Abstract
BACKGROUNDS: Previously, we demonstrated that neutralizing capacity but not the concentration of GM-CSF autoantibody was correlated with the disease severity in patients with autoimmune pulmonary alveolar proteinosis (PAP)1-3. As abrogation of GM-CSF bioactivity in the lung is the likely cause for autoimmune PAP4,5, it is promising to measure the neutralizing capacity of GM-CSF autoantibodies for evaluating the disease severity in each patient with PAP.
Until now, neutralizing capacity of GM-CSF autoantibodies has been assessed by evaluating the growth inhibition of human bone marrow cells or TF-1 cells stimulated with GM-CSF6-8. In the bioassay system, however, it is often problematic to obtain reliable data as well as to compare the data from different laboratories, due to the technical difficulties in maintaining the cells in a constant condition.
OBJECTIVE: To mimic GM-CSF binding to GM-CSF receptor on the cell surface using cell-free receptor-binding-assay.
METHODS: Transgenic silkworm technology was applied for obtaining a large amount for recombinant soluble GM-CSF receptor alpha (sGMRα) with high purity9-13. The recombinant sGMRα was contained in the hydrophilic sericin layers of silk threads without being fused to the silk proteins, and thus, we can easily extract from the cocoons in good purity with neutral aqueous solutions14,15. Fortunately, the oligosaccharide structures, which are critical for binding with GM-CSF, are more similar to the structures of human sGMRα than those produced by other insects or yeasts.
RESULTS: The cell-free assay system using sGMRα yielded the data with high plasticity and reliability. GM-CSF binding to sGMRα was dose-dependently inhibited by polyclonal GM-CSF autoantibody in a similar manner to the bioassay using TF-1 cells, indicating that our new cell-free assay system using sGMRα is more useful for the measurement of neutralizing activity of GM-CSF autoantibodies than the bioassay system using TF-1 cell or human bone marrow cells.
CONCLUSIONS: We established a cell-free assay quantifying the neutralizing capacity of GM-CSF autoantibody.
Protocol
1. Production and purification of sGMRα
Amplify cDNA of sGMRα from human placenta cDNA library by polymerase chain reaction (PCR).
Add 50 base 5'-UTR sequence of baculovirus polyhedrin end and 27 base coding RGS His-tag to the 3' end to the PCR amplicon by another PCR.
Insert the amplified PCR product into a plasmid pMSG1.1MG.
Inject the construct psGMR/M1.1MG with the helper vector pHA3PIG into eggs of silkworm pnd-w1 strain.
Rear the hatched G0 larvae to moths at 25°C. Screen G1 embryos obtained by mating among siblings or with pnd-w1 for MGFP expression in the eyes to obtain transgenic silkworms bearing the sGMRα gene.
Extract recombinant sGMRα from the cocoons of transgenic silkworms with phosphate-buffer containing 500mM NaCl and stir at 4°C for 24h.
Centrifuge sample at 20000 g for 15min to remove a precipitates.
Apply supernatant to a Nickel-affinity column.
Wash the column with 10mM imidazole, 500mM NaCl and 20mM Sodium phosphate, pH6.3, and elute with a linear gradient of imidazole from 10mM to 250mM. Pool the fractions containing the purified sGMRα and dialyze against phosphate-buffered saline (pH7.4) (PBS).
2. Biotinylation of recombinant GM-CSF
Remove sorbitol preparation by dialysis against PBS.
Add 1ml of 20mM cold sodium meta-periodate solution at 4°C to the solution on ice.
Incubate the sample for 30min on ice in the dark.
Add glycerol to the solution at a final concentration of 15mM.
Dialyze the solution against 100mM sodium acetate buffer (pH5.5)
Add biotin hydrazide to the solution at a final concentration of 5mM and continuously agitate the solution for 2h at room temperature.
Remove the non-reacted material by dialysis against PBS, pH7.4.
3. A cell-free GM-CSF receptor-ligand-binding assay
Coat a microtiter plate with 50μl of monoclonal anti-polyHistidine antibody overnight at 4°C.
Wash five times with 500μl of PBST.
Add 100μl of a blocking solution and incubate for 3h at 4°C.
Wash five times with 500μl of PBST.
Add 50μl of sGMRα in the blocking solution and incubate overnight at 4°C.
Wash five times with 500μl of PBST.
Add 25μl of the samples containing GM-CSF autoantibody and then 25μl of biotinylated GM-CSF. Incubate for 1h at 4°C to form sGMRα-GM-CSF complex.
Wash five times with 500μl of PBST.
Add 50μl of 0.4mM streptavidin alkaline phosphatase for 1hour at 4°C to detect the biotinylated GM-CSF bound sGMRα.
