Abstract
Repair of acute injury to the cell membrane is an elemental process of normal cellular physiology, and defective membrane repair has been linked to many degenerative human diseases. The recent discovery of MG53 as a key component of the membrane resealing machinery allows for a better molecular understanding of the basic biology of tissue repair, as well as for potential translational applications in regenerative medicine. Here we detail the experimental protocols for exploring the in vivo function of MG53 in repair of muscle injury using treadmill exercise protocols on mouse models, for testing the ex vivo membrane repair capacity by measuring dye entry into isolated muscle fibers, and for monitoring the dynamic process of MG53-mediated vesicle trafficking and cell membrane repair in cultured cells using live cell confocal microscopy.
Keywords: Cell Biology, Issue 52, mouse, cell membrane, muscle injury, tissue repair, treadmill, MG53, confocal microscopy, vesicle trafficking
Protocol
1. Treadmill Running for Revealing the Extent of Muscle Injury in Mouse Models
Establish the angle of the treadmill surface for use during the running protocol. Generally, a flat level or an angle between 7°and 15°degrees downhill or uphill is used. Some treadmills have in integral apparatus to adjust the incline while others require the treadmill to be elevated through other means.
Place a tray or a blue lab pad underneath the treadmill before putting the animals in the treadmill to collect any waste from the animals during the running protocol.
Before running mice should be acclimated to the environment of the treadmill. This involves placing the animals in the treadmill for 15 minutes with the electric grid off and the belt drive motor on but the belt not moving (i.e. with the speed set at 0 m/s).
Turn on the motivational electric grid. The intensity and frequency of the pulses used can usually be controlled on treadmills but varies from one manufacturer to another. Generally, maximum intensity is not required to motivate mice to run while a high frequency (at least once every two seconds) will improve compliance.
Activate the treadmill belt to begin the running protocol. An initial speed of approximately 5 meters/min should be used for a warm up period for the mice. The speed of the treadmill can be accelerated slowly, usually by adding 1-2 meters/min for each minute following the start of the treadmill.
Compliance of animals in most protocols can be improved by conducting a series of short runs (5 minutes) at warm up speed for 3 to 10 days before the experimental running begins.
An acute exhaustion exercise will usually involve increasing the speed of the treadmill with time until a maximum speed (generally < 30 m/m) is achieved and maintained until the mice show signs of exhaustion. Criteria for judging exhaustion vary but generally include a mouse spending more than half of the time on the electric grid or 5 consecutive seconds on the electric grid without returning to the treadmill surface. After an individual mouse is exhausted it can be removed from the treadmill and the total time of running can be recorded.
Changing the angle of the treadmill can be used in acute running trials to increase the exercise load (with an uphill angle) or to induce damaging eccentric contractions (with a downhill angle) that tear the sarcolemmal membranes of muscle fibers1. Evans blue dye can be injected into animals before such procedures for use as a histological dye to identify damaged muscle fibers2,3.
Endurance training running protocols provide an exercise load to the animal over a prolonged period of time (up to months) in order to produce remodeling of the muscle or the cardiovascular system. Warm up procedure is the same as above (step 1.5) however the maximum speed is generally lower and the running times can be quite long (>1 hour).
After completion of running the mice can be returned to their cages. Treadmills usually become quite dirty while the mice are running, particularly over long protocols. It is necessary to clean the treadmill, usually with a 70% ethanol spray, following each use.
2. Ex Vivo Assay of Membrane Repair Capacity of Isolated Muscle Fibers Following UV-laser Damage
Dissect out the superficial layer of the flexor digitorum brevis (FDB) muscle from the mouse foot. First, cut the skin of the sole open at the median line while taking care not to damage the muscle underneath, then make horizontal cuts and remove the skin. The flat white tendon of FDB muscle (proximal tendon) can be seen attached to the calcaneus bone. Bluntly separate the tendon by forceps and sever the tendon close to site of attachment, grab the free tendon by forceps and pull it up gently to separate the FDB from surrounding tissues while cutting any connective tissues that remain attached. Upon seeing the distal tendons branch to the individual digits the FDB may be removed by cutting where these tendons connect to the deeper layer of muscle.
