.92, −0.17) | .6073 | −0.22 (−0.50, 0.06) |
| Motor coordination | ||||||||
| Purdue-both hands | 11.03 (0.30) | 12.82 (0.20) | 13.47 (0.18) | <.0001 | <.0001 | −1.29 (−1.68, −0.89) | .1849 | −0.34 (−0.61, −0.06) |
Note: NAARs, nonaffected adult relatives; CPT-II, Continuous Performance Test-II; CVLT, California Verbal Learning Test; WCST, Wisconsin Card Sorting Test-128 cards.
To account for possible correlation among subjects within the same sibship, a multilevel model was carried out using the MIXED procedure of SAS (version 9.1.3; SAS Institute Inc.). Sibships nested in the group were used as the second level and modeled according to a random effect. Degrees of freedom were obtained by the method of Kenward–Roger24 which is available with the option DDFM = KR in the MODEL statement of the MIXED procedure.
Global P values were obtained using the method of Hochberg.27
P values were obtained for the cognitive functions showing a global corrected P value < 0.05 using the method of Hochberg.27
Effect sizes (ESs) were calculated using the difference of adjusted means (LSMeans) between the experimental and control groups standardized by a pooled SD. The pooled SD was obtained by dividing the SE of the difference of LSMeans by the square root of 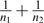 .25
.25
Confidence intervals (CIs) for the ESs were obtained using the noncentrality interval estimation approach based on a t distribution.26 The lower and upper bounds of the 95% CI are calculated by multiplying the 2.5% and 97.5% percentiles, respectively, of the noncentral t distribution by the square root of 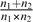 .
.
Global IQ was reported in standardized scores, all the other neuropsychological tests were reported in raw scores corrected for age and gender.
For these subtests, an elevated score indicates a subject’s poor performance. Note that the ESs have been inverted for these subtests.
Statistical Analysis
First, the average raw scores on the cognitive tests were compared for each of the experimental groups (young offspring, nonaffected adult relatives, and patients) to the appropriate control group by means of analyses of covariance (ANCOVAs). Age and gender were selected as covariables in all our statistical analyses. To account for the nonindependence of observations within the same sibship, a multilevel regression analysis was applied with the MIXED procedure of SAS (version 9.1.3; SAS Institute Inc., Cary, NC). Sibships nested in the group were used as the second level and modeled according to a random effect. Degrees of freedom were obtained by the method of Kenward–Roger24, ie, available with the option DDFM = KR in the MODEL statement of the MIXED procedure. ESs were calculated using the difference of adjusted means (LSMeans) between the experimental and control groups standardized by a pooled SD. The pooled SD was obtained by dividing the SE of the difference of LSMeans by the square root of 25 Confidence intervals (CIs) for the ESs were obtained using the noncentrality interval estimation approach based on a “t” distribution.26 The lower and upper bounds of the 95% CI were calculated by multiplying the 2.5% and 97.5% percentiles, respectively, of the noncentral “t” distribution by the square root of .
Multiple Testing.
To account for the multiple tests involved, we calculated corrected P values using the method of Hochberg27 which consists of ordering the raw P values and adjusting the sequence of value according to a step-up fashion. The significance level was fixed at .05.
