Abstract
Conserved nutrient sensing mechanisms exist between mammal and fruit fly where peptides resembling mammalian insulin and glucagon, respectively function to maintain glucose homeostasis during developmental larval stages 1,2. Studies on largely post-mitotic adult flies have revealed perturbation of glucose homeostasis as the result of genetic ablation of insulin-like peptide (ILP) producing cells (IPCs) 3. Thus, adult fruit flies hold great promise as a suitable genetic model system for metabolic disorders including type II diabetes. To further develop the fruit fly system, comparable physiological assays used to measure glucose tolerance and insulin sensitivity in mammals must be established. To this end, we have recently described a novel procedure for measuring oral glucose tolerance response in the adult fly and demonstrated the importance of adult IPCs in maintaining glucose homeostasis 4,5. Here, we have modified a previously described procedure for insulin injection 6 and combined it with a novel hemolymph extraction method to measure peripheral insulin sensitivity in the adult fly. Uniquely, our protocol allows direct physiological measurements of the adult fly's ability to dispose of a peripheral glucose load upon insulin injection, a methodology that makes it feasible to characterize insulin signaling mutants and potential interventions affecting glucose tolerance and insulin sensitivity in the adult fly.
Protocol
1. Insulin Solution Preparation
Prepare fresh bovine insulin solution by dissolving insulin in PBS to achieve the concentration of 0.01 mg/ml. Both insulin/PBS and control PBS solutions must be kept on ice throughout the injection procedure. These solutions should be prepared with 0.5% (v/v) FD&C Blue no. 1 food coloring.
2. Needle Preparation and Injection Set-up
Prepare capillary glass needles using a micropipette puller.The following puller settings produce needles of sufficient quality for injection: Heat, 345; Pull, 210; Velocity, 100; Time, 200 (100ms). Freshly pulled needles must be blunted by pressing the tip through a Kimwipe tissue. This blunting process removes the elongated high resistance tip and produces a stouter tip with a larger pore diameter.
Place blunted needles tip-up in a microcentrifuge tube containing the insulin/PBS solution or PBS alone. Capillary action results in back-filling of each needle. Observe the tip of the needle under a stereomicroscope to ensure that no debris or air bubbles appear at the tip. Discard any needles that do not fill cleanly.
Insert filled needles into the manual microinjector needle holder and position the needle holder with a micromanipulator so that the needle tip is visible through a stereomicroscope. Apply positive pressure on the fluid column in the needle by turning the manual microinjector micrometer knob attached to the plunger on the microinjector syringe.
Verify that sufficient pressure for fluid displacement has been applied by touching a Kimwipe to the tip of the needle and confirming fluid flow.
Once the needle has been prepared for injection, affix a calibration chart to the needle shaft with clear tape, and bring the needle tip into focus through a stereomicroscope under 20X magnification.
3. Fly Preparation
Ten-day-old female flies are used for injection experiments. Collect flies within 24 h of eclosion. Anesthetize flies with humidified CO2 on a gas pad, sort out males and place female flies into vials containing standard or treatment diet. Maintain female flies on experimental diets for 10 days.
On the tenth day following eclosion and separation on to experimental diets, transfer flies from their food-containing vials to vials containing a 5 ml plug of 2% agar and starve them for 12-16 h.
Transfer starved flies to vials containing 10% glucose soaked filters for 1 hour prior to insulin injection.
Briefly anesthetize the flies with humidified CO2 after glucose feeding and then cold immobilize them on ice.
4. Injection Procedure
Gently grasp a cold-immobilized fly with a pair of fine forceps and hold it close to the needle tip so that the tip is adjacent to the anterior region of the left side of the fly's thorax. Bring both the needle tip and the fly's thorax into focus under the stereomicroscope.
Gently move the fly toward the needle tip so that the needle tip touches the center of the protuberance of the left prescutum region of the fly's thorax (Fig. 1). Once the fly is properly oriented and aligned with the needle tip, continue to advance the fly onto the needle so that the needle impales the center of the prescutum.
Apply positive pressure as needed by advancing the syringe plunger with the microinjector's micromanipulator knob until 0.1 μl of fluid has been injected into the fly. The flow of fluid into the fly's hemocoel will impart a blue color to the left side of the anterior thorax. Occasionally one must retract and advance several times to dislodge any needle tip blockage and allow for fluid flow.
Allow injected flies to recover in 2% agar vials for designated time points before hemolymph extraction.
5. Hemolymph Collection
Briefly anesthetize flies with humidified CO2 at desired post-injection time points and position each fly dorsal-side-down on double-sided tape affixed to the surface of a CO2 pad. Arrange flies in even rows with head capsules aligned. One can use a trimmed wooden applicator stick to press their wings into the tape adhesive and ensure immobilization.