Put the FDB muscle bundle in 1 ml of collagenase solution pre-warmed to 37°C in a 1.5 ml Eppendorf tube, tape the tube in a horizontal orientation in a 37°C orbital shaker and shake at 200 rpm for 65 min. Times of incubation may have to be adjusted to ensure sufficient digestion, which is indicated by a frayed appearance and pale color of the muscle.
Carefully transfer the digested FDB bundle into a 1.5 μl centrifuge tube containing ~600 μl of 2.5 Ca2+ Tyrode via a 1 ml pipette tip with a cut end that has a diameter sufficient to allow the digested bundle to easily pass through without disruption. Gently flip the tube to shake the loose fibers from the bundle.
Cut a 30 μL pipette tip so that the diameter is only slightly smaller than the muscle bundle (between 15 - 20 μm in diameter).Use the pipettor to gently draw the bundle into the pipette and then push it back into the solution. Repeat this process until the majority of the fibers are disassociated from the bundle.
Mix disassociated fibers well by gently turning the tube, take desired amount and drop onto a glass-bottom delta T dish. The volume to load will vary based on the efficiency of isolation and the number of useful fibers that are needed on a dish for a particular protocol. The remaining fibers can be stored in the tube at 4°C for approximately 6 hours for additional studies.
Allow the dish to sit undisturbed for 5 minutes. This allows the fibers to adhere to the glass bottom of the dish.
Place the glass bottomed dish on a confocal microscopy equipped with a UV laser. Observe the fibers under phase microscopy at >100X magnification to check for the presence of a normal striation pattern and a straight, rod like shape.
Add FM1-43 or FM4-64 styrylpyridinium dyes4to solution to a final concentration of 2.5 μM5.
To induce damage to the fiber position it in the imaging window so the fiber is oriented at a 45° angle from the top left of the field of view to the bottom right (Figure 1). Irradiate a 5x5 pixel area (approximately 0.9μmx0.9μm)of the plasma membrane using a UV laser (80 mW or greater, 351/364 nm) set to maximum power for 5 seconds. The irradiated region should split the plasma membrane; that is, half of the 5x5 box should be inside the fiber, while the remainder should be outside the fiber (Figure 1).
Capture images of dye entry into the fiber at intervals of 5 seconds each for up to 5 minutes.
Repeat step 2.8 for no more than 3 fibers per dish, as the fluorescent dye will eventually be endocytosed into the fiber and complicate any data analysis. After 3 fibers a new dish should be prepared from step 2.5.
Analyze data by calculating the change in fluorescence intensity (ΔF/F0) between each captured frame to measure the amount of dye entry. This requires measuring the mean fluorescence of an area approximately 200 μm2 using analysis software such as the publically available ImageJ (http://rsbweb.nih.gov/ij/). For comparison between fibers, the following calculation should be made for each frame: ΔF/F0, where F0 is the mean fluorescence of the region of interest in the first captured frame (t = 0; prior to injury), and ΔF is the change in fluorescence of each subsequent frame (F-F0).
3. Live Cell Confocal Imaging to Monitor the Dynamic Process of Cell Membrane Repair
Micropipettes were made from PYREX Capillaries. The capillary was placed on a micropipette puller and pulled by pre-set program (Heat=695; Pull=50; Vel.=55; Time=250).
Micropipette was attached to a 3 axis (xyz) micromanipulator.
Cells are transfected with plasmid DNA using normal methods on a glass bottom delta T dish. Media should be switched to 2.5mM Ca2+ Tyrode buffer before experiments begin.
Glass dishes containing transfected cells were placed on a laser scanning confocal microscope with 40X 1.3NA oil immersion objective.
The acute live-cell damage was performed by inserting the micropipette into the cell membrane and then quickly retract the micropipette out of the cell3,6. Consecutive live cell images were obtained at an interval of 1.54 s/frame.
For the saponin induced membrane disruption assay, 0.005% saponin (Sigma) in 2.5mM Ca2+ Tyrode buffer was perfused by a gravity-flow custom-assembled perfusion system7. The perfusion rate is approximately 1 ml/min and the perfusion tip should be placed directly above the cell being imaged.
