Abstract
Objectives
The use of complementary and alternative medicine (CAM), a group of health care practices and products that are not considered part of conventional medicine, has increased in recent years, particularly among individuals with human immune deficiency virus (HIV). Assessing the prevalence and predictors of CAM use among HIV-positive populations is important because some CAM therapies may adversely affect the efficacy of conventional HIV medications. Unfortunately, CAM use is not comprehensively or systematically assessed among HIV-positive populations. Therefore, the aim of the present study was to evaluate the quality of the instruments employed in observational studies assessing CAM use among HIV-positive populations by examining the degree to which these studies (1) evaluated the psychometric properties of their CAM instruments and (2) assessed the multidimensional nature of CAM use.
Design
A systematic review of studies was undertaken and specific review criteria were used to guide the inclusion of studies. Specifically, articles were included that were published in English and in a peer-reviewed journal between 1997 and 2007, recruited HIV-positive study participants, and assessed CAM use. Thirty-two (32) studies met these inclusion criteria.
Results
Results suggest that CAM assessment among HIV-positive populations continues to be problematic. For example, approximately 20% of the studies assessed the reliability and 3% assessed the validity of the CAM instrument employed.
Conclusions
CAM assessment—regardless of the specific study population—is a complex and challenging task. However, CAM instruments will not become more refined over time in the absence of rigorous psychometric evaluation. Future research must assess reliability and validity and report these data in a clear and nuanced manner.
Introduction
Recent estimates from the Centers for Disease Control and Prevention suggest that approximately 1.1 million adolescents and adults in the United States were living with human immunodeficiency virus (HIV) at the end of 2006.1 Given the magnitude of this epidemic and the absence of an effective vaccine, timely and appropriate treatment, such as with highly active antiretroviral therapy (HAART), is critical in extending the length and quality of life of those infected. Many HIV-positive individuals, however, still seek out alternative treatment modalities, with approximately 60% using complementary and alternative medicine (CAM),2 typically defined as “a group of diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine.”3
Several studies indicate that several CAM therapies show promising results. For example, evidence suggests that acupuncture can reduce reported pain, improve the duration and quality of sleep, and alleviate many HIV-associated symptoms.4–6 Nutritional and plant-based supplements have been efficacious at improving appetite and increasing body weight in HIV-positive populations.7–11 Evidence also indicates that HIV-positive patients who consume supplements and vitamins may also have improved clinical outcomes, specifically as indexed by an improvement in CD4 and CD8 cell counts and decreased viral load.12–14 Furthermore, patients also consistently report that they believe that CAM therapies are “extremely” or “quite a bit” helpful15 and that these therapies are as or even more effective than conventional treatments.16
Although CAM use among HIV-positive populations is common and can be an effective treatment modality, research suggests that, in some cases, its use may be problematic. For example, recent evidence suggests that St John's wort, garlic, and vitamin C may reduce the concentrations of HAART in the blood, thus potentially lowering its effectiveness in controlling HIV viral load.17–19 Furthermore, some studies have reported that HIV-positive CAM users may be less likely to adhere to their conventional treatment regimens, although this literature is conflicting.2,20,21 Given the possibility of CAM–drug interactions and the critical importance of HAART adherence for the health and well-being of HIV-positive patients,22 it is imperative that CAM use be consistently and rigorously assessed among this population.
Unfortunately, there has been a lack of consensus regarding the best way to measure CAM use, in general, and among HIV-positive populations, in particular. The tendency in quantitative research has been to list CAM modalities and ask study participants to report use/nonuse without giving them the opportunity to self-identify therapies they perceive as CAM. The resulting omissions cause the underestimation of the types of therapies used.23 By contrast, much of the qualitative literature argues that the definition of CAM should be developed with the beliefs of the individual as the defining source.24 This perspective can result in the CAM net being cast too wide, such that every therapy that is not considered conventional medicine is “thrown into the [CAM] basket,”23 thereby overestimating the types of therapies used. In fact, one recent study on CAM use reported that participants used over 1600 different types of therapies.25 This subjective approach becomes problematic when decisions about CAM measurement must be addressed. Furthermore, few studies report the reliability and/or validity of CAM measures. In their review of 12 studies on CAM use among patients with breast cancer, Lengacher and colleagues reported that none of these studies cited any psychometric indices assessing the reliability or validity for CAM instruments.26 These measurement-related limitations consequently diminish the degree to which research findings can be compared across studies27 and subsequently disseminated to HIV health care providers.
