Abstract
Purpose
The clinical success of treating corneas with total limbal stem cell deficiency using limbal biopsy explants cultured on intact amniotic membrane (iAM) relies on ex vivo expansion of limbal epithelial progenitor cells. However, the ultimate fate of limbal epithelial progenitor cells in the explant remains unclear.
Methods
Human limbal explants were cultured on iAM for 2 weeks and then removed and transferred to a new iAM until passage 3. The outgrowth surface area of each passage was measured and compared. For each passage, clonogenicity on 3T3 fibroblasts feeder layers was compared among progenitor cells removed from the outgrowth, the explant surface, and the remaining stroma. Cryosections of the explant and the outgrowth were detected with p63, vimentin, pancytokeratin, and the basement membrane components type VII and IV collagen and laminin 5 antibodies.
Results
The outgrowth surface area significantly decreased from passage (P)1 to P3. The total number of epithelial cells that were isolated from the explant surface also decreased from before culture (P0) to P1, became stable from P1 to P2, but was uncountable at P3. Clonogenicity significantly declined from P1 to P3 for the epithelium derived from the explant surface and the outgrowth epithelium; the extent was less in the former than in the latter at P2 and P3. In addition, groups of epithelial cells invaded the limbal stroma of the explants from P1 to P3; p63(+)/pancytokeratin(−) and p63(+)/vimentin(+) cells also presented in the limbal stroma. Increasing fibroblast, but not epithelial, colonies were observed from cells isolated from the remaining limbal stroma when seeded on 3T3 fibroblast feeder layers from P1 to P3.
Conclusions
During ex vivo expansion on iAM, some limbal epithelial progenitor cells indeed migrate onto iAM from the explant surface, whereas some also invade the limbal stroma, very likely undergoing epithelial-mesenchymal transition. This new information should be taken into account in formulating new strategies to improve the expansion protocol.
The epithelial progenitor cells including stem cells of the cornea are located in the limbal basal layer and serve as the ultimate source for constant corneal epithelial renewal.1 Like those in other tissues, limbal epithelial progenitor cells are supported by a unique stromal microenvironment called the stem cell niche, which presumably consists of certain extracellular matrix components, cell membrane-associated molecules, and unique cytokine dialogues.2 Destructive loss of limbal stem cells or dysfunction of their stromal niche causes many corneas to have blinding limbal stem cell deficiency.1,2
Transplantation of cryopreserved amniotic membrane (AM) is sufficient to treat partial limbal stem cell deficiency,3,4 suggesting that AM may be used as an ideal substrate to promote expansion of limbal epithelial stem cells in vivo. Nevertheless, this surgical procedure cannot be used to treat the total limbal stem cell deficiency caused by chemical and thermal injuries, Stevens-Johnson syndrome (SJS), and ocular cicatricial pemphigoid, without transplantation of autologous5 or allogeneic6 limbal epithelial stem cells (for reviews, see Refs. 7–9).
One emerging surgical strategy for restoring a normal corneal epithelial surface on these limbal-deficient corneas is to transplant limbal corneal epithelial progenitor cells expanded ex vivo on AM.10–14 To achieve this objective, one protocol is to cultivate a limbal biopsy specimen on an intact cryopreserved AM (iAM).15–17 The resultant AM composite graft with ex vivo expanded limbal epithelial progenitor cells has successfully reconstructed corneas with total limbal stem cell deficiency in a rabbit study after 1 year of follow-up14,18,19 and in several human studies.10,20 Other practicing protocols differ from the aforementioned one in the use of epithelially denuded AM,21,22 suspension of epithelial cells rather than explants,21,22 or cocultivation of 3T3 fibroblast feeder layers.22,23 At the present time, no study been conducted to compare these cultivation variables thoroughly, to determine which one(s) is vital in achieving effective expansion of limbal epithelial progenitor cells.
One critical factor that may influence the long-term clinical outcome of this surgical procedure is whether a sufficient number of limbal epithelial progenitor cells has been successfully expanded. Because the expanded epithelium from limbal explants on iAM adopts a limbal epithelial phenotype whereas that expanded on epithelially denuded AM reveals a corneal epithelial phenotype,17 we have assumed that limbal epithelial progenitor cells have migrated from the explant onto iAM during cultivation. Even if this were the case, it remains unclear whether such migration continues indefinitely. Recently, we reported that limbal basal epithelial progenitor cells can also invade the limbal stroma when rabbit limbal explants are cultured on a collagen-coated substrate at the air–medium interface.24 Furthermore, these invading epithelial progenitor cells undergo epithelium–mesenchymal transition (EMT) into fibroblasts.24 Therefore, it also remains unclear whether such a phenomenon also takes place in human limbal explants cultured on iAM. To answer these questions, we undertook the investigation described herein and discovered that there was a progressive decline in the number of epithelial progenitor cells migrating from the explant and that such a decline was attributable in part to the cells’ invading the limbal stroma. This important finding underscores the necessity of further modifying this ex vivo expansion protocol to achieve a higher success in clinical transplantation.
Materials and Methods
HEPES-buffered Dulbecco’s modified Eagle’s medium (DMEM), Ham’s/F12 medium, amphotericin B, gentamicin, fetal bovine serum (FBS), newborn calf serum (NCS), mouse epidermal growth factor (EGF), and 0.05% trypsin-0.53 mM EDTA were purchased from Invitrogen (Carlsbad, CA). Hydrocortisone, dimethyl sulfoxide, cholera toxin, insulin-transferrin-sodium selenite media supplement, mitomycin C, bovine serum albumin (BSA), Triton X-100, mouse monoclonal anti-pancytokeratin, type IV and VII collagen antibodies, FITC-conjugated goat anti-mouse IgG antibody, 3% hydrogen peroxide, propidium iodide (PI), Hoechst 33342 dye, and 4% formalin were all from Sigma-Aldrich (St. Louis, MO). Dispase II was obtained from Roche (Indianapolis, IN); Swiss albino 3T3 fibroblasts from ATCC (Manassas, VA); a cell viability kit (LIVE/DEAD) from Invitrogen-Molecular Probes (Eugene, OR); an ABC kit (Vectastain Elite) for mouse IgG and mounting medium (Vectashield) from Vector Laboratories (Burlingame, CA); mouse monoclonal anti-p63 antibody (Clone 4A4) and diaminobenzidine (DAB) from DakoCytomation (Carpinteria, CA); rabbit monoclonal anti-vimentin antibody from abcam (Cambridge, MA); mouse monoclonal anti-laminin 5 antibody from Chemicon International, Inc. (Temecula, CA); and optimal cutting temperature (OCT; Tissue-Tek) compound from Sakura Finetek (Torrance, CA).
