Abstract
Imaging glycans in vivo has recently been enabled using a bioorthogonal chemical reporter strategy by treating cells or organisms with azide- or alkyne-tagged monosaccharides1, 2. The modified monosaccharides, processed by the glycan biosynthetic machinery, are incorporated into cell surface glycoconjugates. The bioorthogonal azide or alkyne tags then allow covalent conjugation with fluorescent probes for visualization, or with affinity probes for enrichment and glycoproteomic analysis. This protocol describes the procedures typically used for noninvasive imaging of fucosylated glycans in zebrafish embryos, including: 1) microinjection of one-cell stage embryos with GDP-5-alkynylfucose (GDP-FucAl), 2) labeling fucosylated glycans in the enveloping layer of zebrafish embryos with azide-conjugated fluorophores via biocompatible Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC), and 3) imaging by confocal microscopy3. The method described here can be readily extended to visualize other classes of glycans, e.g. glycans containing sialic acid4 and N-acetylgalactosamine5, 6, in developing zebrafish and in other living organisms.
Protocol
1. Egg Collection and Dechorionation
Collect and transfer zebrafish eggs to 35mm petri dish, remove as much water as possible and then add 1 mg/ml Pronease E in E3 embryo medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2·2H2O, 0.33 mM MgSO4, pH = 7.4) to digest the chorion.
After 3-5 minutes, merge the dish into a beaker filled with fish water (60 mg "instant Ocean" per liter distilled H2O), and gently transfer the eggs to the beaker and allow the eggs to "fall" into water.
Rinse the eggs with fish water three times. Most eggs will be released from their chorion.
Using a fire-polished glass Pasteur pipette, transfer the dechorionated eggs to agarose-coated petri dishes filled with E3 embryo medium.
2. Microinjection with GDP-FucAl
Prepare the injection dishes following the reported protocol7.
Transfer dechorionated eggs into injection dishes filled with E3 embryo medium.
Prepare the needle, and load with 2 μL injection solution. This solution contains 20 mM GDP-FucAl synthesized chemoenzymatically8 and either Alexa Fluor 594-dextran (5% w/v) as a tracer or phenol red loading dye (0.1% w/v) in 0.2 M KCl. As a negative control, replace GDP-FucAl with GDP-fucose in injection solution.
Break the needle and adjust the injection pressure and duration to yield a 1 nL drop9.
Inject the eggs with 1 nL of either solution.
Transfer the eggs into agarose-coated petri dishes filled with E3 embryo medium.
Incubate eggs at 28 °C and remove the unfertilized eggs within three to four hours after fertilization.
3. BTTES-Cu(I)-catalyzed Click Chemistry Reaction
When the embryos reach desired developmental stages (e.g. late gastrula, tissue segmentation and early larva), coat the base of a 96-well plate with agarose.
Add 92 μL E3 embryo medium to each well, followed by addition of 4 μL Alexa Fluor-488 azide (from a 2.5 mM stock in H2O), 2 μL BTTES-CuSO4 6:1 complex, and shake gently to mix.
Transfer embryos into the well containing the click chemistry reagent using a fire-polished glass Pasteur pipette. Each well should contain less than five embryos.
Add 2.5 μL freshly prepared sodium ascorbate (from a 100 mM stock in H2O) to initiate the click reaction3. Final concentration of each reagent: Alexa Fluor-488 azide: 100 μM; CuSO4: 50 μM; BTTES: 300 μM; sodium ascorbate: 2.5 mM.
After 3 min, add 2 μL bathocuproine sulphonate (50 mM stock in H2O), a biocompatible copper chelator, to quench the reaction then dilute immediately with 100 μL E3 embryo medium.
Transfer the embryos to a glass petri dish and wash the treated embryos 2 times with 15 mL E3 embryo medium.
4. Imaging
Place a drop of ultralow melting point agarose (1.2% (w/v) in E3 embryo medium) on a MatTek glass bottom microwell dish, and place an embryo into the agarose drop.