In spite of recent evidence that suggests that quality assessment of observational studies in systematic reviews is essential, it is conducted infrequently.28 Therefore, the purpose of this systematic review was to evaluate the quality of the instruments employed in observational studies assessing CAM use among HIV-positive populations.
Methods
Search strategy
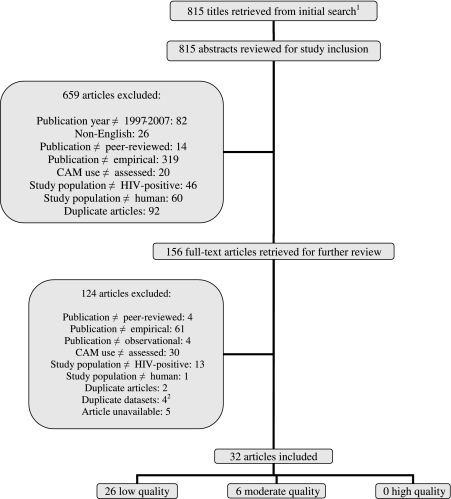
A multistep search process based on recommended strategies29 was utilized to identify relevant studies. First, a comprehensive search of the literature was conducted under the guidance of an experienced research librarian using combinations of the keywords complementary medicine/medication/therapy or alternative medicine/medication/therapy or integrative medicine/medication/therapy or self-treatment with Human Immunodeficiency Virus/HIV or Acquired Immune Deficiency Syndrome/AIDS. This initial search produced 815 abstracts. Second, these 815 abstracts were evaluated for inclusion. Specifically, articles had to meet all of the following criteria for inclusion in the review: (1) publication year between 1997 and 2007, (2) published in English and (3) in a peer-reviewed journal, (4) empirical, (5) quantitative, (6) observational, (7) study population human and (8) HIV-positive and (9) complementary and/or alternative medicine assessed. Six hundred and fifty-nine (659) articles were excluded after the initial abstract review, as their ineligibility was unambiguous from the abstract. The remaining 156 articles were retrieved for further review because study inclusion could not be determined from the abstract (Fig. 1).
FIG. 1.
Search strategy. HIV, human immune deficiency virus; CAM, complementary and alternative medicine. 1Databases used: MEDLINE,® EMBASE, CINAHL, Alternative Health Watch, Global Health, EBM Reviews, PsychInfo, Sociological Abstracts, and Health and Sociological Abstracts. 2In four cases, there were two articles published from the same dataset (identified by having the same study sponsor, location and time period of data collection, methodology, and number of study participants). In these cases, the first article published chronologically was selected for inclusion.
One hundred and fifty-one (151) articles were reviewed for study inclusion by the first author (5 articles could not be retrieved because the journals were not available through any University-affiliated library). Though prior systematic reviews have not evaluated intercoder reliability at the study inclusion stage (only at the article coding stage),30,31 there has been a recent call for researchers to assess intercoder reliability at both stages.32,33 Therefore, the second author reviewed a randomly selected subsample of the 151 abstracts (approximately 20%; N=30) to evaluate intercoder reliability for study inclusion. Intercoder reliability between the two reviewers, adjusting for chance agreement, was satisfactory (κ=0.864). From the 151 reviewed articles, 119 were excluded, resulting in 32 remaining included articles (Fig. 1). Each of the included articles employed only 1 CAM instrument.
Article Coding
Reliability and validity
The quality of the 32 included articles was first assessed by examining the degree to which each assessed and/or reported any psychometric properties of the CAM use instrument(s) employed. Using an approach similar to that developed by Noar and colleagues,31 each article was assigned higher numeric values if it was strong on a characteristic and lower values if it was weak on a characteristic. For example, if an article reported information about the reliability and validity of the CAM instrument, it was assigned a 2; if an article reported information about the reliability or validity of the instrument, it was assigned a 1. If this information was not reported or the assessment/reporting was unclear, it was assigned a 0 (maximum score=2).