Human Tissue Preparation
Human tissue was handled according to the Declaration of Helsinki. Corneoscleral tissues from human donor eyes were obtained from the Florida Lions Eye Bank (Miami, FL) immediately after the central corneal button had been used for corneal transplantation. The tissue was rinsed three times with DMEM containing 50 μg/mL gentamicin and 1.25 μg/mL amphotericin B. After careful removal of excessive sclera, conjunctiva, iris, and corneal endothelium, the remaining tissue was placed in a culture dish and exposed to 10 mg/mL Dispase II in supplemented hormonal epithelial medium (SHEM) at 37°C under humidified 5% CO2 for 10 minutes. After three rinses with SHEM for 10 minutes each, each corneoscleral rim was trimmed to obtain 12 limbal tissue cubes of 1-clock-hour width (i.e., an approximately 1 × 1.5 × 2.5-mm size). The whole experiment was conducted using five corneoscleral rims which generated a total of 60 explants.
Human Limbal Explant Cultures on Intact Amniotic Membrane
Cryopreserved human AM was kindly provided by Bio-Tissue (Miami, FL) and was managed according to the Declaration of Helsinki. After thawing at room temperature, AM with the epithelium intact and facing up was fastened to a culture insert, as previously reported.25 On the center of each AM insert, a human limbal explant was cultured in SHEM made of an equal volume of HEPES-buffered DMEM and Ham’s F12. The medium was supplemented with 5% FBS, 0.5% dimethyl sulfoxide, 2 ng/mL mouse EGF, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 0.5 μg/mL hydrocortisone, 1 nM cholera toxin, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B. Cultures were incubated at 37°C in 5% CO2 and 95% air, and the medium was changed every 2 to 3 days. After 2 weeks of culture (defined as passage [P]1), the outgrowth area was measured by scanning the border of the outgrowth. Some explants were removed and embedded in OCT compound for cryosectioning, and some were removed for isolation of the surface epithelial sheet, as described later. The remaining explants were passaged to new AM inserts and cultured in the same manner for 2 weeks (defined as P2). Again, cultures and explants were terminated in the same manner as described earlier. The same method of transfer and culturing was performed until the end of P3.
Harvesting of Epithelial Sheets from Explants and the Outgrowth on AM
Limbal epithelial sheets were isolated directly from limbal explants before and after cultivation, based on our modified digestion method using 10 mg/mL Dispase II in SHEM at 4°C for 16 hours.26 Under a dissecting microscope, the limbal epithelial sheet was separated by inserting and sliding a noncutting, flat, stainless-steel spatula into a plane between the limbal epithelium and the stroma. Epithelial sheets were also isolated from the outgrowth expanding on iAM by first making a trephine cut of 7 mm in diameter including the underlying AM and then by incubating it in 10 mg/mL Dispase II in SHEM at 37°C for 15 minutes. The epithelial sheet was then separated under the dissecting microscope using the technique just described.
Epithelial Colony Formation Assay on 3T3 Fibroblast Feeder Layers
Epithelial sheets harvested from the surface of limbal explants and from the outgrowth on AM were treated with 0.05% trypsin/0.53 mM EDTA at 37°C for 15 minutes to be rendered into single cells. The remaining limbal explant stroma was digested with 1 mg/mL collagenase A in SHEM at 37°C for 16 hours to isolate cells remaining in the stroma. A colony formation assay for epithelial progenitor cells followed the method reported by Rheinwald and Green27 for epidermal keratinocytes and modified and adopted by us for limbal epithelial cells.28 In brief, Swiss albino 3T3 fibroblasts, grown in DMEM containing 10% NCS at 80% subconfluence, were treated with 5 μg/mL mitomycin C for 2 hours and then trypsinized and plated at a density of 2 × 104 cells/cm2 in 60-mm dishes. Epithelial cells isolated from the surface epithelium of the explant and the outgrowth as mentioned earlier were seeded at a density of 30 cells/cm2 in 60-mm dishes on 3T3 fibroblasts feeder layers in SHEM. Entire cells isolated from the remaining stroma were also seeded on 3T3 fibroblasts feeder layers. Cultures were incubated at 37°C under 5% CO2 and 95% humidity, and the medium was changed every 2 to 3 days. Colonies were fixed by 4% formalin 12 days later, stained with crystal violet, and photographed.
Histology and Immunostaining
Frozen sections of 4 μm thickness obtained from the explants as well as the epithelial outgrowth on AM were fixed in acetone at −20°C for 10 minutes and stained with hematoxylin for histology. For immunofluorescence staining, fixed sections were incubated in 0.2% Triton X-100 for 10 minutes. After three rinses with PBS for 5 minutes each and preincubation with 2% BSA to block nonspecific staining, sections were incubated with primary antibodies for 1 hour. After three washes with PBS for 15 minutes, the sections were incubated with an FITC-conjugated secondary antibody (goat anti-mouse IgG at 1:200) for 1 hour. After three additional PBS washes for 5 minutes each, the sections were mounted with an antifade solution and photographed with an epifluorescence microscope. For double staining of p63 and pancytokeratin or vimentin, immunohistochemical staining of p63 was conducted first. After fixation, the endogenous peroxidase activity was blocked by 0.6% hydrogen peroxide for 10 minutes. Nonspecific staining was blocked by 1% normal goat serum for 30 minutes. The sections were then incubated with anti-p63 monoclonal (4A4) antibody (1:50) for 1 hour. After three washes with PBS for 15 minutes, the sections were incubated with biotinylated anti-mouse IgG (1:100) for 1 hour followed by incubation with ABC reagent for 30 minutes, the reaction product was then developed with DAB for 2 minutes. After that, the sections were washed with PBS for 15 minutes and blocked again with 2% BSA, followed by immunofluorescence staining with pancytokeratin or vimentin. Nuclei were counterstained with PI or Hoechst 33342, and the sections were mounted with antifade solution. Images were photographed at the same location with an epifluorescence and a light microscope, separately.
Statistical Analysis
Summary data are reported as the mean ± SD. Group means were compared using the appropriate version of Student’s unpaired t-test. Test results are two-tailed, where P < 0.05 is considered statistically significant.