Once flies are fixed to the tape, grasp each fly's proboscis with fine forceps and gently pull it towards the ventral and then posterior region of the fly so that the front of the head capsule is visible through the stereo microscope.
With your other hand and while holding the proboscis in position, puncture the center of the head capsule just above the ptilinal suture using a sharp tungsten needle (Fig. 2). One must be careful not to insert the needle too far as it is easy to punch the tip of the needle through the other side of the head capsule and subsequently lose control of hemolymph flow. Puncture all flies in an injection group before collecting hemolymph.
Once the head capsule has been punctured, use a trimmed wooden applicator stick to gently apply pressure to the fly's abdomen. It may be helpful to roll the fly over so that it rests on its ventral side as you apply pressure. This procedure will produce a droplet of hemolymph from the head capsule puncture site.
Touch one end of a 1μl microcapillary tube to the hemolymph droplet emerging from the head capsule puncture site. The hemolymph will enter the tube via capillary action. Determine and record the amount of hemolymph collected by checking the fluid column within the microcapillary tube against a graduated volume chart.
6. Hemolymph Glucose Determination
Eject hemolymph samples into microplate wells containing 100 μl of Infinity Glucose Reagent. Keep plate on ice while loading hemolymph samples.
Establish a standard curve by loading separately 1 μl each of glucose stock solutions at 50mM, 25mM, 12.5mM, 6.25mM and 3.125mM.
Incubate samples at 37°C for 10 minutes.
Detect absorbance at 340 nm.
Account for hemolymph volume collected and determine sample glucose concentration based on the standard curve with known glucose concentrations.
7. Representative Results
A typical insulin tolerance response is detected in insulin-injected flies where a drop in circulating glucose levels is detected 15 minutes post-injection. In contrast, such response is not seen in PBS injected flies (Fig. 3). This response in peripheral glucose disposal continues in insulin-injected flies up to 30 minutes post-injection. We routinely extract 0.2-0.5 μl of hemolymph per 4-5 flies in each injection group. Three injection groups are included in each experiment.
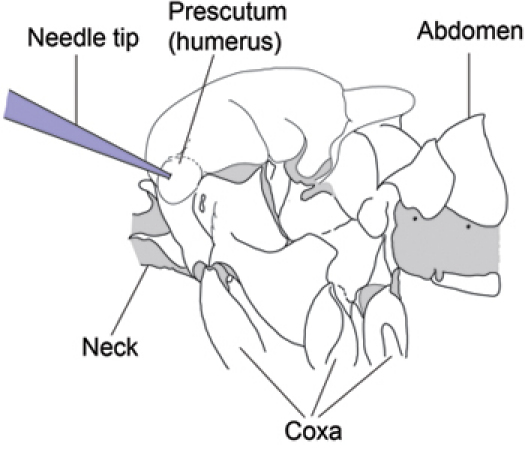 Figure 1. Left side of Drosophila thorax showing needle insertion site (Modified from Demerec, 1950) 7. Insert the needle through the center of the prescutum on the anterior, dorsal region of the left side of the thorax.
Figure 1. Left side of Drosophila thorax showing needle insertion site (Modified from Demerec, 1950) 7. Insert the needle through the center of the prescutum on the anterior, dorsal region of the left side of the thorax.
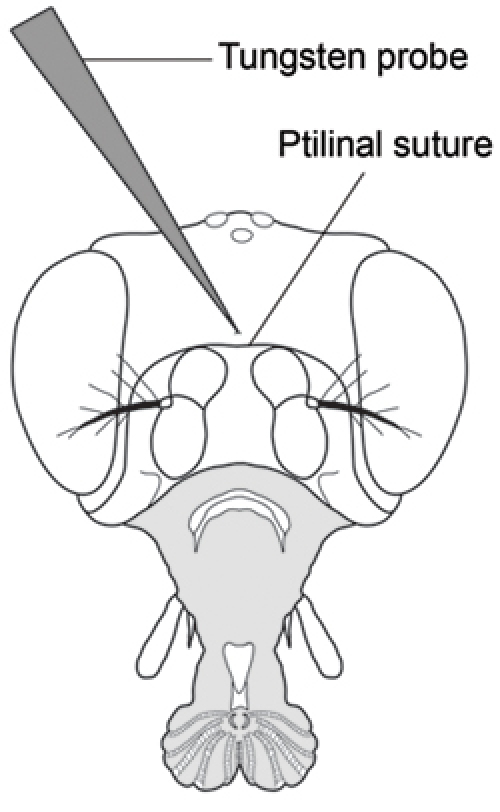 Figure 2. Frontal view of Drosophila head showing puncture location for hemolymph extraction (Modified from Demerec, 1950) 7. Puncture the head capsule with a finely sharpened tungsten probe in the center of the head capsule just above the ptilinal suture.
