Abstract
Biomolecules, including peptides,1-9 proteins,10,11 and antibodies and their engineered fragments,12-14 are gaining importance as both potential therapeutics and molecular imaging agents. Notably, when labeled with positron-emitting radioisotopes (e.g., Cu-64, Ga-68, or F-18), they can be used as probes for targeted imaging of many physiological and pathological processes.15-18 Therefore, significant effort has devoted to the synthesis and exploration of 18F-labeled biomolecules. Although there are elegant examples of the direct 18F-labeling of peptides,19-22 the harsh reaction conditions (i.e., organic solvent, extreme pH, high temperature) associated with direct radiofluorination are usually incompatible with fragile protein samples. To date, therefore, the incorporation of radiolabeled prosthetic groups into biomolecules remains the method of choice.23,24
N-Succinimidyl-4-[18F]fluorobenzoate ([18F]SFB),25-37 a Bolton-Hunter type reagent that reacts with the primary amino groups of biomolecules, is a very versatile prosthetic group for the 18F-labeling of a wide spectrum of biological entities, in terms of its evident in vivo stability and high radiolabeling yield. After labeling with [18F]SFB, the resulting [18F]fluorobenzoylated biomolecules could be explored as potential PET tracers for in vivo imaging studies.1 Most [18F]SFB radiosyntheses described in the current literatures require two or even three reactors and multiple purifications by using either solid phase extraction (SPE) or high-performance liquid chromatography (HPLC). Such lengthy processes hamper its routine production and widespread applications in the radiolabeling of biomolecules. Although several module-assisted [18F]SFB syntheses have been reported,29-32, 41-42 they are mainly based on complicated and lengthy procedures using costly commercially-available radiochemistry boxes (Table 1). Therefore, further simplification of the radiosynthesis of [18F]SFB using a low-cost setup would be very beneficial for its adaption to an automated process.
Herein, we report a concise preparation of [18F]SFB, based on a simplified one-pot microwave-assisted synthesis (Figure 1). Our approach does not require purification between steps or any aqueous reagents. In addition, microwave irradiation, which has been used in the syntheses of several PET tracers,38-41 can gives higher RCYs and better selectivity than the corresponding thermal reactions or they provide similar yields in shorter reaction times.38 Most importantly, when labeling biomolecules, the time saved could be diverted to subsequent bioconjugation or PET imaging step.28,43 The novelty of our improved [18F]SFB synthesis is two-fold: (1) the anhydrous deprotection strategy requires no purification of intermediate(s) between each step and (2) the microwave-assisted radiochemical transformations enable the rapid, reliable production of [18F]SFB.
Protocol
1. Initial preparations
A V-vial (5-mL) RV1 (with stirring bar) is used as the main reaction vessel for performing microwave synthesis. It is connected to a PEEK adaptor with seven inlet/outlet ports connects and placed inside the microwave cavity (see Figure 2). RV2 is connected to SPE cartridge (I) to collect the crude [18F]SFB. RV3 is connected to SPE cartridge (II) for collecting the final [18F]SFB solution. It can be placed in a warm water bath (40°C) to concentrate the corresponding solution before reconstituting in PBS buffer, especially for the downstream radiolabeling of biomolecules.
Setup for collecting crude [18F]SFB: Fill up MeCN/H2O [6 mL; 1:4 (v:v)] solution, 5% aqueous AcOH (8 mL), MeCN (2 mL) for reservoir A,B and C, respectively. Then activate a SPE cartridge (I) (polystyrene, Merck LiCholut EN) with ethanol (10 mL), followed by 5% aqueous AcOH (10 mL) washing.
Setup for collecting purified [18F]SFB: Prepare reservoir D and E filled up with 10 ml of H2O and 3 ml of diethyl ether respectively. The second SPE cartridge (II) (polystyrene, Merck LiCholut EN) is activated by the same procedure mentioned above.
Start the HPLC (elution buffer: MeCN/ H2O, 1:1 (v/v) containing 0.2% TFA; flow rate: 3 ml/min) for pre-conditioning the HPLC column [a reverse-phase semi-prep column (Luna, 5 μm C18(2) 100 Å, 250 x 10 mm), Phenomenex, Torrance, CA, USA].