4. Representative Results:
A representative movie of the mouse treadmill running can be made during filming. The movie will illustrate the reduced running capacity of the mg53-/- mice due to damage to the skeletal muscle.
The extent of damage caused by the UV-laser damage protocol detailed above depends on the membrane repair capacity of the fiber analyzed. This capacity is affected by the genotype of the mouse from which the fiber was isolated, extracellular conditions, and any alterations to protein expression (transfection or infection). A normal wild type fiber will allow slight to moderate dye entry (Figure 2a). Muscle fiber derived from the mg53-/- mice with compromised membrane repair capacity will display more significant entry of dye into the fiber (Figure 2b).
Typical results for microelectrode injury show translocation of MG53-containing vesicles to the injury site. Figure 3a and 3b show GFP-MG53 transfected C2C12 myotubes damaged by insertion of the micropipette into the cell membrane. In the cell before micropipette damage, GFP-MG53 is located on both plasma membrane and cytoplasm3. Following micropipette penetration, GFP-MG53 containing vesicles moved towards damage sites (as indicated by arrowhead). Figure 3c shows a GFP-MG53 transfected C2C12 myoblasts treated with 0.005% of saponin, a detergent that can permeabilize the plasma membrane. Upon saponin treatment, GFP-MG53 translocated from cytosol to the cell membrane.
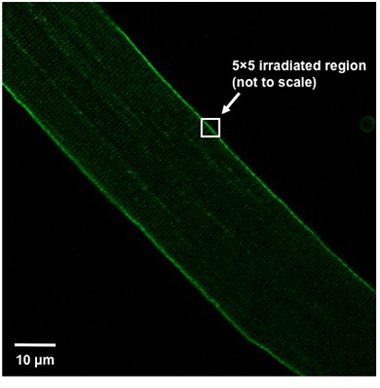 Weisleder, et al. Figure 1. Fiber alignment and injury region definition. Proper orientation of the isolated muscle fiber within the field of view and definition of the injury region shown.
Weisleder, et al. Figure 1. Fiber alignment and injury region definition. Proper orientation of the isolated muscle fiber within the field of view and definition of the injury region shown.
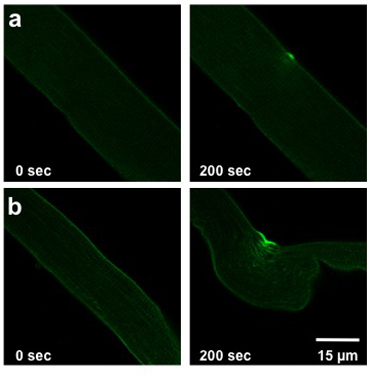 Weisleder, et al. Figure 2. UV laser damage of FDB fiber. Images show fibers prior to injury (0 sec, left) and at 200 sec post injury (right). Fibers from a wild type mouse (a) show slight injury. Fibers from mice with compromised membrane repair (b) allow more dye to enter.
Weisleder, et al. Figure 2. UV laser damage of FDB fiber. Images show fibers prior to injury (0 sec, left) and at 200 sec post injury (right). Fibers from a wild type mouse (a) show slight injury. Fibers from mice with compromised membrane repair (b) allow more dye to enter.
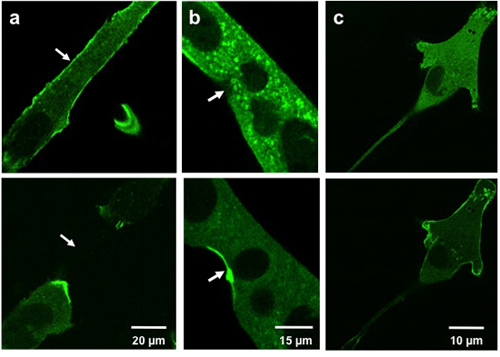 Weisleder, et al. Figure 3. Microelectrode and saponin damage to cultured cells. Images show C2C12 myotubes (a, b) and a C2C12 myoblast (c) prior to injury (above) and post injury (bottom). Cells injured with a microelectrode (a, b) show MG53 translocation to injury sites (arrows). Cells treated with saponin (c) show MG53 translocation to the cell membrane.