Wash five times with 500μl of PBST.
Add 50μl of a chemiluminescent substrate to the solution and incubated for 1h at room temperature.
Using a chemiluminescence plate reader, detect the chemiluminescence activity.
4. Representative Results:
The first step in the GM-CSF receptor signal transduction pathway is the binding of GM-CSF to GM-CSF receptor alpha on the cell surface. Because GM-CSF autoantibody specifically binds GM-CSF and block its binding to the receptor in vitro 16,17, we hypothesized that the autoantibodies inhibit this first reaction by directly binding to GM-CSF. As described previously (Ref. 9-15), we applied transgenic silkworm technology to obtain a large amount of recombinant sGMRα with high purity.
When the silkworm-derived recombinant sGMRα was loaded on SDS-PAGE under non-reducing conditions, the sGMRα showed both monomeric (45 kDa) and dimeric (90 kDa) forms, whereas only the monomeric form was detected under reducing conditions (Figure 1B), indicating that the recombinant sGMRα was a mixture of monomers and disulfide-linked dimmers18.
Using the cell-free system (Fig. 2A), we evaluated the inhibition of GM-CSF binding to sGMRα by GM-CSF autoantibodies (Fig. 2B and Fig. 2C). These methods were described in reference 19 by Urano et al. The growth inhibition was closely correlated with the binding inhibition (r=0.988, p=0.002) at various concentrations of the GM-CSF autoantibody (Fig. 3A)19. Similarly, the binding inhibition was significantly correlated with the growth inhibition. The binding inhibition increased in a dose-dependent manner by GM-CSF autoantibodies (Fig. 3B)19. These two parameters for the serum IgG fractions from different patients were correlated each other (r=0.589 p=0.006, Fig. 3C). Neither binding inhibition nor growth inhibition correlated with Kd values, and thus, both parameters were unaffected by binding affinity19. Consequently reproducibility of data between binding and growth inhibition obtained through three independent experiments on three different samples was evaluated. The coefficient of variation indicated that the cell-free system was more excellent than that of bioassay (table 1).
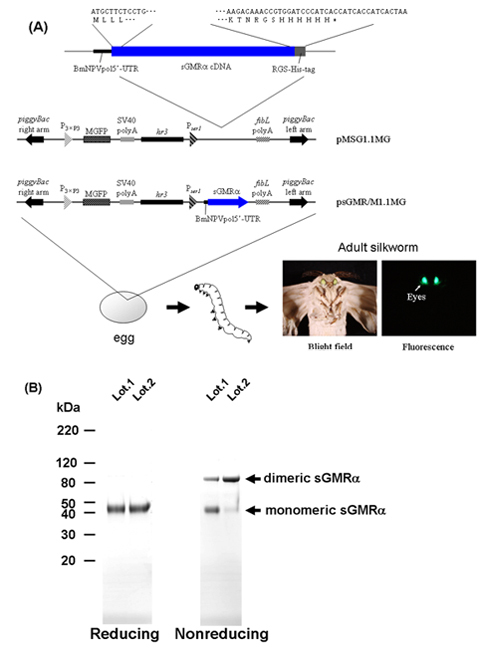 Figure 1. Flow chart of the procedure for the production of sGMRα using transgenic silkworms. A) Structures of the transformation vectors9-11. B) SDS-PAGE and Coomassie Brilliant Blue-staining of the purified sGMRα under reducing and non-reducing conditions. sGMRα, soluble GM-CSF receptor alpha; MGFP, monster green fluorescent protein; BmNPVpol5'-UTR, 5'-untranslated region sequence of Bombyx mori nuclear polyhedrosis virus polyhedrin; SV40 polyA, SV40 polyA signal sequence; P3xP3, 3xP3 promoter; Pser1, ser1 promoter; fibL polyA, fibroin L-chain polyA signal sequence; hr3, Bombyx mori nuclear polyhedrosis virus hr3 enhancer.
Figure 1. Flow chart of the procedure for the production of sGMRα using transgenic silkworms. A) Structures of the transformation vectors9-11. B) SDS-PAGE and Coomassie Brilliant Blue-staining of the purified sGMRα under reducing and non-reducing conditions. sGMRα, soluble GM-CSF receptor alpha; MGFP, monster green fluorescent protein; BmNPVpol5'-UTR, 5'-untranslated region sequence of Bombyx mori nuclear polyhedrosis virus polyhedrin; SV40 polyA, SV40 polyA signal sequence; P3xP3, 3xP3 promoter; Pser1, ser1 promoter; fibL polyA, fibroin L-chain polyA signal sequence; hr3, Bombyx mori nuclear polyhedrosis virus hr3 enhancer.