2. Preparation of dried [i.e. non-carrier-added, (n.c.a)] [18F]fluoride
[18F]fluoride solution in [18O]H2O (100 μL) was added to a mixture of Kryptofix 222 (20 mg), 1M aqueous K2CO3 (26 μL) and MeCN (0.8 mL) in an Eppendorf tube. The entire solution is then mixed well before transferring to the RV1 via inlet line 1. The [18F]fluoride solution can be also passed through an anionic exchange cartridge (e.g. QMA-light Sep-Pak from Waters) to trap the fluoride-18 and then eluted out with a mixture of K2CO3 and Kryptofix in MeCN.
Execute the Drying sequence (20W, 3 min) under the Microwave control program to remove residual water in RV1 [under vacuum]. After the cooling down as system temperature is below 50°C, additional MeCN (1.0 mL) was introduced into the reactor and the sequence is repeated once.
3. Synthesis of ethyl 4-[18F]fluorobenzoate
To a DMSO solution (0.4 mL) containing ethyl 4-(N,N,N-trimethylammonium)benzoate triflate (1.5 mg) was added into RV1 via inlet line 2.
Execute the Labeling sequence (50W, 1 min) under the Microwave control program with stirring, vessel cooling and all valves closed to afford ethyl 4-[18F]fluorobenzoate ([18F]2).
4. Synthesis of potassium 4-[18F]fluorobenzoate
To a DMSO solution (0.5 mL) containing KOtBu (13 mg) was added into RV1 via inlet line 3.
Execute Deprotect program (40 W, 1 min) under the Microwave control program with stirring, vessel cooling and all valves closed to afford the 4-[18F]fluorobenzoate salt ([18F]3).
5. Synthesis of crude [18F]SFB
To an acetonitrile solution (2.5 mL) containing TSTU (30 mg) was added to RV1 via inlet line 6. TSTU is moisture- and light-sensitive. It should be aliquoted into small vial and stored at 4°C in a closed container covered by aluminum foil.
Execute Coupling sequence (30W, 2 min) under the Microwave control program with stirring, vessel cooling and all valves closed to afford the crude [18F]SFB.
6. The Preparation of SPE-purified [18F]SFB
5% aqueous AcOH (1.0 mL) was added to RV1 via inlet line 7 to neutralize the reaction mixture. The solution was then transferred into vial B containing 8 ml of 5% aqueous AcOH (Figure 2).
Pass the diluted reaction mixture through SPE cartridge (I) to trap crude [18F]SFB using nitrogen (10 psi).
WASH SPE cartridge (I) with a mixture of MeCN and H2O [10 mL, 1:4 (v:v)] from reservoir A.
[18F]SFB was eluted out into RV2 using MeCN (2 mL) from reservoir C.
7. Purification of Crude [18F]SFB with Radio-HPLC
Dilute either crude [18F]SFB or SPE-purified [18F]SFB with H2O (2 mL) in RV2 and transfer the mixture into the HPLC loop (5 mL). The the solution was injected into radio-HPLC [MeCN/ H2O, 1:1 (v/v) containing 0.2% TFA; flow rate: 3 mL/min].
Collect the fraction containing purified [18F]SFB (retention time: 8-10 minutes) into the vial D (pre-filled with 10 ml of H2O) (Figure 2). Critical step: If performed correctly, the fraction volume collected here should be 4-5 ml.
Pass the diluted reaction mixture through SPE cartridge (II) to trap purified [18F]SFB using nitrogen (10 psi). Dry the cartridge with a stream of nitrogen for 2-3 minutes.
[18F]SFB was eluted out into RV3 using diethylether (3 mL) from reservoir E..
Evaporate the solvent in RV3 to dryness by a gentle stream of nitrogen gas (10 psi) using a water bath (40° C). The final dried [18F]SFB can be reconstituted into PBS buffer for the downstream application.