Weisleder, et al. Figure 3. Microelectrode and saponin damage to cultured cells. Images show C2C12 myotubes (a, b) and a C2C12 myoblast (c) prior to injury (above) and post injury (bottom). Cells injured with a microelectrode (a, b) show MG53 translocation to injury sites (arrows). Cells treated with saponin (c) show MG53 translocation to the cell membrane.
Discussion
Treadmill running is a useful methodology at providing an exercise load to the treated animals. As a methodology for measuring muscle function or endurance capacity it is a notoriously noisy approach that is difficult to execute in a consistently reproducible fashion8. In general, times to exhaustion can be used as an endpoint to resolve major differences between experimental groups, such as those between some knockout mouse lines and wild type mice9. Experimental manipulations that produce more subtle difference, such as some pharmaceutical trials, may not resolve differences above the noise associated with this technique. In order to maximize the reproducibility and sensitivity of these techniques it is important to maintain the conditions for session to session very closely. The time of day the animals are exercised, as well as the warm up period conditions used, are important factors and should stay consistent from trial to trial.
Strain effects can have significant impact on the design of treadmill protocols as certain strains of mice can run for much more time on treadmill than other strains and respond differently to endurance exercise10. As a general guideline, many mice will be able to run 200-300 total meters in an exhaustion study with constant increase in the speed of the treadmill (5 m/m initial speed with 1 m/m added for each minute after start). For an endurance exercise the mice may be run between 9 to 12 m/m for 30 minutes 2 or 3 times a week. However, individual studies will usually require some optimization of the protocol to match individual experimental needs.
The UV-laser damage procedure has been shown to be an effective method for measuring the membrane repair capacity of skeletal muscle fibers3,6,11. One vital component to the success of these experiments is to use only muscle fibers that display a straight, rod shaped appearance with a regular pattern of striations and a smooth sarcolemma that does not appear wrinkled. If other muscle fibers are selected membrane damage may already be present in these fibers and interpretation of results from these experiments may be complicated. It is also important that the orientation of the fiber and definition of the irradiated region be as defined in Figure 1. This provides an injury of reproducible size, thus allowing comparison between fibers. If the defined region of injury lies completely within the fiber, an internal injury will be created rather than disrupting the sarcolemma. Additionally, the value calculated for ΔF/F0 is very sensitive to the region of interest selected for measurement of mean fluorescence. An area of approximately 200μm2, adjacent to and including the injury site, is appropriate. Unlike the region defined for injury, this area should lie completely within the fiber. If you include space outside the fiber, the resulting blank area will increase ΔF/F0 in fibers with little dye entry while lowering ΔF/F0 in fibers with a more severe phenotype. This decreases the sensitivity of the assay, thus making comparison between fibers more difficult.
For the micropipette damage assay, the angle between the micropipette and glass bottom dish should be approximately 45 degree in order to effectively damage the cell membrane. The cells were chosen for micropipette damage should be tightly attached to the bottom of the dish and rounded cells should not be used since most likely they will detach after micropipette poking. Saponin permeabilzation experiments are less technically demanding and relatively quicker to perform compared to micropipette penetration assays. However, there are several limitations to saponin damage, including that only one cell can be used per dish since saponin will diffuse and damage other cells in that dish.
Disclosures
No conflicts of interest declared.
References
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Miyake K, Vogel SS. The endomembrane requirement for cell surface repair. Proc Natl Acad Sci U S A. 2003;100:4592–4597. doi: 10.1073/pnas.0736739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res. 2010;107:76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- Knab AM. Repeatability of exercise behaviors in mice. Physiol Behav. 2009;98:433–440. doi: 10.1016/j.physbeh.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics. 2005;23:72–78. doi: 10.1152/physiolgenomics.00020.2005. [DOI] [PubMed] [Google Scholar]
- Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:1006–1013. doi: 10.1152/ajpregu.00476.2004. [DOI] [PubMed] [Google Scholar]
- Bansal D. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]