Results
Successful Separation of Epithelial Sheets from Limbal Explants and Outgrowth on iAM
From a limbal explant tissue, a limbal epithelial sheet (Fig. 1A) was successfully isolated from the remaining stroma (Fig. 1B) using our reported modified method of Dispase II digestion.26 Complete removal of epithelial cells was verified by pancytokeratin staining of the isolated epithelial sheet (Fig. 1C), whereas the remaining stroma did not contain pancytokeratin(+) epithelial cells (Fig. 1D). For further confirmation further that limbal epithelial progenitor cells had been totally separated from the limbal stroma, trypsinized epithelial cells from the limbal epithelial sheet were seeded on 3T3 fibroblasts feeder layers, cultured for 12 days, and generated vivid clonal growth (Fig. 1E). In contrast, cells isolated from the remaining stroma by collagenase digestion did not reveal any epithelial colony on 3T3 fibroblast feeder layers (Fig. 1F).
Figure 1.
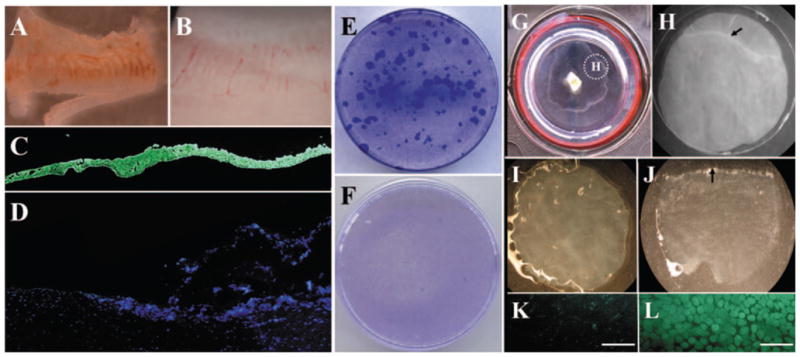
Isolation and clonal culture of human limbal epithelial cells and outgrowth on iAM. From limbal tissue, an epithelial sheet (A) was successfully isolated from the remaining stroma (B) by a modified method of Dispase II digestion. Complete removal of epithelial cells was confirmed by pancytokeratin staining of the isolated epithelial sheet (C), whereas the remaining stroma did not contain pancytokeratin(+) epithelial cells (D). Epithelial cells from the limbal sheet showed vivid clonal growth (E). In contrast, cells isolated from the remaining stroma did not form any epithelial colony on 3T3 fibroblast feeder layers (F). Epithelial outgrowth generated by limbal explant culture on iAM was visible after 2 weeks’ cultivation (G). A 7-mm trephine was used to remove the outgrowth together with the underlying AM (G, inset), and the section was subjected to Dispase II digestion (H, dotted circle in G). The remaining AM (I) did not contain any epithelial cells, as shown by cell-viability assay (K). In contrast, the isolated epithelial sheet (J) contained 100% viable cells, according to the viability assay (L). It should be noted that trephination was intentionally performed to include the border of the outgrowth (H, arrow) and to verify the completion of such isolation by showing such a border of the isolated epithelial sheet (J, arrow). For the data presented in the study, trephination was performed to include only the epithelial outgrowth. Bars, 100 μm.
The limbal explant was then cultured on iAM for 2 weeks and generated a visible epithelial outgrowth (Fig. 1G). A 7-mm trephine was used to remove the epithelial outgrowth together with the underlying AM and the sections were subjected to Dispase II digestion (Fig. 1H), resulting in successful isolation of the entire epithelial sheet from the remaining AM (Figs. 1I, 1J). Isolated epithelial cells retained 100% viability (LIVE/DEAD cell-viability assay; Invitrogen-Molecular Probes; Figs. 1K, 1L). These results collectively confirmed that our isolation methods were capable of removing the entire epithelial sheet from the surface of the limbal explant and from the AM after cultivation.
Migration of Limbal Epithelial Progenitor Cells from Limbal Explants to iAM
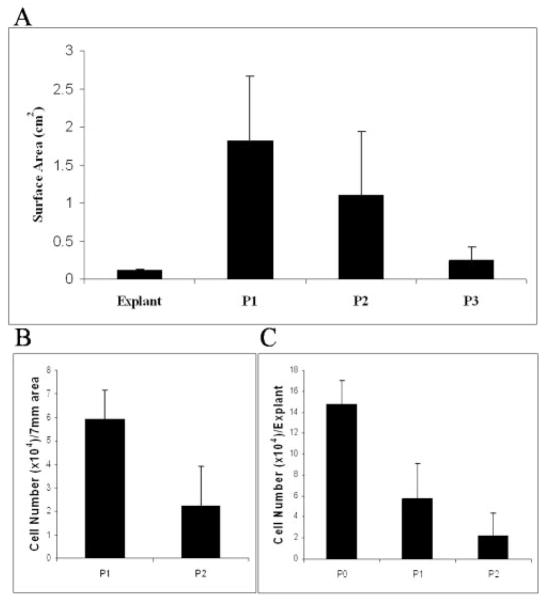
To investigate whether epithelial cells continuously migrated from the limbal explant onto the iAM, we cultured 50 limbal explants on iAM and successively passaged them until P3. The surface area of epithelial outgrowth was measured at the end of a 2-week cultivation for each of the three passages. The results showed that the surface area decreased significantly from P1 (n = 41) to P2 (n = 23) and to P3 (n = 10; P1 vs. P2, P = 0.002; P2 vs. P3, P < 0.001; Fig. 2A). Cells isolated from the same surface area of the epithelial outgrowth by a 7-mm trephine were counted. The result showed that the total number of cells also significantly decreased from P1 to P2 (Fig. 2B, P = 0.004, n = 5). Cells isolated from the surface of the entire limbal explant were also counted. The results showed that the total number of cells per explant decreased significantly from P0 to P1 (P = 0.002, n = 5), that there was not a significant difference between P1 and P2 (P = 0.08, n = 5), and that the cells were uncountable at P3 (Fig. 2C). These results collectively indicated that the extent of epithelial outgrowth migrated from the explant progressively declined with an increase in the cell size when cultured on iAM and that the number of epithelial cells remaining on the surface of the explant also progressively declined with time.
Figure 2.
Decline of limbal epithelial cell outgrowth from limbal explants during successive passages. The surface area of epithelial outgrowth was measured when the same limbal explant was transplanted to a new AM every 2 weeks for three passages. (A) The surface area decreased significantly from P1 to P3 (P = 0.002 for P1 vs. P2 and P < 0.001 for P2 vs. P3; n = 41 for P1, n = 23 for P2, n = 10 for P3). (B) Cells from the outgrowth were isolated and counted, and the number of cells from the unit surface area (7 mm diameter) also significantly decreased from P1 to P2 (P = 0.004, n = 5). (C) The number of cells from the surface of the explant also decreased significantly from P0 to P1 (P = 0.002, n = 5), but did not show a significant difference between P1 and P2 (P = 0.08, n = 5).