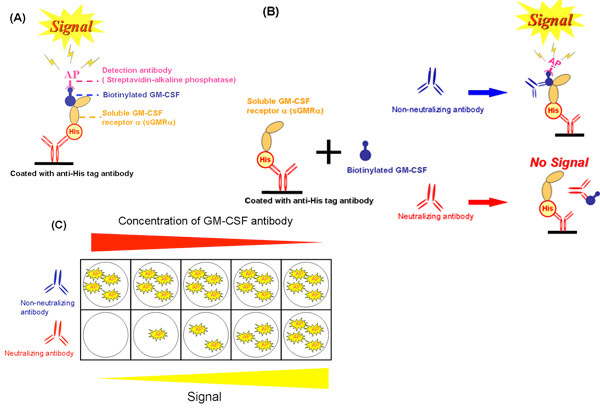 Figure 2. Scheme of the cell-free assay system. A) A competitive binding assay using sGMRα produced by silkworm. B) Effect of neutralizing and non-neutralizing antibodies on the binding inhibition by cell-free system. C) The difference of binding inhibition between various concentrations of neutralizing antibodies and non-neutralizing antibodies. GM-CSF, granulocyte-macrophage colony-stimulating factor; sGMRα, soluble GM-CSF receptor alpha; His, RGS-His-tag; AP, alkaline phosphatase.
Figure 2. Scheme of the cell-free assay system. A) A competitive binding assay using sGMRα produced by silkworm. B) Effect of neutralizing and non-neutralizing antibodies on the binding inhibition by cell-free system. C) The difference of binding inhibition between various concentrations of neutralizing antibodies and non-neutralizing antibodies. GM-CSF, granulocyte-macrophage colony-stimulating factor; sGMRα, soluble GM-CSF receptor alpha; His, RGS-His-tag; AP, alkaline phosphatase.
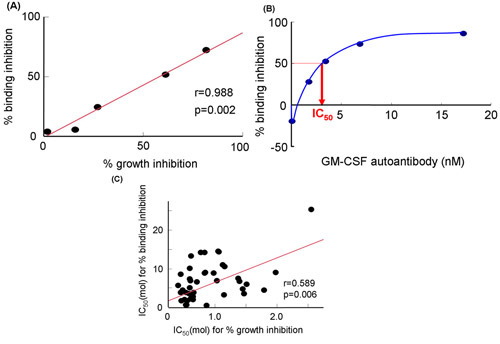 Figure 3. GM-CSF binding inhibition to sGMRα by effect of GM-CSF polyclonal antibodies or the serum IgG fractions from patients with autoimmune PAP. A) Relationship between binding inhibition and growth inhibition by GM-CSF autoantibody. B) Binding inhibition at various concentrations of GM-CSF autoantibody. C) Relationship between IC50 for percent binding inhibition and percent growth inhibition by the serum IgG fractions19. IC50, 50% inhibitory concentration; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Figure 3. GM-CSF binding inhibition to sGMRα by effect of GM-CSF polyclonal antibodies or the serum IgG fractions from patients with autoimmune PAP. A) Relationship between binding inhibition and growth inhibition by GM-CSF autoantibody. B) Binding inhibition at various concentrations of GM-CSF autoantibody. C) Relationship between IC50 for percent binding inhibition and percent growth inhibition by the serum IgG fractions19. IC50, 50% inhibitory concentration; GM-CSF, granulocyte-macrophage colony-stimulating factor.
| Sample | A | B | C |
| Inter-assay | |||
| # of determinations | 3 | 3 | 3 |
| Mean value ( % binding inhibition ) | 61.6 | 63.8 | 69.7 |
| Mean value ( % growth inhibition ) | 21.0 | 73.4 | 82.8 |
| Coefficient of variation (%) (% binding inhibition) | 6.0 | 5.6 | 8.3 |
| Coefficient of variation (%) (% growth inhibition) | 64.4 | 18.3 | 10.4 |
Both assays were performed at 5 ng/ml of GM-CSF and a concentration equal to GM-CSF autoantibody.
Table 1. Comparison of coefficient of variations between percent binding and growth inhibitions obtained through three independent experiments.
Discussion
The cell-free assay estimated the neutralizing capacity of GM-CSF autoantibodies with excellent reproducibility and rapidity. The binding inhibition by GM-CSF autoantibodies or the patient's serum IgG fractions was evaluated by this assay. The data showed a correlation between the binding inhibition of the cell-free assay and the growth inhibition of a bioassay using TF-1 cells, respectively. The bioassay has been widely utilized, but harbored difficulties in comparing data between different facilities and different time points, which we can avoid by using this new system.