Results
Comparisons of Young Offspring, Adult Relatives, and Patients to Their Respective Controls
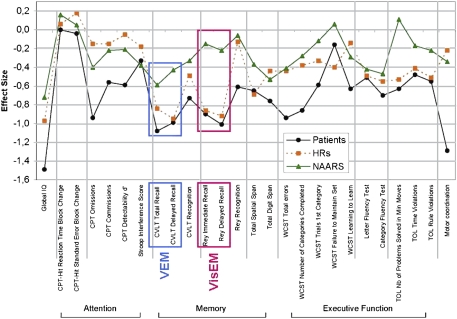
For the “young offspring,” the comparisons in the present enlarged sample yielded results similar to those recently reported,10 and episodic memory showed the largest ES across the domains assessed by our battery (see figure 1 and table S1). The ESs were large not only for VEM (respectively, ES of −0.84, PANCOVA = 0.0001 and −0.95, PANCOVA < 0.0001 on the CVLT total recall and delayed recall) but also for VisEM on the “Rey figures immediate” and delayed recall (respectively, ES = −0.86, PANCOVA = 0.0001 and −0.92, PANCOVA < 0.0001). To assess the possibility that the memory differences might be mainly due to a subgroup being in a prodromal state, we stratified the offspring sample into those under 17 years (N = 25) vs those over 17 (N = 35). The ES were large in these 2 age strata, respectively, VEM CVLT total recall: ES = −0.69, PANCOVA < 0.05 and ES = −0.96, PANCOVA < 0.05, and for VisEM Rey delayed recall: ES = −1.32, PANCOVA < 0.05, and ES = −0.71, PANCOVA < 0.05. The “nonaffected adult relatives” (figure 1 and table 1) showed also a significant difference from controls for VEM on the CVLT total recall (ES = −0.59, PANCOVA = 0.0013) and the CVLT delayed recall (ES = −0.43, PANCOVA = 0.0422). In contrast, VisEM showed no significant differences between nonaffected relatives and controls on the Rey immediate recall (ES = −0.15, PANCOVA = 0.68) and on the Rey delayed recall (ES = −0.22, PANCOVA = 0.61). Finally, “patients” affected by SZ or BP showed large ESs in both VEM and VisEM (ES ∼ −1.0) (see figure 1 and table 1).
Fig. 1.
Mean Effect Sizes for the Full Neuropsychological Battery in Young High-Risk Offspring, Nonaffected Adult Relatives, and Patients in Comparison With Normal Controls. Effect sizes (ESs) were calculated using the difference of adjusted means (LSMeans) between the experimental and control groups standardized by a pooled SD. The pooled SD was obtained by dividing the SE of the difference of LSMeans by the square root of .25 High-risk (HR) offspring are represented by an orange box (▪), patients are represented by a black circle (•), and nonaffected adult relatives (NAARs) are represented by a green triangle (▴). The sign of the ES values was changed for some tests in order to have the dysfunctional poles in negative values. On this figure, the zero value indicates d = 0, ie, no difference with controls. Verbal episodic memory (VEM) is included in a blue box and refers to the California Verbal Learning Test (CVLT) total recall and delayed recall subtests. Visual episodic memory (VisEM) is represented by a pink box and refers to the Rey Complex Figure Test immediate and delayed recall subtests.
Shared Memory Profiles in SZ and BP
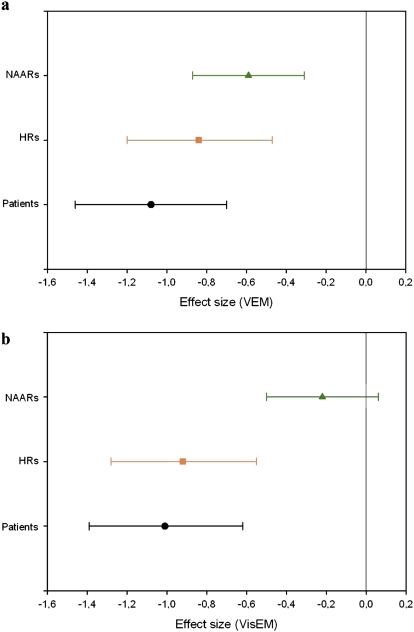
We tested whether there were differences between the 2 disorders, SZ and BP, in our samples. VEM and VisEM deficits were observed in both SZ and BP patients, the latter showing significant differences from controls but with smaller ESs than SZ: for the CVLT total recall, ES = −1.45, PANCOVA < 0.05 in SZ patients vs ES = −0.94, PANCOVA < 0.05 in BP patients (figure 2a), whereas for the Rey delayed recall, ES = −1.17, PANCOVA < 0.05 in SZ patients vs ES = −0.85, PANCOVA < 0.05 in BP patients (figure 2b). As regards “the adult relatives,” VEM deficits were comparable in relatives of SZ (ES = −0.60, PANCOVA < 0.05) and relatives of BP (ES = −0.56, PANCOVA < 0.05). In contrast, as found in the total sample of adult relatives, VisEM showed no significant differences from controls in relatives of SZ and relatives of BP. For “the high-risk offspring,” congruent with what was previously reported,10 offspring of SZ and offspring of BP showed very similar deficits both on VEM (respectively, ES = −0.70, PANCOVA < 0.05 and ES = −0.88, PANCOVA < 0.05) and VisEM (respectively, ES = −0.84, PANCOVA < 0.05 and ES = −0.88, PANCOVA < 0.05). Given these findings, in the subsequent analyses, subjects were grouped notwithstanding their SZ vs BP diagnosis (for patients), and young offspring and nonaffected relatives were grouped notwithstanding the diagnoses of their affected relative.