To determine whether the decline in the number of cells and the enlargement of the cell size was a reflection of limbal epithelial progenitor cells, we isolated cells from the surface epithelium of the limbal explant and from the explant outgrowth at each passage and seeded the same density of 30 cells/cm2 on 3T3 fibroblasts feeder layers in 60-mm dishes in triplicate for 12 days. A total of five explants, each derived from a different donor limbal rim, were performed for each passage, and the representative crystal violet–stained dishes from different passages of the same donor limbal rim are shown in Figure 3A. Epithelial colonies notably decreased from P1 to P3 in both the surface epithelium of the explant (Fig. 3A, left column) and in the epithelial outgrowth (Fig. 3A, middle column). Epithelial colonies from the explant surface were larger than those from the outgrowth at P1, whereas there were more epithelial colonies from the outgrowth than the explant surface at P2 and P3. At P3, there was no colony formed from epithelial cells isolated from the explant surface, whereas some small epithelial colonies could still be generated from the outgrowth epithelium. These results collectively indicated that limbal epithelial progenitor cells indeed migrated from the limbal explant to iAM during ex vivo expansion. However, such progenitor cells progressively declined in number after each passage. Furthermore, limbal epithelial progenitor cells remaining on the surface of the explant also declined in number after each passage, suggesting that there was an overall loss of the potency of these cells. Of note, cells isolated from the remaining stroma by collagenase yielded only increasing fibroblasts, but not epithelial cell colonies, from P1 to P3 when seeded on 3T3 fibroblasts feeder layers (Fig. 3A, right column). Phase-contrast photographs showed typical epithelial colonies generated from the explant (Fig. 3B) and outgrowth (Fig. 3C) epithelial cells, whereas cells from explant stroma generated only fibroblast colonies (Fig. 3D).
Figure 3.
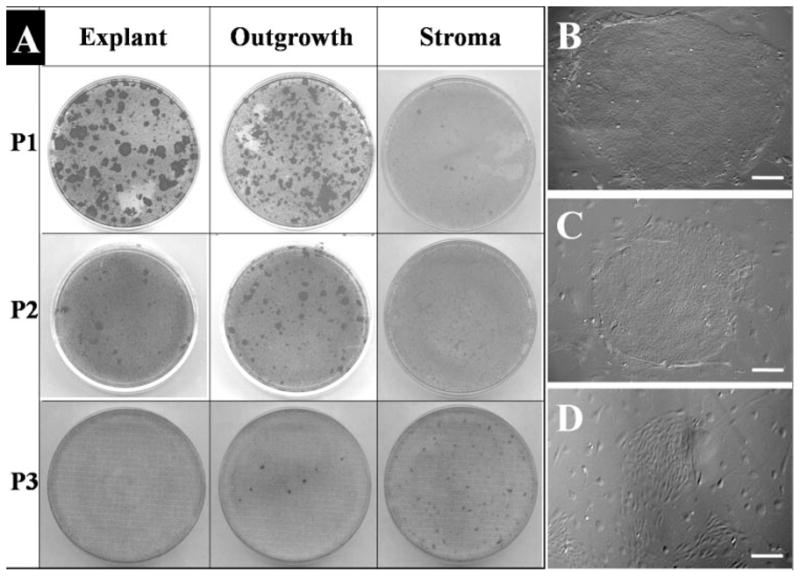
Epithelial clonogenicity of explant surface epithelium, outgrowth epithelium, and remaining explant stroma. The same number of cells isolated from the surface epithelium of the limbal explant and from the explant outgrowth was seeded on 3T3 fibroblast feeder layers for 12 days. Epithelial colonies visualized by crystal violet staining notably decreased from P1 to P3 for both the epithelium on explants (A, left column) and that from the outgrowth (A, middle column). As a comparison, cells isolated from the remaining stroma only yielded increasing fibroblast colonies from P1 to P3 (A, right column). Phase-contrast images showed representative colonies from P1 explant (B), P1 epithelial outgrowth (C), and P3 stroma cells (D). Bars, 200 μm.
Intrastromal Invasion of Limbal Epithelial Progenitor Cells during Successive Passages on AM Culturing
Because limbal epithelial progenitor cells invade the limbal stroma when rabbit limbal explants are cultured on a collagen-coated substrate and exposed to the air–medium interface,24 we wondered whether a similar phenomenon might also occur in human limbal explants cultured on iAM in a submerged manner. To do so, we removed five limbal explants from each passage, each from a different donor limbal rim, at the end of 2 weeks of culturing and subjected them to frozen sectioning. Hematoxylin staining showed that the surface of the control limbal explant before cultivation (P0) was covered by a stratified epithelium of four to six cell layers (Figs. 4A, 4B). In contrast, the number of epithelial cell layers on the explant surface progressively decreased from P1 to P3, to a level where the cells did not form a continuous layer. This finding helps explain why there was a progressive decline of the total number of cells that could be isolated from the explant surface (Fig. 2C). In an intriguing finding, groups of epithelioid cells were observed in the limbal stroma of P1 (Figs. 4C, 4D) and P2 (Figs. 4E, 4F) explants, but not in those at P0 and P3 (Figs. 4G, 4H).
Figure 4.
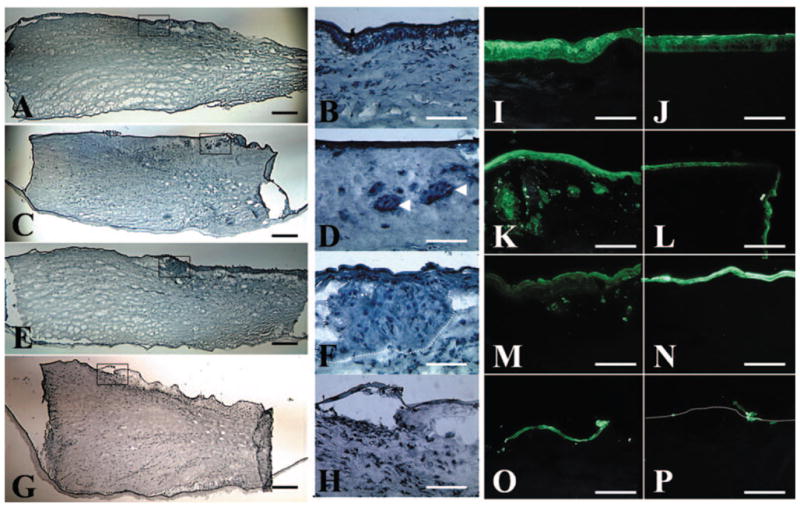
Migration and invasion of limbal epithelial cells. (A, B) Hematoxylin staining of the explant showed a stratified epithelium before cultivation (P0). The number of epithelial cell layers on the surface of the explant decreased from P1 (C, D) and P2 (E, F) to P3 (G, H). Intriguingly, groups of epithelioid cells were found in the limbal stroma of P1 and P2 explants (D, F, arrowheads and dotted lines, respectively), but not at P0 (B) and P3 (H). (B, D, F, H are the boxed areas of A, C, E, G, respectively). Pancytokeratin staining showed that epithelial cells were only noted on the surface of the limbal (I) and the peripheral corneal (J) region of the explant before cultivation (P0). In contrast, epithelial cells migrated onto the corneal cut edge of the explant at P1 (L). Epithelial cell layers on both the limbal (K, M, O, represent P1, P2, and P3, respectively) and the peripheral (L, N, P, represent P1, P2, and P3, respectively) corneal regions decreased in successive passages from P1 to P3, resulting in only sporadic epithelial cells left on the surface of P3 explant. Groups of epithelial cells were found in the limbal stroma at P1 (K), decreased at P2 (M), and became negligible at P3 (O). Bar, 100 μm.