8. Representative Results:
We developed a simplified, rapid, one-pot method for synthesizing [18F]SFB using a deprotection strategy under anhydrous conditions and microwave heating during each radiochemical/chemical transformation. Figure 1 presents the details of our radiosynthesis. The identity of final product was confirmed by comparison of HPLC retention time with a non-radioactive SFB reference. The purified [18F]SFB was also analyzed through radio-TLC and -HPLC to determine its radiochemical and chemical purity. The RCY of [18F]SFB was 35 ± 5% within 60 min after HPLC purification (n > 30), with high radiochemical purity (>99%) and good chemical purity (see the UV trace in the HPLC profile, Figure 3). The specific activity was ca. 67-330 GBq/μmol (1.8-9.0 Ci/μmol), depending on the starting radioactivity.
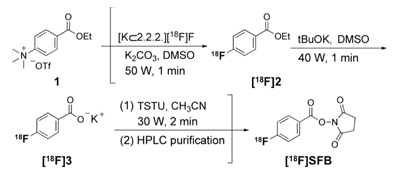 Figure 1. Microwave-assisted one-pot radiosynthesis of [18F]SFB. First, the radiofluorination of ethyl 4-(N,N,N-trimethylammonium)benzoate triflate (1) was performed under microwave heating (50 W, 1 min) in the presence of [K⊃2.2.2][18F]F- complex in dimethylsulfoxide (DMSO) to afford ethyl 4-[18F]fluorobenzoate ([18F]2). Without purification, a DMSO solution of potassium tert-butoxide (tBuOK) was added and the reaction vessel was microwave irradiated (40 W, 1 min) to complete the anhydrous deprotection. The final conversion of [18F]3 into [18F]SFB was achieved using O-(N-succinimidyl)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TSTU) activation. TSTU in acetonitrile was added to the reaction mixture containing the 4-[18F]fluorobenzoate ([18F]3) salt; this last synthetic step yielded crude [18F]SFB after heating (30 W, 2 min).
Figure 1. Microwave-assisted one-pot radiosynthesis of [18F]SFB. First, the radiofluorination of ethyl 4-(N,N,N-trimethylammonium)benzoate triflate (1) was performed under microwave heating (50 W, 1 min) in the presence of [K⊃2.2.2][18F]F- complex in dimethylsulfoxide (DMSO) to afford ethyl 4-[18F]fluorobenzoate ([18F]2). Without purification, a DMSO solution of potassium tert-butoxide (tBuOK) was added and the reaction vessel was microwave irradiated (40 W, 1 min) to complete the anhydrous deprotection. The final conversion of [18F]3 into [18F]SFB was achieved using O-(N-succinimidyl)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TSTU) activation. TSTU in acetonitrile was added to the reaction mixture containing the 4-[18F]fluorobenzoate ([18F]3) salt; this last synthetic step yielded crude [18F]SFB after heating (30 W, 2 min).
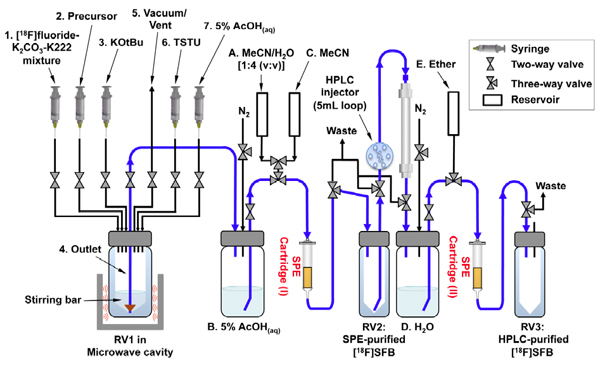 Figure 2. The schematic diagram of setup for microwave-assisted one-pot [18F]SFB synthesis.
Figure 2. The schematic diagram of setup for microwave-assisted one-pot [18F]SFB synthesis.
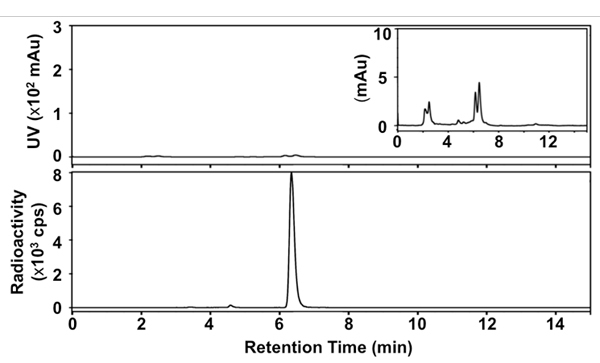 Figure 3. Radio-HPL chromatograms of final [18F]SFB. Top: UV signal at 254 nm; bottom: radioactive signal; inset: UV signal at 254 nm (x 33.3).