Fig. 2.
Confidence Intervals (95%) of Effect Sizes Obtained in Offspring, Nonaffected Adult Relatives, and Patients. Each of the 3 subsamples was compared with its healthy control group. Panel A. Verbal episodic memory (VEM) (California Verbal Learning Test [CVLT] total recall). Panel B. Visual episodic memory (VisEM) (Rey delayed recall). High-risk (HR) offspring are represented by an orange box (▪), patients are represented by a black circle (•), and nonaffected adult relatives (NAARs) are represented by a green triangle (▴).
Generational Comparisons
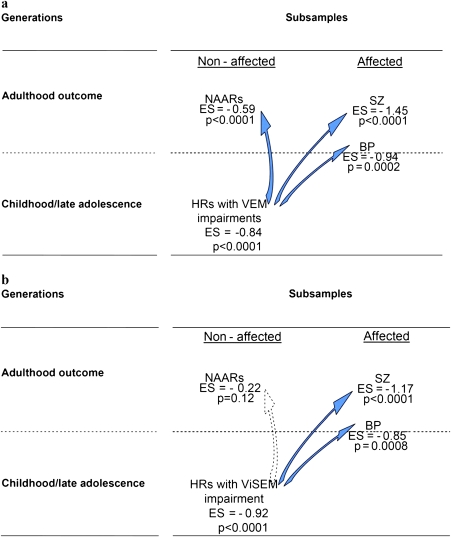
The patterns for VEM and VisEM differed across the 3 subsamples. figure 2 illustrates the ESs observed in young offspring, in nonaffected adult relatives, and in patients with their 95% CIs. For VEM, all 3 subsamples showed significant ESs starting at −0.59 with the additional feature that patients appeared more impaired than offspring and the latter worse than adult relatives. In other words, each of the 3 subsamples showed deficits in comparison with controls and the young offspring position was between patients and adult relatives (although their 95% CI did overlap), as offspring will eventually become nonaffected relatives or patients. For VisEM, the nonaffected relatives appeared different from the young offspring and patients, the 2 latter groups having overlapping values in terms of difference with controls. figure 3 illustrates further the 2 distinct generational patterns observed for VEM and for VisEM.
Fig. 3.
Trajectories Associated with Verbal Episodic Memory (VEM) and Visual Episodic Memory (VisEM) According to a Generational Pattern. VEM was measured by the California Verbal Learning Test (CVLT) total recall (panel A), and VisEM was measured by the Rey delayed recall (panel B). Affected means adult subjects affected by SZ or BP. Nonaffected means subjects not affected by SZ or BP, ie, nonaffected adult relatives (NAARs) and high-risk (HR) offspring.