To verify that the aforementioned intrastromal epithelioid cells were indeed epithelial cells, cryosections were stained with pancytokeratin antibody, a marker that recognizes different keratins in simple and stratified epithelium. For the control explant before cultivation (P0), positive epithelial staining was noted only on the surface of limbal (Fig. 4I) and peripheral corneal regions (Fig. 4J). For the explant at P1, epithelial cells indeed migrated onto the corneal cut edge (Fig. 4L). Epithelial cell layers on both limbal and peripheral corneal regions decreased after successive passages from P1 to P3, with only sporadic epithelial cells left on the P3 explant surface (Figs. 4O, 4P). Groups of epithelioid cells found in the limbal stroma by hematoxylin staining were confirmed to be epithelial cells by positive pancytokeratin staining. Such cells were abundant at P1 (Fig. 4K) but decreased at P2 (Fig. 4M) and became negligible at P3 (Fig. 4O). In contrast, epithelial cells were found only on the surface but not in the stroma of the peripheral cornea at all passages. These results collectively indicate that besides migration to AM, some limbal epithelial cells also invaded the limbal stroma during ex vivo expansion.
To illustrate further the intrastromal invasion of limbal epithelial cells, we investigated the basement membrane components on P0 and P1 explants. As expected, type VII collagen and laminin 5 exhibited linear band staining in the limbal and peripheral corneal epithelia, type IV collagen presented in the limbus but not the peripheral cornea in P0 explant before culture as reported29 (data not shown). After 2 weeks of culturing, all these basement membrane components were partially dissolved and broken down both in the limbus (Figs. 5A–C) and the peripheral cornea (Figs. 5D–F). However, intrastromal invading cells that were surrounded by basement membrane components were found only in the limbal stroma, but not in the peripheral corneal stroma, indicating that intrastromal invasion was restricted to limbal epithelial cells.
Figure 5.
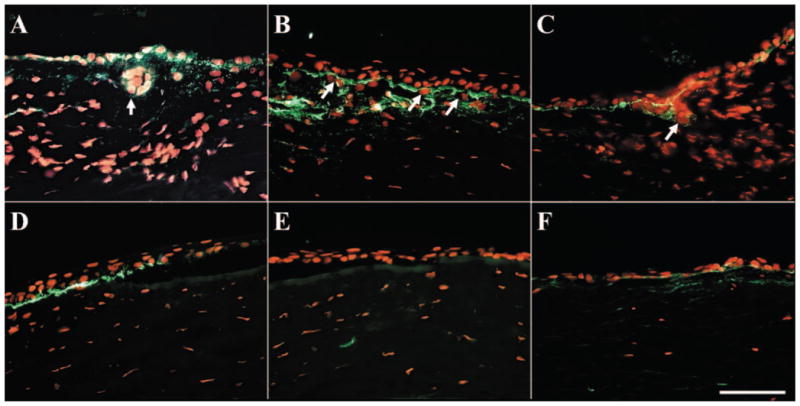
Dissolution of basement membrane components during explant cultures. Immunostaining of type VII collagen (A, D), type IV collagen (B, E), and laminin 5 (C, F) in P1 explants showed that the basement membrane was partially dissolved in both the limbus (A–C) and the peripheral cornea (D–F) after 2 weeks of culturing. However, cell invasion only happened in the limbus (A–C, arrows) but not in the peripheral cornea. Bar, 100 μm.
To examine whether these invading epithelial cells contained epithelial progenitor cells, we performed double staining of p63, a marker thought to be expressed by basal epithelial progenitor cells of all stratified epithelia30–33 and pancytokeratin. For P0 explant before cultivation, as expected and shown in Figure 4I, all cells in the surface epithelium showed positive staining for pancytokeratin (Fig. 6A). In these multiple epithelial cell layers, most basal and some suprabasal epithelial cells showed positive nuclear staining for p63 (Fig. 6B). No cell in the limbal stroma showed positive staining for pancytokeratin or p63. In contrast, for P1 and P2 explants, pancytokeratin staining revealed groups of epithelial cells in the stroma (Figs. 6C, 6E, respectively). For P1 explants, some of these pancytokeratin(+) epithelial cells, either as a group or as single cells, retained p63(+) nuclear staining (Figs. 6C, 6D, respectively). However, some cells with positive nuclear staining for p63 were negative for pancytokeratin staining (Figs. 6C, 6D, arrows). In P2 explants, cells showing positive nuclear staining for p63 but negative staining for pancytokeratin were located at the periphery of the invading epithelial cluster (Figs. 6E, 6F, arrows). In contrast, cells in the center of the invading cluster were positive for pancytokeratin staining but negative for p63 staining. In the outgrowth epithelium, all epithelial cells were positive for pancytokeratin whereas some also expressed nuclear staining for p63 in all passages (data not shown). These results confirm that some invading cells indeed were p63-expressing epithelial progenitor cells. However, some invading cells had lost the keratin-expressing epithelial characteristic.
Figure 6.