Figure 3. Radio-HPL chromatograms of final [18F]SFB. Top: UV signal at 254 nm; bottom: radioactive signal; inset: UV signal at 254 nm (x 33.3).
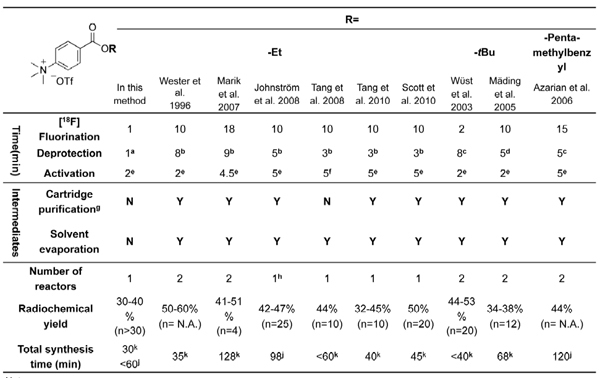 Table 1. Summary of [18F]SFB radiosyntheses reported in the literature using alkyl 4-(trimethylammonium)benzoate triflate as precursors.
Table 1. Summary of [18F]SFB radiosyntheses reported in the literature using alkyl 4-(trimethylammonium)benzoate triflate as precursors.
Discussion
This simplified three-step, one-pot radiosynthesis of the 18F-acylation reagent [18F]SFB is developed based on non-aqueous chemistry. This process has excellent reproducibility and could be used reliably for the production of [18F]SFB in automated radiochemistry modules, owing to two key modifications described as followings: 1. We employ a deprotection/saponification step in anhydrous KOtBu/DMSO system to replace the common aqueous basic or acidic solution. Our non-aqueous deprotection strategy enables sequential addition of reagents without intermediate SPE purification or solvent evaporation/exchange. This modification eliminates the need for a significant number of extra components and control units associated with a second reactor and SPE modules that have been required in other synthetic routes. Therefore, it reduces system complexity while increasing its reliability. Our process can be performed in a simple manual setup or in an automated radiochemistry module possessing a basic single-reactor configuration. Furthermore, elimination of SPE purification(s) between steps shortens the total synthesis time. 2. The use of microwave heating also enables the rapid, reliable production of [18F]SFB (see Table 1 for comparison with other methods). We apply microwaves in every step during this one-pot [18F]SFB synthesis: F-18 drying, radiofluorination, deprotection, and activation. As indicated in Figure 1, each transformation was complete within 1-2 min under microwave heating; in comparison, it generally requires 5 - 10 minutes when using conventional conduction heating (e.g., oil bath or heater block).29-32, 41-42
With a significant reduction in its overall complexity, we believe that this improved and concise synthesis will make the routine production of [18F]SFB more practical and attractive for adoption in automated modules, enabling the use of 18F-labeled biomolecules43 as research tools to accelerate biomedical discovery and enhance clinical studies.
Disclosures
This method has been submitted for US patent application.
Acknowledgments
This study was supported by the US Department of Energy (DE-FG02-09ER09-08 and DE-PS02-09ER09-18), the Jonsson Comprehensive Cancer Center at UCLA, and the Industry-University Cooperative Research Program (UC Discovery Grant, bio07-10665). We thank Dr. Nagichettiar Satyamurthy and staffs at the UCLA Biomedical Cyclotron Facility for providing the F-18 radioisotope and many insightful discussions. We thank Drs. Michael Collins, Greg Leblanc, Joseph Lambert, and Keller Barnhardt from CEM for their technical advice and support. We thank Dirk Williams, Darin Williams, Drs. Joseph Hong Dun Lin, and Michael van Dam for designing and machining parts to modify the CEM microwave reactor and for SPE purification modules.