Discussion
Memory Impairments in Offspring, Nonaffected and Affected Adults
In these members of Eastern Québec kindreds densely affected by SZ or BP, we observed sizeable episodic memory deficits, both verbal and visual. The present sample of young offspring, as our former smaller sample,10 showed deficits in both VEM and VisEM. Byrne et al28 in the Edinburgh HR Study also observed memory impairments in offspring of SZ. The observed impairments in our offspring are unlikely to be due to a prodromal state because the memory deficits were seen in both the very young and in the older offspring of our sample. It should be noted that the young offspring subsample is much younger than the age of disease incidence and consequently includes many future patients. This is not the case for the nonaffected adult relatives who are on average older than the age of incidence and are unlikely to develop the disease later. This might partly explain why some of the high ESs observed in the offspring are higher than those found in the nonaffected adult relatives.
In the adult relatives, the observed VEM deficits (ES ∼ −0.6) were very similar to those already reported in meta-analyses of studies of relatives of SZ or BP.3,5,29 Among the many domains assessed with our battery, the largest cognitive difference in relatives and in offspring appeared to be for VEM which is again congruent with the previous meta-analyses of relatives. The subgroup of nonaffected adult relatives with a nonpsychotic axis I diagnosis and the subgroup without showed nearly the same differences from controls in terms of ES (result not shown) which is congruent with the report of Gur et al.30
In our kindreds, the patients affected by SZ or BP also presented large ESs for VEM and VisEM that were comparable with those obtained in meta-analyses of studies of SZ1 and BP3 patients. Also, congruent with former studies,31 the memory impairments in the present BP patients were of a lesser magnitude, lying in an intermediate position between controls and SZ patients. It is noteworthy that we replicate the recent VisEM findings of Skelley et al,5 who showed the presence of VisEM deficits in SZ patients contrasting with an absence of such deficits in their nonaffected adult relatives. Overall, the observed cognitive impairments are consistent with findings reported in other samples in which familial loads for psychosis were less marked.
Developmental/Generational Memory Deficits From the Young Offspring up to the Adult Generation
The current study is the first, to our knowledge, to use such cross-sectional family data to inspect potential differential patterns between VEM and VisEM impairments across generations, ie, in the period preceding disease onset up to adulthood. Our observations have implications in 3 respects, as discussed below: (1) the distinct developmental trajectories of the verbal and the visual domains, (2) the use of VEM or VisEM as phenotypes in genetic studies, and (3) the potential heterogeneity in pathogenesis starting early in the life in the individual at risk. Overall, our findings suggest that all cognitive domains cannot be treated or modeled in the same manner in prevention or genetic studies.32
The presently observed pattern indicates that altered VEM can reflect, at the neuropsychological level, elements of risk shared in SZ and BP and transmitted within these high-risk families. VEM impairments would stand as an intermediate phenotype allowing the detection of gene carriers not expressing the DSM syndrome among nonaffected adult relatives and unaffected offspring.32,33 Such a pattern is suggested by the findings of figure 2 in which each of the 3 subsamples showed deficits in comparison with controls with the offspring position located between that of patients and adult relatives (although their 95% CI did overlap), as young offspring will eventually become nonaffected adult relatives or patients.
In contrast, VisEM deficits were absent in the nonaffected adult relatives but were apparent both in patients and young offspring. This suggests that the trajectory associated with VisEM would offer a better potential than VEM to predict future risk of developing the disease.
Although VisEM has so far been less investigated than VEM in major psychiatric disorders,5 the observed differences in generational patterns might reflect 2 relatively independent or heterogeneous developmental pathways with different gene-environment risk mechanisms from the early years to adulthood. To further test this hypothesis, we computed the correlation between VEM and VisEM performance in the offspring which was low and nonsignificant (r = .17, P = .21). Interestingly, the recent results from the Dunedin birth cohort34 also detected 2 different longitudinal pathways leading to psychosis, characterized by 2 different clusters of cognitive dysfunctions. Hence, as already suggested,21,35,36 determinants of the components of the disease might have roots in the early years of life in individuals at risk.