Position the embryos dorsally or laterally and place the dish on ice for 5 min to solidify the agarose drop. Add E3 embryo medium gently to the dish until it covers the agarose drop.
Fluorescence and bright field images are acquired sequentially using a confocal microscope. All embryo images are acquired using a 5 μm step interval. Composite figures are prepared using ImageJ software.
5. Representative Results
Figure 1 shows the workflow of our two-step labeling strategy. Figure 2 shows the labeling of zebrafish embryos via BTTES-mediated CuAAC at 9.5 hours post fertilization (hpf). BTTES is a tris (triazolylmethyl) amine-based ligand. It accelerates CuAAC dramatically when coordinating with the in situ generated Cu(I), and promotes the cycloaddition reaction rapidly in living systems without apparent toxicity. Immediately following a 3-min click reaction, we are able to detect robust labeling of the GDP-FucAl treated embryos (Figure 2, left panels). Only background fluorescence is detected for control embryos microinjected with GDP-fucose (Figure 2, right panels).
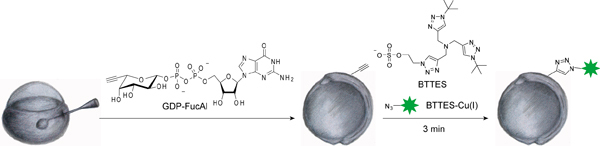 Figure 1.The strategy of labeling fucosylated glycans in the enveloping layer of zebrafish embryos.
Figure 1.The strategy of labeling fucosylated glycans in the enveloping layer of zebrafish embryos.
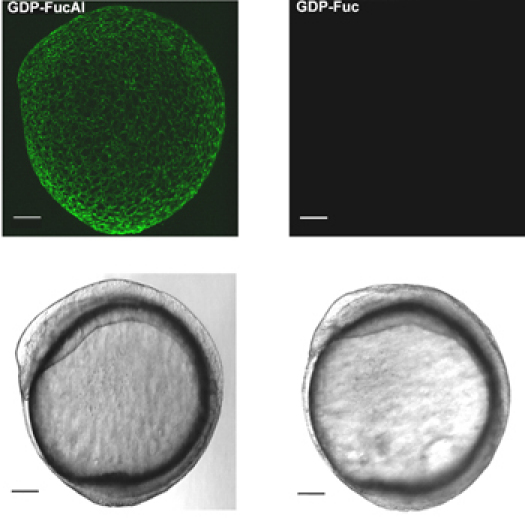 Figure 2.
In vivo imaging of fucosylated glycans during zebrafish embryogenesis via BTTES-Cu(I)-catalyzed click chemistry. One-cell stage zebrafish embryos are microinjected with a single dose of GDP-FucAl and allowed to develop to 9.5 hpf. The embryos are then reacted with Alexa Fluor 488-azide catalyzed by BTTES-Cu(I). Reacted embryos are imaged using confocal microscopy. Maximum intensity z-projection images of Alexa Fluor 488 fluorescence (upper panel); Bright field (lower panel). Scale bar: 100 μm.
Figure 2.
In vivo imaging of fucosylated glycans during zebrafish embryogenesis via BTTES-Cu(I)-catalyzed click chemistry. One-cell stage zebrafish embryos are microinjected with a single dose of GDP-FucAl and allowed to develop to 9.5 hpf. The embryos are then reacted with Alexa Fluor 488-azide catalyzed by BTTES-Cu(I). Reacted embryos are imaged using confocal microscopy. Maximum intensity z-projection images of Alexa Fluor 488 fluorescence (upper panel); Bright field (lower panel). Scale bar: 100 μm.