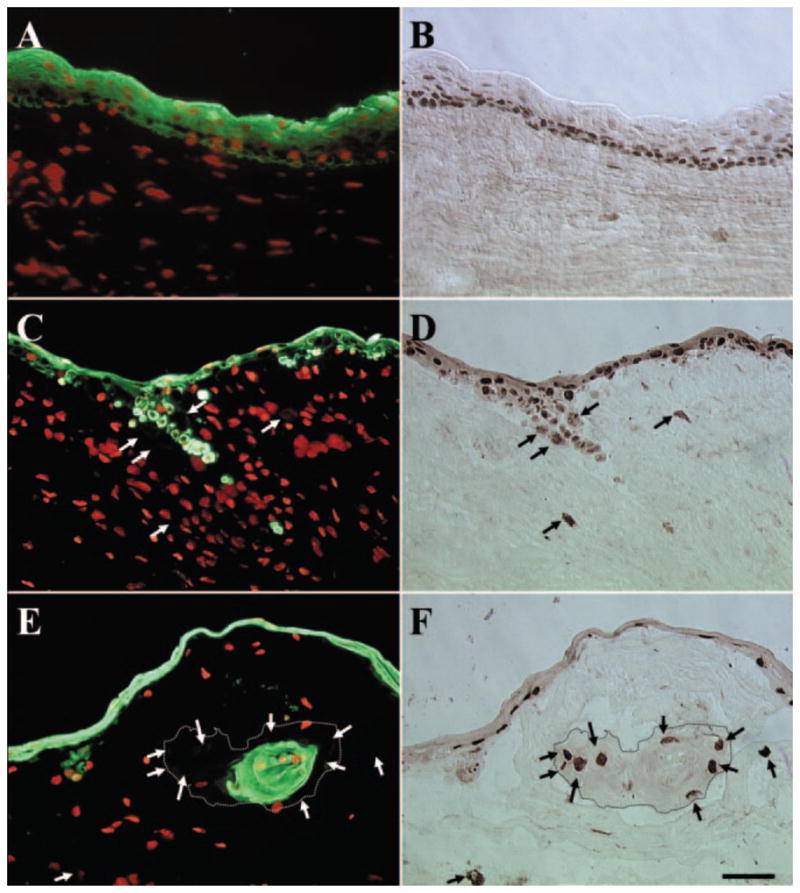
Double staining of pancytokeratin and p63. For P0 explant before cultivation, all cells in the surface epithelium were pancytokeratin(+) (A), whereas most of the basal and some suprabasal epithelial cells showed p63(+) nuclear staining (B). No cell in the limbal stroma showed positive staining for pancytokeratin or p63. In contrast, in P1 and P2 explants, pancytokeratin staining revealed groups of epithelial cells in the stroma (C, E, respectively). In P1 explants, some of these pancytokeratin(+) epithelial cells retained p63(+)nuclear staining (C, D). However, some cells with p63(+) nuclear staining were pancytokeratin(−) (C, D, arrows). Cells showing p63(+) staining but pancytokeratin(−) staining were located at the periphery of the invading epithelial cluster (E, F, arrows, dotted lines outline the border of invading epithelial clusters) for P2 explants. In contrast, cells in the center of invading epithelial clusters were positive for pancytokeratin but negative for p63. Bar, 50 μm.
To determine whether the invading limbal epithelial progenitor cells had undergone the epithelial–mesenchymal transition, we performed double-staining of p63 and vimentin in the explant. Results showed vimentin (Figs. 7A, 7C, 7E) and p63 (Figs. 7B, 7D, 7F) double-stained cells were in the limbal stroma from all the passages except P0. Furthermore, there were also some p63(+) cells in the limbal stroma that did not express vimentin. Vimentin and p63 double-stained cells were also presented on the surface of P2 explant (Figs. 7C, 7D). Consistent with the previous report,34,35 vimentin(+)/p63(−) cells are also present in the limbal basal layer of the explant before culture (data not shown).
Figure 7.
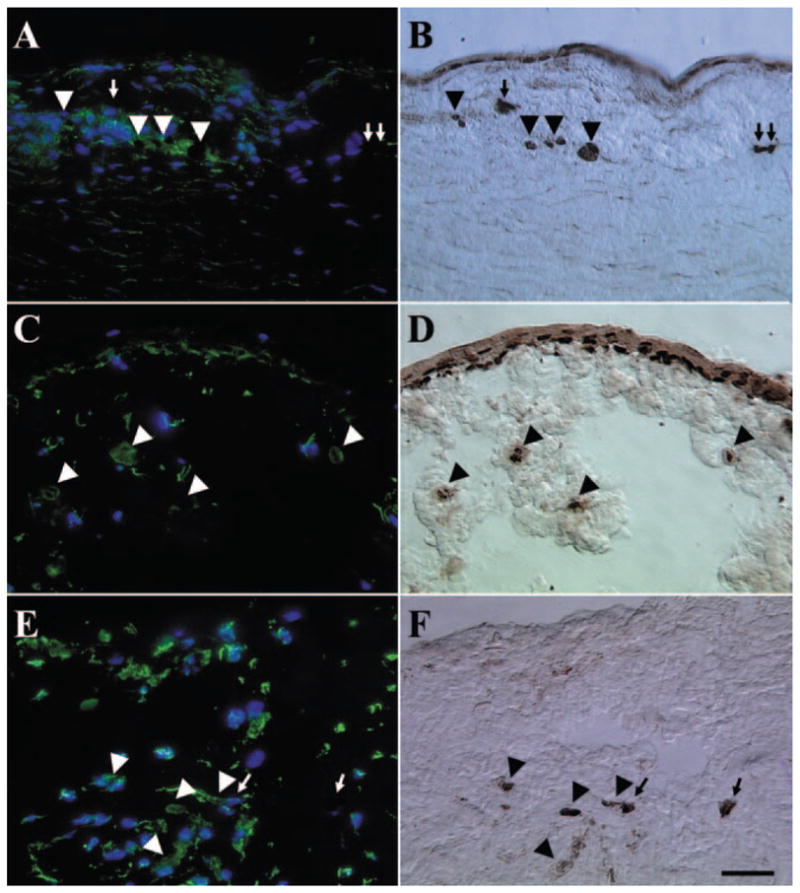
Double staining of vimentin and p63. Vimentin (A, C, E) and p63 (B, D, F) double-stained cells (arrowheads) were found in the limbal stroma of P1 (A, B), P2 (C, D), and P3 (E, F) explants, whereas some p63(+) cells (arrows) did not express vimentin. Vimentin and p63 double-stained cells also presented on the surface of P2 explants (C, D). There was an overall increase of vimentin(+) cells in the limbal stroma from P1 to P3. Bar, 100 μm.
Discussion
Our experiments clearly demonstrated two fates of limbal epithelial progenitor cells in the limbal explant during cultivation on iAM (Fig. 8). The first fate was migration of some progenitor cells from the explant onto AM. These progenitor cells expressed nuclear p63 (data not shown), a finding consistent with an earlier report by Hernandez et al.,31 and generated epithelial colonies on 3T3 fibroblast feeder layers (Fig. 3C). This result is consistent with our previous report that limbal epithelial progenitor cells are preserved during ex vivo expansion on iAM.15–17,36 This baseline information also constitutes the basis for us to investigate whether limbal epithelial progenitor cells migrate from explant to iAM in serial passages.