Little is known about the potential adverse effect of cognitive impairments in VEM or VisEM on the social adaptation of children or adolescents at risk.37–39 Future research must take into consideration that the putative heterogeneous pathways marked by VisEM or VEM deficits may result not only from gene-environment mechanisms affecting neurodevelopment but also from longitudinal mechanisms impacting negatively the social development and self-image that can accentuate the risk of later developing the disorder.
Cognitive Memory Patterns Shared by SZ and BP
Even though this was not the main goal of this report, it is noteworthy that impairments in VEM and VisEM were present in both the SZ and BP patients, as in former studies.9,31 We had already reported such commonality in memory impairments in the offspring of SZ or BP parents from these kindreds.10 This suggests that common dysfunctional neurodevelopmental pathways, influenced by genes or/and environment, may have an expression in cognition a long time before disease onset.10,35 In continuity, other shared phenotypic and genetic susceptibility loci or genes have been extensively documented for SZ and BP.12,40–43 These commonalities do not necessarily indicate that SZ and BP are the same disorder but rather that they would share some genetic and neurodevelopmental causes, as this has also been recently suggested by our physiological data in electroretinography in these young offspring.36 These phenotypic characteristics offer several promising avenues for future etiology and prevention research focusing on the longitudinal trajectories of different disease precursors.
Strengths and Limitations
This study strengths reside in the longitudinal follow-up of a large number of affected kindreds that permitted the thorough characterization of different phenotypes, both DSM diagnosis and neurocognition. The design also allowed us to test cognitive functioning cross-sectionally, using a generational paradigm looking at the young offspring unaffected by SZ or BP vs the adult family members who were either affected or nonaffected by the disease. However, one must first remember that the present young offspring had not yet reached the age of disease incidence and that the present study was not a long-term longitudinal study of these offspring. Second, it is unlikely that the observed generational patterns in visual and verbal were due to peculiarities of our young and adult control groups. table 1 and Supplementary table S1 report an overall 3 point difference of performance in raw scores on the RCF Test between the young and adult control groups. Incidentally, such a drop in the raw scores between the age 17 and 40 is also reported in the normative values (Supplementary table S2, supplemental data).44 Third, we cannot rule out the possibility that differences in the proportions of sibs, offspring, or parents in the different subsamples may have biased our results. However, such a possibility is unlikely given the recent meta-analysis of Snitz et al45 which showed that cognitive dysfunctions in nonaffected adult relatives did not vary with the different types of relatives. Finally, one must keep in mind that, due to the heavy risk setting related to the familial loading of the present sample, the generalization of the results to all the patients, the nonaffected adult relatives, or the HR offspring of the general population requires caution.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Canada Research Chair (#950-200810) in psychiatric genetics of which M.M is the Chair; Canadian Institute of Health Research grant (#MOP-74430).
Supplementary Material
Acknowledgments
We are grateful to our professional research assistants, Linda René, Marie-Claude Boisvert, Claudie Poirier, Lisette Gagnon, Louise Bélanger, Nicole Leclerc, Pierrette Boutin, Julie Boutin, Rosalie Ouellet, Karine Létourneau, and to the family members, adults and children, who participated in this study. We also thank l'Institut interuniversitaire de recherches sur les populations for their collaboration regarding the BALSAC database. The funding organizations had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Financial disclosures: M.M. and M.A.R. have been consultants for GlaxoSmithKline (GSK) and Eli Lilly and have received research funding from GSK, Eli Lilly and AstraZeneca that are not related to the material of this study. R.H.B. has been consultant and/or received funding from Eli Lilly, Pfizer, AstraZeneca, and Janssen Ortho that are not related to the material of this study. N.R., C.M., M.B., C.M., V.J., E.G., C.C., A.A., T.P., and M.E.P. have no financial support from pharmaceutical companies.