Figure 2. Frontal view of Drosophila head showing puncture location for hemolymph extraction (Modified from Demerec, 1950) 7. Puncture the head capsule with a finely sharpened tungsten probe in the center of the head capsule just above the ptilinal suture.
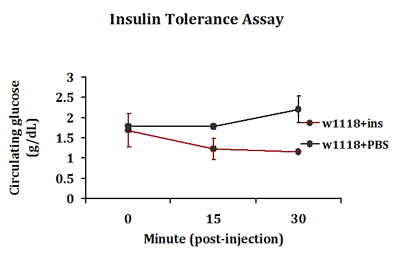 Figure 3. A typical insulin tolerance response detected in control adult flies. Control w1118 flies were injected with bovine insulin (1 ng in PBS) or PBS only. Flies in replicate groups were then allowed to recover for 0, 15 or 30 minutes and circulating glucose levels were measured.
Figure 3. A typical insulin tolerance response detected in control adult flies. Control w1118 flies were injected with bovine insulin (1 ng in PBS) or PBS only. Flies in replicate groups were then allowed to recover for 0, 15 or 30 minutes and circulating glucose levels were measured.
Discussion
The technique described in this report is potentially useful in any study that investigates physiological processes resulting in detectable alterations in Drosophila hemolymph composition. By combining injection and hemolymph collection in this manner, it is possible to ascertain the immediate physiologically relevant effects of a particular experimental treatment or manipulation. The primary advantage of this "bloodletting" technique in hemolymph collection over previous techniques involving decapitation 2 is that this technique minimizes contamination of hemolymph samples with gut contents.If care is taken not to collect any pericerebral fat body tissue, which may sometimes appear in the exuded hemolymph droplet, then samples should accurately reflect the state of in vivo circulatory fluids.
A potentially confounding factor inherent in any injection experiment performed at this scale is the initial dilution of total circulatory fluids following injection. This can easily be controlled for, however, by injecting the same volumes of PBS in experimental flies or insulin into age-matched controls and normalizing results. To ensure reproducible circulating glucose readings, the quality of hemolymph droplets is of most importance. Only clear droplets of hemolymph devoid of pericerebral fat body or other tissue debris should be collected for glucose determination. It is also noteworthy that only up to 8 samples should be collected before determining relative glucose concentrations. We noticed a "drift" in OD340 readings when the hemolymph samples were left on ice for an extended period of time.
Our protocol leaves much room for modification based on available equipment. Pipet puller settings may need to be modified based on the model and condition of the puller. We have found that settings adequate for producing intracellular recording electrode needles are ideal for injection needles. Additionally, while we employed a three-axis micromanipulator for maintaining needle position under the stereoscope, this may also be achieved with a sturdy ringstand and clamp system as the needle remains in a fixed position throughout the injection procedure. The high resistance of the needles used necessitated the development of an alternative method for determining the volume of injected fluid as a 1:1 syringe plunger movement to volume displacement was not achievable. We developed a graduated scale that could be printed and attached to the side of the needle shaft. Depending on the system used, it may be necessary to displace some fluid from the needle beyond the needle holder of the microinjector so that the fluid meniscus may be aligned with graduations the calibration card affixed to the side of the needle shaft.
Finally, two limitations of this technique are noted in our protocol. First, two persons are usually required to coordinate both injection and hemolymph extraction steps in order to maximize the number of samples processed. Second, variations in insulin tolerance response are sometimes observed within the same genotype. We believe this is due to variable responses individual flies may have following ice immobilization and recovery on agar. Therefore, a sufficient number of samples should be examined before reliable conclusions can be drawn.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by grants from the NIA to Y-W.C.F (AG21068, AG31086).
References
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1118. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–316. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Broughton SJ. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3105. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton A. Partial ablation of adult Drosophila insulin-producing neurons modulates glucose homeostasis and extends life span without insulin resistance. Cell Cycle. 2010;9:3063–3063. doi: 10.4161/cc.9.15.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton AT, Fridell YW. Adult Drosophila melanogaster as a model for the study of glucose homeostasis. Aging (Albany NY) 2010;2:523–523. doi: 10.18632/aging.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgacem YH, Martin JR. Disruption of insulin pathways alters trehalose level and abolishes sexual dimorphism in locomotor activity in Drosophila. J Neurobiol. 2006;66:19–19. doi: 10.1002/neu.20193. [DOI] [PubMed] [Google Scholar]
- Demerec M. Biology of Drosophila. New York: John Wiley & Sons; 1950. [Google Scholar]