References
- Okarvi SM. Recent progress in fluorine-18 labeled peptide radiopharmaceuticals. Eur. J. Nucl. Med. 2001;28:929–938. doi: 10.1007/s002590100508. [DOI] [PubMed] [Google Scholar]
- Chen XY, Park R, Hou YP, Khankaldyyan V, Gonzales-Gomez I, Tohme M, Bading JR, Laug WE, Conti PS. MicroPET imaging of brain tumor angiogenesis with 18F-labeled PEGylated RGD peptide. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:1081–1089. doi: 10.1007/s00259-003-1452-2. [DOI] [PubMed] [Google Scholar]
- Wu Z, Li Z-B, Chen K, Cai W, He L, Chin FT, Li F, Chen X. MicroPET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide. J. Nucl. Med. 2007;49:1536–1544. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Yin D, Zhang L, Li G, Wang M, Li S, Zheng M, Cai H, Wang Y. Radiolabeling and in vitro and in vivo characterization of [18F]FB-[R8,15,21, L17]-VIP as a PET imaging agent for tumor over-expressed VIP receptors. Chem. Biol. Drug Des. 2006;68:319–325. doi: 10.1111/j.1747-0285.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- Cheng D, Yin D, Zhang L, Wang M, Li G, Wang Y. Preparation of the novel fluorine-18-labeled VIP analog for PET imaging studies using two different synthesis methods. J. Fluorine Chem. 2007;128:196–201. [Google Scholar]
- Fredriksson A, Johnstroem P, Stone-Elander S, Jonasson P, Nygren P-A, Ekberg K, Johansson B-L, Wahren J. Labeling of human C-peptide by conjugation with N-succinimidyl-4-[18F]fluorobenzoate. J. Label. Compd. Radiopharm. 2001;44:509–519. [Google Scholar]
- Bergmann R, Scheunemann M, Heichert C, Mäding P, Wittrisch H, Kretzschmar M, Rodig H, Tourwe D, Iterbeke K, Chavatte K. Biodistribution and catabolism of 18F-labeled neurotensin(8-13) analogs. Nucl. Med. Biol. 2002;29:61–72. doi: 10.1016/s0969-8051(01)00284-0. [DOI] [PubMed] [Google Scholar]
- Guenther KJ, Yoganathan S, Garofalo R, Kawabata T, Strack T, Labiris R, Dolovich M, Chirakal R, Valliant JF. Synthesis and in vitro evaluation of 18F- and 19F-labeled insulin: a new radiotracer for PET-based molecular imaging studies. J. Med. Chem. 2006;49:1466–1474. doi: 10.1021/jm0509344. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cai W, Cao F, Schreibmann E, Wu Y, Wu JC, Xing L, Chen X. 18F-labeled bombesin analogs for targeting GRP receptor-expressing prostate cancer. J. Nucl. Med. 2006;47:492–501. [PubMed] [Google Scholar]
- Murakami Y, Takamatsu H, Taki J, Tatsumi M, Noda A, Ichise R, Tait JF, Nishimura S. 18F-labelled annexin V: a PET tracer for apoptosis imaging. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:469–474. doi: 10.1007/s00259-003-1378-8. [DOI] [PubMed] [Google Scholar]
- Yagle KJ, Eary JF, Tait JF, Grierson JR, Link JM, Lewellen B, Gibson DF, Krohn KA. Evaluation of 18F-annexin v as a PET imaging agent in an animal model of apoptosis. J. Nucl. Med. 2005;46:658–666. [PubMed] [Google Scholar]
- Vaidyanathan G, Zalutsky MR. An improved synthesis of N-succinimidyl 4-[18F]fluorobenzoate and its application to the labeling of a monoclonal antibody fragment. Bioconjugate Chem. 1994;5:352–356. doi: 10.1021/bc00028a012. [DOI] [PubMed] [Google Scholar]
- Garg PK, Garg S, Zalutsky MR. Fluorine-18 labeling of monoclonal antibodies and fragments with preservation of immunoreactivity. Bioconjugate Chem. 1991;2:44–49. doi: 10.1021/bc00007a008. [DOI] [PubMed] [Google Scholar]
- Cai W, Olafsen T, Zhang X, Cao Q, Gambhir SS, Williams LE, Wu AM, Chen X. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic antigen diabody. J. Nucl. Med. 2007;48:304–310. [PubMed] [Google Scholar]
- Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 2008;49:113–128. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- Jong Mde, Breeman WA, Kwekkeboom DJ, Valkema R, Krenning EP. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc. Chem. Res. 2009;42:873–880. doi: 10.1021/ar800188e. [DOI] [PubMed] [Google Scholar]
- Fani M, André JP, Maecke HR. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging. 2008;3:53–63. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- Shokeen M, Anderson CJ. Molecular imaging of cancer with copper-64 radiopharmaceuticals and positron emission tomography (PET. Acc. Chem. Res. 42:832–841. doi: 10.1021/ar800255q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Sharkey RM, Karacay HC, D'Souza A, Rossi EA, Laverman P, Chang C-H, Boerman OC, Goldenberg DM. A novel method of 18F radiolabeling for PET. J. Nucl. Med. 2009;50:991–998. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- Becaud J, Mu L, Karramkam M, Schubiger PA, Ametamey SM, Graham K, Stellfeld T, Lehmann L, Borkowski S, Berndorff D, Dinkelborg L, Srinivasan A, Smits R, Koksch B. Direct one-step 18F-labeling of peptides via nucleophilic aromatic substitution. Bioconjugate Chem. 2009;20:2254–2261. doi: 10.1021/bc900240z. [DOI] [PubMed] [Google Scholar]
- Mu L, Höhne A, Schubiger PA, Ametamey SM, Graham K, Cyr JE, Dinkelborg L, Stellfeld T, Srinivasan A, Voigtmann U, Klar U. Silicon-based building blocks for one-step 18F-radiolabeling of peptides for PET imaging. Angew. Chem. Int. Ed. 2008;47:4922–4925. doi: 10.1002/anie.200705854. [DOI] [PubMed] [Google Scholar]
- Schirrmacher R, Bradtmöller G, Schirrmacher E, Thews O, Tillmanns J, Siessmeier T, Buchholz HG, Bartenstein P, Wängler B, Niemeyer CM, Jurkschat K. 18F-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew. Chem. Int. Ed. 2006;45:6047–6050. doi: 10.1002/anie.200600795. [DOI] [PubMed] [Google Scholar]
- Olberg DE, Hjelstuen OK, Solbakken M, Arukwe J, Karlsen H, Cuthbertson A. A novel prosthetic group for site-selective labeling of peptides for positron emission tomography. Bioconjugate Chem. 19:1301–1308. doi: 10.1021/bc800007h. [DOI] [PubMed] [Google Scholar]
- Wuest F, Köhler L, Berndt M, Pietzsch J. Systematic comparison of two novel, thiol-reactive prosthetic groups for 18F labeling of peptides and proteins with the acylation agent succinimidyl-4-[18F]fluorobenzoate ([18F]SFB. Amino Acids. 2009;36:283–295. doi: 10.1007/s00726-008-0065-2. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan G, Zalutsky MR. Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an agent for labeling proteins and peptides with 18F. Nat. Protocols. 1:1655–1661. doi: 10.1038/nprot.2006.264. [DOI] [PubMed] [Google Scholar]
- Guhlke S, Coenen HH, Stöcklin G. Fluoroacylation agents based on small N.C.A. [18F]fluorocarboxylic acids. Appl. Radiat. Isot. 1994;45:715–727. [Google Scholar]
- Wester HJ, Hamacher K, Stöcklin G. A comparative study of N.C.A. Fluorine-18 labeling of proteins via acylation and photochemical conjugation. Nucl. Med. Biol. 1996;23:365–372. doi: 10.1016/0969-8051(96)00017-0. [DOI] [PubMed] [Google Scholar]
- Wüst F, Hultsch C, Bergmann R, Johannsen B, Henle T. Radiolabeling of isopeptide NE epsilon-(γ-glutamyl)-L-lysine by conjugation with N-succinimidyl-4-[18F]fluorobenzoate. Appl. Radiat. Isot. 2003;59:43–48. doi: 10.1016/s0969-8043(03)00161-1. [DOI] [PubMed] [Google Scholar]