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 3.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113(1–2):1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl. 2007;116(434):17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 5.Skelley SL, Goldberg TE, Egan MF, Weinberger DR, Gold JM. Verbal and visual memory: characterizing the clinical and intermediate phenotype in schizophrenia. Schizophr Res. 2008;105(1–3):78–85. doi: 10.1016/j.schres.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidman LJ, Lanca M, Kremen WS, Faraone SV, Tsuang MT. Organizational and visual memory deficits in schizophrenia and bipolar psychoses using the Rey-Osterrieth complex figure: effects of duration of illness. J Clin Exp Neuropsychol. 2003;25:949–964. doi: 10.1076/jcen.25.7.949.16482. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre AA, Cellard C, Tremblay S, et al. Familiarity and recollection processes in patients with recent-onset schizophrenia and their unaffected parents. Psychiatry Res. 2009;175(1–2):15–21. doi: 10.1016/j.psychres.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pradhan BK, Chakrabarti S, Nehra R, Mankotia A. Cognitive functions in bipolar affective disorder and schizophrenia: comparison. Psychiatry Clin Neurosci. 2008;62:515–525. doi: 10.1111/j.1440-1819.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- 9.Schretlen DJ, Cascella NG, Meyer SM, et al. Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry. 2007;62(2):179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maziade M, Rouleau N, Gingras N, et al. Shared neurocognitive dysfunctions in young offspring at extreme risk for schizophrenia or bipolar disorder in eastern quebec multigenerational families. Schizophr Bull. 2009;35:919–930. doi: 10.1093/schbul/sbn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prinzie P, Onghena P. Cohort sequential design. In: Everitt BS, Howell DC, editors. Encyclopedia of Statistics in Behavioral Science. Chichester, UK: John Wiley & Sons, Ltd; 2005. pp. 319–322. [Google Scholar]
- 12.Maziade M, Chagnon YC, Roy MA, Bureau A, Fournier A, Mérette C. Chromosome 13q13-q14 locus overlaps mood and psychotic disorders: the relevance for redefining phenotype. Eur J Hum Genet. 2009;17:1034–1042. doi: 10.1038/ejhg.2008.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mérette C, Roy MA, Bureau A, et al. Replication of linkage with bipolar disorder on chromosome 16p in the Eastern Quebec population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:737–744. doi: 10.1002/ajmg.b.30673. [DOI] [PubMed] [Google Scholar]
- 14.Maziade M, Roy MA, Chagnon YC, et al. Shared and specific susceptibility loci for schizophrenia and bipolar disorder: a dense genome scan in Eastern Quebec families. Mol Psychiatry. 2005;10:486–499. doi: 10.1038/sj.mp.4001594. [DOI] [PubMed] [Google Scholar]
- 15.Maziade M, Roy MA, Fournier JP, et al. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: results from the Quebec pedigree studies. Am J Psychiatry. 1992;149:1674–1686. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- 16.Maziade M, Roy MA, Martinez M, et al. Negative, psychoticism, and disorganized dimensions in patients with familial schizophrenia or bipolar disorder: continuity and discontinuity between the major psychoses. Am J Psychiatry. 1995;152:1458–1463. doi: 10.1176/ajp.152.10.1458. [DOI] [PubMed] [Google Scholar]
- 17.Roy MA, Lanctôt G, Mérette C, et al. Clinical and methodological factors related to reliability of the best-estimate diagnostic procedure. Am J Psychiatry. 1997;154:1726–1733. doi: 10.1176/ajp.154.12.1726. [DOI] [PubMed] [Google Scholar]
- 18.Maziade M, Chagnon YC, Roy MA, Bureau A, Fournier A, Merette C. Chromosome 13q13-q14 locus overlaps mood and psychotic disorders: the relevance for redefining phenotype. Eur J Hum Genet. 2009;17:1034–1042. doi: 10.1038/ejhg.2008.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children—present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I disorders (Patient ed.) Washington, DC: New York State Psychiatric Institute: American Psychiatric Press; 1998. [Google Scholar]