Troubleshooting
| Problem | Cause | Remedy |
| The embryos look unhealthy | The microinjected solution contains contaminants | The purity of the nucleotide sugars should be greater than 85%. Check your source. |
| There is no labeling after the reaction | Copper concentration is below 30 μM | Be careful not to dilute the reaction solution by adding excess E3 embryo medium when adding the embryos, as the reaction rate drops significantly when the copper concentration is below 30 μM. |
| The embryos die after the reaction | Improper handling of the embryos | Make sure the pipet is fire-polished and the reaction vessel is coated with a very thin layer of 0.5% agarose. |
| The images look spotty | The reagents are not properly mixed before adding the embryos | Follow the recommended order of addition for the reagents, and make sure that the click chemistry reagents have been properly mixed before adding the embryos. |
| The images show the embryos are damaged | Vigorous shaking damages the embryos during the reaction | Gently shake for less than 10 seconds once the embryos are in the solution. |
Discussion
Imaging biomolecules in vivo provides critical insights of their biological activities in their native environments. In this video, we demonstrate how the labeling of fucosylated glycans in the enveloping layer of zebrafish embryos is realized by microinjecting one-cell stage embryos with GDP-FucAl and a second-step fluorophore conjugation via BTTES-mediated biocompatible CuAAC3. Robust labeling can be achieved within 2-3 minutes, and the labeled glycans are detectable as early as 2.5 hpf. Importantly, a single dose of GDP-FucAl can yield a detectable alkyne-dependent fluorescent signal till 96 hpf. The same approach can be extended to multicolor time-lapse imaging experiments to monitor the trafficking of the alkyne-tagged fucosides in developing zebrafish via two sequential CuAAC. However, labeling is only achieved in the enveloping layer of the blastodisc due to the low tissue penetration depth of the click chemistry reagents. By fixing and permeabilizing the embryos metabolically treated with GDP-FucAl, we can achieve labeling of the interior body parts as well. Since transplantation can be easily performed from alkyne labeled donor embryos to unlabeled hosts between blastula stages (4 hpf) and the onset of gastrulation (5.3 hpf) following the zebrafish fate map10, 11, exploiting this manipulation may allow us to follow the allocation of these glycans in specific lineages of the embryo during development.
As many azide- and alkyne-functionalized reagents are commercially available, only metabolic manipulations are required to generate the tagged glycans in vivo for this procedure of click modification. Therefore, the chemical tools described here can be directly applied for studying other glycans, e.g. glycans containing sialic acid4 and N-acetylgalactosamine5, 6, in zebrafish embryos and in other living systems (the azide-tagged metabolic precursors of these monosaccharides are commercially available from Invitrogen).
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was partially supported by the National Institutes of Health (GM093282 to P.W.; 3U54AI057158-06S1 to R.D.S.) and startup funds from Albert Einstein College of Medicine.
References
- Laughlin ST, Bertozzi CR. Imaging the glycome. Proc. Natl. Acad. Sci. U. S. A. 2009;106:12–17. doi: 10.1073/pnas.0811481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin JM, Bertozzi CR. Bioorthogonal click chemistry: Covalent labeling in living systems. Qsar Comb. Sci. 2007;26:1211–1219. [Google Scholar]
- Soriano del Amo D. Biocompatible copper(I) catalysts for in vivo imaging of glycans. J. Am. Chem. Soc. 2010;132:16893–16899. doi: 10.1021/ja106553e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PV. Metabolic labeling of sialic acids in living animals with alkynyl sugars. Angew. Chem. Int. Ed. 2009;48:4030–4033. doi: 10.1002/anie.200806319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin JM, Dehnert KW, Laughlin ST, Amacher SL, Bertozzi CR. Visualizing enveloping layer glycans during zebrafish early embryogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10360–10365. doi: 10.1073/pnas.0912081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp HA, Carmany-Rampey A, Moens C. Generating chimeric zebrafish embryos by transplantation. J. Vis. Exp. 2009 doi: 10.3791/1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Chemoenzymatic synthesis of GDP-L-fucose and the Lewis X glycan derivatives. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16096–16101. doi: 10.1073/pnas.0908248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JN, Sweeney MF, Mably JD. Microinjection of zebrafish embryos to analyze gene function. J. Vis. Exp. 2009 doi: 10.3791/1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. THE ZEBRAFISH BOOK, A guide for the laboratory use of zebrafish (Danio rerio) 5th Edition. Eugene: University of Oregon Press; 2007. [Google Scholar]
- Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]