- Zijlstra S, Gunawan J, Burchert W. Synthesis and evaluation of a 18F-labelled recombinant annexin-V derivative, for identification and quantification of apoptotic cells with PET. Appl. Rad. Isot. 2003;58:201–207. doi: 10.1016/s0969-8043(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Mäding P, Füchtner F, Wüst F. Module-assisted synthesis of the bifunctional labeling agent N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB. Appl. Rad. Isot. 2005;63:329–332. doi: 10.1016/j.apradiso.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Marik J, Sutcliffe JL. Fully automated preparation of N.C.A. 4-[18F]fluorobenzoic acid and N-succinimidyl 4-[18F]fluorobenzoate using a Siemens/CTI chemistry process control unit (CPCU) Appl. Rad. Isot. 2007;65:199–203. doi: 10.1016/j.apradiso.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Johnström P, Clark JC, Pickard JD, Davenport AP. Automated synthesis of the generic peptide labelling agent N-succinimidyl 4-[18F]fluorobenzoate and application to 18F-label the vasoactive transmitter urotensin-II as a ligand for positron emission tomography. Nucl. Med. Biol. 2008;35:725–731. doi: 10.1016/j.nucmedbio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Tang G, Zeng WB, Yu MX, Kabalka G. Facile synthesis of N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) for protein labeling. J Label. Compd. Radiopharm. 51:68–71. [Google Scholar]
- Azarian V, Gangloff A, Seimbille Y, Delaloye S, Czernin J, Phelps ME, Silverman DHS. Synthesis and liposome encapsulation of a novel 18F-conjugate of ω-conotoxin GVIA for the potential imaging of N-type Ca2+ channels in the brain by positron emission tomography. J. Label. Compd. Radiopharm. 2006;49:269–283. [Google Scholar]
- Toretsky J, Levenson A, Weinberg IN, Tait JF, Uren A, Mease RC. Preparation of F-18 labeled annexin V: a potential PET radiopharmaceutical for imaging cell death. Nucl. Med. Biol. 2004;31:747–752. doi: 10.1016/j.nucmedbio.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Glaser M, Arstad E, Luthra SK, Robins EG. Two-step radiosynthesis of [18F]N-succinimidyl-4-fluorobenzoate ([18F]SFB. J. Label. Compd. Radiopharm. 2009;52:327–330. [Google Scholar]
- Carroll M, Yan R, Aigbirhio F, Soloviev D, Brichard L. The first nucleophilic synthesis of 3-[18F]fluoroethylbenzoate. J. Nucl. Med. 2008;49:303P–303P. [Google Scholar]
- Stone-Elander S, Elander N. Microwave application in radiolabeling with short-lived positron-emitting radionuclides. J. Label. Compd. Radiopharm. 2002;45:715–746. [Google Scholar]
- Guo N, Alagille D, Tamagnan G, Price RR, Baldwin RM. Microwave-induced nucleophilic [18F]fluorination on aromatic rings: synthesis and effect of halogen on [18F]fluoride substitution of meta-halo (F, Cl, Br, I)-benzonitrile derivatives. Appl. Rad. Isot. 2008;66:1396–1402. doi: 10.1016/j.apradiso.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandap KS, Ido T, Kiyono Y, Kobayashi M, Lohith TG, Mori T, Kasamatsu S, Kudo T, Okazawa H, Fujibayashi Y. Development of microwave-based automated nucleophilic [18F]fluorination system and its application to the production of [18F]flumazenil. Nucl. Med. Biol. 2009;36:403–409. doi: 10.1016/j.nucmedbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Scott PJH, Shao X. Fully automated, high yielding production of N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB), and its use in microwave-enhanced radiochemical coupling reactions. J. Label. Compd. Radiopharm. 2010;53:586–591. [Google Scholar]
- Tang G, Tang X, Wang X. A facile automated synthesis of N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) for 18F-labeled cell-penetrating peptide as PET tracer. J. Label. Compd. Radiopharm. 2010;53:543–547. [Google Scholar]
- Olma S, Liu K, Chen Y-C, Dam Rvan, Shen CK-F. Microfluidic Droplet Mixer for Fluorine-18 Labeling of Biomolecules. J. Label. Compd. Radiopharm. 2009;52:S10–S10. [Google Scholar]
- Olma S, Lambert J, Barnhardt E, Liu K, Shen CK-F, van Dam R. A compact microwave system for rapid, semi-automated radiosyntheses. J. Label. Compd. Radiopharm. 2009;52:S509–S509. [Google Scholar]