GM-CSF binds to sGMRα with low affinity and form a binary complex20. Recent studies demonstrated that the human GM-CSF binds to GM-CSF receptor alpha and GM-CSF receptor beta forming a dodecameric complex with high-affinity on the cell surface21. Thus, an assay system based on ternary complexes consisting of monomeric GM-CSF receptor alpha and dimeric GM-CSF receptor beta would be a future candidate system to improve the cell-free assay.
Disclosures
We have nothing to disclose.
Acknowledgments
We are very grateful to K. Nakagaki, Dr. H. Ishii, Dr. K. Suzuki, A. Yamagata, K. Oofusa for their valuable contributions.
References
- Arai T. Serum neutralizing capacity of GM-CSF reflects disease severity in a patient with pulmonary alveolar proteinosis successfully treated with inhaled GM-CSF. Respir Med. 2004;98:1227–1230. doi: 10.1016/j.rmed.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Tazawa R. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2005;171:1142–1149. doi: 10.1164/rccm.200406-716OC. [DOI] [PubMed] [Google Scholar]
- Inoue Y. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008;177:752–762. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–1098. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- Sakagami T. Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med. 2009;361:2679–2681. doi: 10.1056/NEJMc0904077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines M. Identification and molecular cloning of a soluble human granulocyte-macrophage colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1991;88:8203–8207. doi: 10.1073/pnas.88.18.8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W, VonFeldt J, Rosenbaum H, Ugen K, Weiner D. Molecular cloning of a soluble form of the granulocyte-macrophage colony-stimulating factor receptor alpha chain from a myelomonocytic cell line. Expression, biologic activity, and preliminary analysis of transcript distribution. Arthritis Rheum. 1994;37:1468–1478. doi: 10.1002/art.1780371010. [DOI] [PubMed] [Google Scholar]
- Prevost J. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and inflammatory stimuli up-regulate secretion of the soluble GM-CSF receptor in human monocytes: evidence for ectodomain shedding of the cell surface GM-CSF receptor alpha subunit. J Immunol. 2002;169:5679–5688. doi: 10.4049/jimmunol.169.10.5679. [DOI] [PubMed] [Google Scholar]
- Iizuka M. Production of a recombinant mouse monoclonal antibody in transgenic silkworm cocoons. FEBS J. 2009;276:5806–5820. doi: 10.1111/j.1742-4658.2009.07262.x. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Tomita M, Shimizu K, Kikuchi Y, Yoshizato K. Translational enhancement of recombinant protein synthesis in transgenic silkworms by a 5'-untranslated region of polyhedrin gene of Bombyx mori Nucleopolyhedrovirus. J Biosci Bioeng. 2008;105:595–603. doi: 10.1263/jbb.105.595. [DOI] [PubMed] [Google Scholar]
- Zou W, Ueda M, Yamanaka H, Tanaka A. Construction of a combinatorial protein library displayed on yeast cell surface using DNA random priming method. J Biosci Bioeng. 2001;92:393–396. doi: 10.1263/jbb.92.393. [DOI] [PubMed] [Google Scholar]
- Tamura T. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol. 2000;18:81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- Tomita M. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat Biotechnol. 2003;21:52–56. doi: 10.1038/nbt771. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tomita M, Shimizu K, Yoshizato K. Generation of a transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon: production of recombinant human serum albumin. J Biotechnol. 2007;128:531–544. doi: 10.1016/j.jbiotec.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Tomita M. A germline transgenic silkworm that secretes recombinant proteins in the sericin layer of cocoon. Transgenic Res. 2007;16:449–465. doi: 10.1007/s11248-007-9087-x. [DOI] [PubMed] [Google Scholar]
- Kitamura T. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N. Lungs of patients with idiopathic pulmonary alveolar proteinosis express a factor which neutralizes granulocyte-macrophage colony stimulating factor. FEBS Lett. 1999;442:246–250. doi: 10.1016/s0014-5793(98)01668-8. [DOI] [PubMed] [Google Scholar]
- Brown C, Pihl C, Murray E. Oligomerization of the soluble granulocyte-macrophage colony-stimulating factor receptor: identification of the functional ligand-binding species. Cytokine. 1997;9:219–225. doi: 10.1006/cyto.1996.0157. [DOI] [PubMed] [Google Scholar]
- Urano S. A cell-free assay to estimate the neutralizing capacity of granulocyte-macrophage colony-stimulating factor autoantibodies. J Immunol Methods. 2010;360:141–148. doi: 10.1016/j.jim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Hayashida K. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990;87:9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen G. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134:496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]