Figure 8.
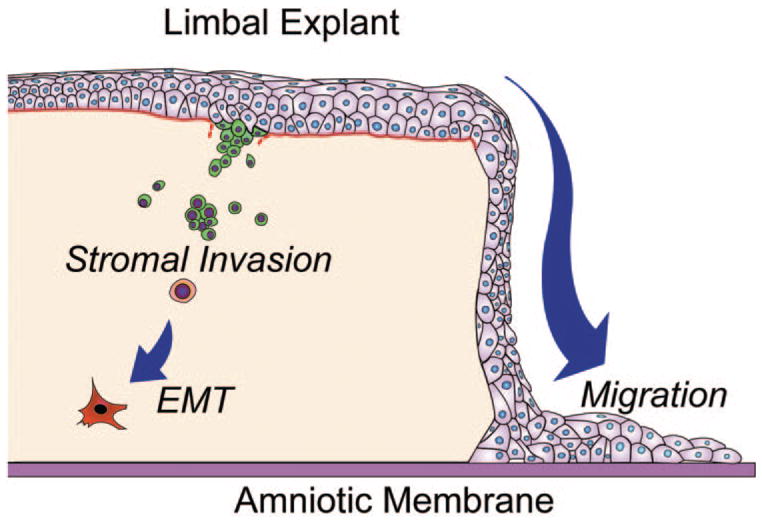
The two fates of limbal epithelial progenitor cells during ex vivo expansion on iAM. The first fate involves the migration of some limbal epithelial progenitor cells from the explant onto the iAM. The second involves invasion of some limbal epithelial progenitor cells into the limbal stroma. The latter process involves degradation of the basement membrane and eventual EMT into fibroblasts.
Our study further noted that clonal growth from the surface epithelium of the explant reached the highest point at P1 (Fig. 4), suggesting that some epithelial progenitor cells still resided in the explant after migration onto the iAM. Colonies generated from the explant surface epithelium were bigger and rounder than those from the outgrowth epithelium at P1, indicating that during the first 2 weeks of culture, cells migrating onto the iAM may contain more transient amplifying cells than stem cells based on keratinocyte experiments.37 Nevertheless, the aforementioned clonogenicity of epithelial cells from the explant surface declined from P1 to P3, eventually resulting in the lack of any colony formation at P3 (Fig. 3A). This finding indicated that epithelial progenitor cells were eventually exhausted during continuous migration from the limbal explant in 6 weeks. Such exhaustion was supported by the progressive decrease in the outgrowth surface area and in the total number of cells at a defined area of 7 mm in diameter (Fig. 2), also implying progressive enlargement of epithelial cells. This interpretation could be explained in part by the decrease in the total number of epithelial cells isolated from the outgrowth (Fig. 2B) and of epithelial cell layers remaining on the explant surface (Fig. 4) from P0 to P3. Of note, there were more colonies generated from epithelial cells obtained from the outgrowth than from the explant surface at P2 and P3 (Fig. 3), suggesting that there might be more epithelial progenitor cells in the outgrowth than those retained on the explant surface after P1. Alternatively, iAM may provide a better niche for limbal progenitor cells to maintain their phenotype than does the limbal stroma in P2 and P3.
The aforementioned gradual exhaustion of limbal epithelial progenitor cells during three successive passages is further caused by the second fate of limbal epithelial progenitor cells that underwent intrastromal invasion. Such a conclusion was first supported by the presence of pancytokeratin(+) epithelial cells in the limbal, but not peripheral corneal, stroma (Fig. 4), a finding resembling that reported in rabbit limbal explant cultures.24 Second, basement membrane components such as type VII and IV collagen and laminin 5 were all partially dissolved both in the limbus and the peripheral cornea after the first 2 weeks of cultivation, whereas epithelial cells that were surrounded by fragmented basement membrane components were seen only in the superficial limbal stroma but not in the peripheral cornea (Fig. 5). These findings indicated that these epithelial cells moved to the limbal stroma, most likely through intrastromal invasion instead of migration from the edge of the explant. This notion was further supported by the fact that all cryosections were harvested from the middle of the explant. Third, in P1, double staining with p63 and pancytokeratin further showed that most of the invading cells were positive for both p63 and pancytokeratin, indicating their status as epithelial progenitor cells (Fig. 6). Moreover, there were some cells that expressed cytokeratins but no longer showed positive nuclear staining for p63 in the limbal stroma at P2 (Figs. 7E, 7F), indicating that these cells were more differentiated and may have lost progenitor status. Because intrastromal invasion only happened in the limbal area, we presume that these p63(−)/pancytokeratin(+) cells were derived from invading progenitor cells.
Of note, double staining of p63 and pancytokeratins also showed that many p63(+) cells no longer expressed pancytokeratin in the limbal stroma, both in P1 and P2 (Fig. 6). Double staining for p63 and vimentin, however, revealed some p63(+)/vimentin(+) cells in the limbal stroma (Fig. 7), indicating the loss of the epithelial cell phenotype. It should be mentioned that although vimentin is widely used as a mesenchymal cell marker38 and has been used to demonstrate EMT,39 it is also expressed in migrating epithelial cells during wound healing.40 In this study, because 3T3 fibroblast feeder layer assays showed that cells harvested from the remaining limbal stroma yielded increasing fibroblastic, but not epithelial, colonies from P1 to P3 (Fig. 3), pancytokeratin(+) cells also decreased, whereas vimentin(+) cells increased in limbal stroma during serial passages, we strongly suspect that such invading cells not only lose the progenitor cell status to generate epithelial cells but also turn into mesenchymal cells. If our interpretation is correct, we speculate that invading epithelial cells may have undergone EMT into fibroblasts in the same way as was observed in the rabbit model.24 In the rabbit model, intrastromal invasion only happened when the explant was exposed to the air–medium interface, but not in submerged culture.24 However, in the present study, intrastromal invasion happened in the submerged culture. Besides the obvious differences in species, cultivation time, and substrate used, we wondered whether the brief treatment with Dispase II used herein but not in the rabbit model facilitated limbal progenitor cell intrastromal invasion. Further studies are needed to test this hypothesis and investigate whether invading epithelial progenitor cells indeed irreversibly transdifferentiate into fibroblasts. Such studies will support the novel hypothesis that EMT of limbal epithelial progenitor cells constitutes the pathogenic basis of developing limbal stem cell deficiency. Furthermore, we believe a new strategy can be developed to enhance the success of the ex vivo expansion protocol for limbal epithelial progenitor cells cultured on iAM by inhibiting the second fate of intrastromal invasion.