Dimensions of CAM use
The quality of the 32 included articles was also assessed by examining the extent to which each article assessed the following dimensions of CAM use: (1) the types of therapies used, (2) the number of therapies used, (3) when therapies were used, (4) the frequency of use, (5) the dose used, (6) the duration of use, (7) how the therapies were used (as a complement or an alternative to conventional medical approaches), (8) the reasons for use, (9) whether use was disclosed to health care providers, (10) satisfaction with use, (11) perceived benefits/efficacy of use, (12) sources of information about use, and (13) use-related expenditures. For each dimension, the values were assigned the following way. For the first dimension, the types of therapies used, articles were assigned a 2 if they assessed the types of CAM used with closed-ended and open-ended questions, a 1 if they used closed-ended or open-ended questions, and a 0 if they did not assess types of CAM used or if this assessment was unclear. For the second dimension, the number of therapies used, articles were assigned a 2 if they assessed ≥5 modalities/≥10 therapies, a 1 if they assessed <5 modalities/<10 therapies, and a 0 if this information was not assessed or was unclear. Articles were assigned an additional 2 points if they also reported >5 modalities/>10 therapies, a 1 if they reported <5 modalities/<10 therapies, and a 0 if this information was not reported or was unclear (maximum score=4). At least five CAM modalities (broad categories of CAM, such as mind–body interventions) and/or 10 CAM therapies (individual therapies such as meditation) were selected as the requirement for the highest quality score as these are the primary modalities/therapies used in the United States as outlined by the National Center for Complementary and Alternative Medicine (NCCAM).3 For the third dimension, when therapies were used, articles were assigned a 1 if they assessed whether CAM was ever used, a 1 if they assessed whether CAM was used since HIV diagnosis, and a 1 if they assessed whether CAM used was used currently* (maximum score=3). Articles were assigned a 1 if this information was assessed but the information was not reported (ANR) or was reported but information about the assessment was not described (RAND). Articles were assigned a 0 if the information was not assessed or reported or was unclear. For each of the remaining 10 dimensions, articles were assigned a 2 if the dimension was assessed, a 1 if the dimension was either ANR or RAND, or a 0 if the information was not assessed/reported or was unclear. The values for each of the above characteristics, including the reliability and validity scores, were summed in order to give a total quality score for which the maximum value was 31.
All 32 included articles were independently evaluated by two coders and then the results were compared. There was evidence of strong reliability between the two coders, even after adjusting for chance agreement (κ=0.853). The coders met to discuss and reconcile all discrepancies.
Results
The 32 included studies had a cumulative N of 16, 925 participants. Most studies utilized convenience sampling (90.6%), were conducted in the United States (59.4%), and predominantly enrolled both male and female participants (75%) from HIV treatment centers (50.0%). Study samples were diverse in racial/ethnic background (Table 1).
Table 1.
Characteristics of the 32 Studies
| Study characteristic | k | % |
|---|---|---|
| Type of sampling | ||
| Convenience | 29 | 90.6 |
| Random | 3 | 9.4 |
| Country of sample | ||
| United States | 19 | 59.4 |
| Country other than United States | 13 | 40.6 |
| Type of sample | ||
| HIV treatment center patients | 16 | 50.0 |
| Other health center/hospital patients | 4 | 12.5 |
| HIV service/advocacy organization participants | 4 | 12.5 |
| Other | 5 | 15.6 |
| Not reported | 3 | 9.4 |
| Gender of participants | ||
| Men | 7 | 21.9 |
| Women | 1 | 3.1 |
| Men and women | 24 | 75.0 |
| Predominant race (>50%) | ||
| White | 9 | 28.1 |
| Black/African American | 8 | 25.0 |
| Asian | 2 | 6.3 |
| Hispanic | 1 | 3.1 |
| Mixed (none greater than 50%) | 7 | 21.9 |
| Not reported | 5 | 15.6 |
k, number of studies; HIV, human immune deficiency virus.
Table 2 contains detailed information about each of the studies, including whether psychometric information was reported, whether each of the other 13 dimensions was addressed, and the calculated quality score. A summary by dimension across articles is provided in Table 3. With respect to the assessment of the psychometric properties of the CAM instruments, approximately 20% of the studies assessed the reliability and 3% assessed the validity of the CAM instrument employed.
Table 2.