- 21.Maziade M, Gingras N, Rouleau N, et al. Clinical diagnoses in young offspring from eastern Quebec multigenerational families densely affected by schizophrenia or bipolar disorder. Acta Psychiatr Scand. 2008;117(2):118–126. doi: 10.1111/j.1600-0447.2007.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 23.Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial (RCFT) Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 24.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 25.Kelley K. Confidence intervals for standardized effect sizes: theory, application, and implementation. J Stat Softw. 2007;20(8):1–24. [Google Scholar]
- 26.Steiger JH, Fouladi RT. Noncentrality interval estimation and the evaluation of statistical models. In: Mulaik SA, Harlow LL, Steiger JH, editors. What if There Were No Significance Tests. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1997. pp. 221–257. [Google Scholar]
- 27.Hochberg Y. A sharper Bonferroni procedure for multiple significance testing. Biometrika. 1988;75:800–803. [Google Scholar]
- 28.Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS) Psychol Med. 1999;29:1161–1173. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- 29.Whyte MC, McIntosh AM, Johnstone EC, Lawrie SM. Declarative memory in unaffected adult relatives of patients with schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2005;78(1):13–26. doi: 10.1016/j.schres.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 31.Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80(2–3):137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Szatmari P, Maziade M, Zwaigenbaum LZ, et al. Informative phenotypes for genetic studies of psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2007;144:581–588. doi: 10.1002/ajmg.b.30426. [DOI] [PubMed] [Google Scholar]
- 33.Braff DL, Freedman R, Schork NJ, Gottesman I. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33(1):21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2009.09040574. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Hebert M, Gagne AM, Paradis ME, et al. Retinal response to light in young nonaffected offspring at high genetic risk of neuropsychiatric brain disorders. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.08.016. doi:10.1016/j.biopsych.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Cicchetti D, Rogosch FA, Toth SL. The efficacy of toddler-parent psychotherapy for fostering cognitive development in offspring of depressed mothers. J Abnorm Child Psychol. 2000;28(2):135–148. doi: 10.1023/a:1005118713814. [DOI] [PubMed] [Google Scholar]
- 38.Rutter M. Psychosocial influences: critiques, findings, and research needs. Dev Psychopathol. 2000;12:375–405. doi: 10.1017/s0954579400003072. [DOI] [PubMed] [Google Scholar]
- 39.Rutter M, Kim-Cohen J, Maughan B. Continuities and discontinuities in psychopathology between childhood and adult life. J Child Psychol Psychiatry. 2006;47:276–295. doi: 10.1111/j.1469-7610.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 40.Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2009;66:748–755. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- 41.Goes FS, Zandi PP, Kuangyi M, et al. Mood-incongruent psychotic features in bipolar disorder: familial aggregation and suggestive linkage to 2p11-q14 and 13q21-33. Am J Psychiatry. 2007;164:236–247. doi: 10.1176/ajp.2007.164.2.236. [DOI] [PubMed] [Google Scholar]
- 42.Maier W, Zobel A, Wagner M. Schizophrenia and bipolar disorder: differences and overlaps. Curr Opin Psychiatry. 2006;19(2):165–170. doi: 10.1097/01.yco.0000214342.52249.82. [DOI] [PubMed] [Google Scholar]
- 43.Berrettini W. Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C Semin Med Genet. 2003;123C(1):59–64. doi: 10.1002/ajmg.c.20014. [DOI] [PubMed] [Google Scholar]
- 44.Meyers J, Meyers K. Rey Complex Figure Test and the Recognition Trial: Professional Manual. Supplement norms for children and adolescents. Odessa, FL: PA Resources; 1996. [Google Scholar]
- 45.Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.