Acknowledgments
Supported by a Grants R01 EY06819 and R01 EY015735 (SCGT) from the National Eye Institute, National Institutes of Health, Bethesda, Maryland.
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, May 2005, Fort Lauderdale, Florida.
Disclosure: W. Li, TissueTech, Inc. (F, E); Y. Hayashida, Tissue-Tech, Inc. (F, E); H. He, TissueTech, Inc. (F, E); C.-L. Kuo, None; S.C.G. Tseng, TissueTech, Inc. (F, I, E), Bio-Tissue, Inc. (P)
References
- 1.Lavker RM, Tseng SC, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Grueterich M, Espana EM, Tseng SCG. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. doi: 10.1016/j.survophthal.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001;85:567–575. doi: 10.1136/bjo.85.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sangwan VS, Matalia HP, Vemuganti GK, Rao GN. Amniotic membrane transplantation for reconstruction of corneal epithelial surface in cases of partial limbal stem cell deficiency. Indian J Ophthalmol. 2004;52:281–285. [PubMed] [Google Scholar]
- 5.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. [DOI] [PubMed] [Google Scholar]
- 6.Tsai RJF, Tseng SCG. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13:389–400. doi: 10.1097/00003226-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Holland EJ, Schwartz GS. Changing concepts in the management of severe ocular surface disease over twenty-five years. Cornea. 2000;19:688–698. doi: 10.1097/00003226-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 9.Espana EM, Di Pascuale M, Grueterich M, et al. Keratolimbal allograft in corneal reconstruction. Eye. 2004;18:406–417. doi: 10.1038/sj.eye.6700670. [DOI] [PubMed] [Google Scholar]
- 10.Tsai RJF, Li L-M, Chen J-K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 11.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001;108:1569–1574. doi: 10.1016/s0161-6420(01)00694-7. [DOI] [PubMed] [Google Scholar]
- 13.Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–1290. doi: 10.1016/s0161-6420(02)01089-8. [DOI] [PubMed] [Google Scholar]
- 14.Ti SE, Grueterich M, Espana EM, et al. Correlation of long term phenotypic and clinical outcomes following limbal epithelial transplantation cultivated on amniotic membrane in rabbits. Br J Ophthalmol. 2004;88:422–427. doi: 10.1136/bjo.2003.026054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meller D, Pires RTF, Tseng SCG. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–471. doi: 10.1136/bjo.86.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002;120:783–790. doi: 10.1001/archopht.120.6.783. [DOI] [PubMed] [Google Scholar]
- 17.Grueterich M, Espana E, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci. 2002;43:63–71. [PubMed] [Google Scholar]
- 18.Ti S-E, Anderson DF, Touhami A, et al. Factors affecting outcome following transplantation of ex vivo expanded limbal epithelium on amniotic membrane for total limbal deficiency in rabbits. Invest Ophthalmol Vis Sci. 2002;43:2584–2592. [PubMed] [Google Scholar]
- 19.Espana EM, Ti SE, Grueterich M, et al. Corneal stromal changes following reconstruction by ex vivo expanded limbal epithelial cells in rabbits with total limbal stem cell deficiency. Br J Ophthalmol. 2003;87:1509–1514. doi: 10.1136/bjo.87.12.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grueterich M, Espana EM, Touhami A, et al. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology. 2002;109:1547–1552. doi: 10.1016/s0161-6420(02)01105-3. [DOI] [PubMed] [Google Scholar]
- 21.Schwab IR. Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc. 1999;97:891–986. [PMC free article] [PubMed] [Google Scholar]
- 22.Koizumi N, Cooper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002;43:2114–2121. [PubMed] [Google Scholar]
- 23.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001;119:298–300. [PubMed] [Google Scholar]
- 24.Kawakita T, Espana EM, He H, et al. Intrastromal invasion by limbal epithelial progenitor cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol. 2005;167:381–393. doi: 10.1016/S0002-9440(10)62983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meller D, Tseng SCG. Conjunctival epithelial cell differentiation on amniotic membrane. Invest Ophthalmol Vis Sci. 1999;40:878–886. [PubMed] [Google Scholar]
- 26.Espana EM, Romano AC, Kawakita T, et al. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003;44:4275–4281. doi: 10.1167/iovs.03-0089. [DOI] [PubMed] [Google Scholar]
- 27.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–337. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 28.Tseng SC, Kruse FE, Merritt J, Li DQ. Comparison between serum-free and fibroblast-cocultured single-cell clonal culture systems: evidence showing that epithelial anti-apoptotic activity is present in 3T3 fibroblast-conditioned media. Curr Eye Res. 1996;15:973–984. doi: 10.3109/02713689609017643. [DOI] [PubMed] [Google Scholar]
- 29.Ljubimov AV, Burgeson RE, Butkowski RJ, et al. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- 30.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Expression of Delta Np63 in response to phorbol ester in human limbal epithelial cells expanded on intact human amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:2959–2965. doi: 10.1167/iovs.02-0776. [DOI] [PubMed] [Google Scholar]
- 32.Koster MI, Kim S, Mills AA, et al. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Iorio E, Barbaro V, Ruzza A, et al. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–9528. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasper M. Patterns of cytokeratins and vimentin in guinea pig and mouse eye tissue: evidence for regional variations in intermediate filament expression in limbal epithelium. Acta Histochem. 1992;93:319–332. doi: 10.1016/s0065-1281(11)80231-x. [DOI] [PubMed] [Google Scholar]
- 35.Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Li W, He H, Kuo CL, et al. Basement membrane dissolution and re-assembly by limbal corneal epithelial cells expanded on amniotic membrane. Invest Ophthalmol Vis Sci. 2006;47:2381–2389. doi: 10.1167/iovs.05-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehtonen E, Virtanen I, Saxen L. Reorganization of intermediate filament cytoskeleton in induced metanephric mesenchyme cells is independent of tubule morphogenesis. Dev Biol. 1985;108:481–490. doi: 10.1016/0012-1606(85)90051-x. [DOI] [PubMed] [Google Scholar]
- 39.Willis BC, Liebler JM, Luby-Phelps K, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1322. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SundarRaj N, Rizzo JD, Anderson SC, Gesiotto JP. Expression of vimentin by rabbit corneal epithelial cells during wound repair. Cell Tissue Res. 1992;267:347–356. doi: 10.1007/BF00302973. [DOI] [PubMed] [Google Scholar]