Characteristics and Quality Scores of Complementary and Alternative Medicine Measures
| Study | Type used | Number used | When used | Frequency |
|---|---|---|---|---|
| Agnoletto (2003) | 0 | 4 | 1 | 0 |
| Bica (2003) | 2 | 4 | 1 | 0 |
| Burg (2005) | 2 | 4 | 2 | 0 |
| Chang (2003) | 2 | 3 | 0 | 0 |
| Cho (2006) | 2 | 2 | 1 | 0 |
| Chou (2004) | 0 | 2 | 0 | 0 |
| Colebunders (2003) | 2 | 2 | 0 | 0 |
| De Visser (2000) | 0 | 2 | 0 | 0 |
| De Visser (2002) | 2 | 2 | 2 | 0 |
| Duggan (2001) | 0 | 2 | 1 | 0 |
| Fitzpatrick (2007) | 2 | 2 | 1 | 0 |
| Fogarty (2007) | 2 | 2 | 1 | 0 |
| Gore-Felton (2003) | 2 | 4 | 1 | 1 |
| Jernewall (2005) | 2 | 4 | 2 | 0 |
| Josephs (2007) | 2 | 0 | 1 | 0 |
| Kaufman (2007) | 2 | 4 | 1 | 0 |
| Kirksey (2002) | 2 | 2 | 1 | 0 |
| Knipples (2000) | 2 | 4 | 2 | 0 |
| Langlois-Klassen (2007) | 2 | 3 | 0 | 0 |
| London (2003) | 2 | 2 | 1 | 1 |
| Mikhail (2004) | 2 | 4 | 1 | 0 |
| Molassiotis (2004) | 2 | 1 | 0 | 0 |
| Nicholas (2007) | 0 | 2 | 1 | 1 |
| Sparber (2000) | 2 | 2 | 2 | 0 |
| Standish (2001) | 2 | 4 | 1 | 0 |
| Suarez (1997) | 2 | 4 | 1 | 0 |
| Suarez (2000) | 2 | 4 | 2 | 0 |
| Sugimoto (2005) | 0 | 4 | 1 | 0 |
| Sukati (2005) | 2 | 1 | 0 | 0 |
| Thomas (2007) | 0 | 1 | 1 | 2 |
| Wanyama (2007) | 2 | 2 | 1 | 0 |
| Wutoh (2001) | 2 | 4 | 1 | 0 |
| Study | Dose | Duration | How used | Reasons |
|---|---|---|---|---|
| Agnoletto (2003) | 0 | 0 | 1 | 2 |
| Bica (20030 | 0 | 0 | 0 | 0 |
| Burg (2005) | 0 | 0 | 0 | 0 |
| Chang (2003) | 0 | 0 | 0 | 0 |
| Cho (2006) | 0 | 0 | 0 | 2 |
| Chou (2004) | 0 | 0 | 1 | 1 |
| Colebunders (2003) | 0 | 0 | 2 | 0 |
| De Visser (2000) | 0 | 0 | 2 | 0 |
| De Visser (2002) | 0 | 0 | 0 | 0 |
| Duggan (2001) | 0 | 1 | 0 | 0 |
| Fitzpatrick (2007) | 0 | 0 | 0 | 0 |
| Fogarty (2007) | 0 | 0 | 0 | 2 |
| Gore-Felton (2003) | 1 | 1 | 1 | 0 |
| Jernewall (2005) | 0 | 0 | 0 | 0 |
| Josephs (2007) | 0 | 0 | 0 | 0 |
| Kaufman (2007) | 0 | 0 | 0 | 0 |
| Kirksey (2002) | 0 | 0 | 0 | 2 |
| Knipples (2000) | 0 | 0 | 0 | 0 |
| Langlois-Klassen (2007) | 0 | 0 | 1 | 3 |
| London (2003) | 0 | 0 | 0 | 0 |
| Mikhail (2004) | 0 | 0 | 0 | 0 |
| Molassiotis (2004) | 0 | 0 | 0 | 0 |
| Nicholas (2007) | 0 | 0 | 1 | 0 |
| Sparber (2000) | 0 | 0 | 1 | 0 |
| Standish (2001) | 0 | 0 | 2 | 1 |
| Suarez (1997) | 0 | 0 | 0 | 0 |
| Suarez (2000) | 0 | 0 | 0 | 0 |
| Sugimoto (2005) | 0 | 0 | 0 | 1 |
| Sukati (2005) | 0 | 0 | 0 | 0 |
| Thomas (2007) | 0 | 0 | 0 | 2 |
| Wanyama (2007) | 0 | 0 | 1 | 0 |
| Wutoh (2001) | 0 | 0 | 1 | 1 |
| Study | Disclosure | Satisfaction | Perceived efficacy | Info source |
|---|---|---|---|---|
| Agnoletto (2003) | 0 | 0 | 1 | 1 |
| Bica (20030 | 0 | 0 | 0 | 0 |
| Burg (2005) | 0 | 0 | 0 | 0 |
| Chang (2003) | 0 | 0 | 0 | 0 |
| Cho (2006) | 2 | 0 | 2 | 2 |
| Chou (2004) | 0 | 0 | 0 | 0 |
| Colebunders (2003) | 0 | 0 | 0 | 0 |
| De Visser (2000) | 0 | 0 | 2 | 2 |
| De Visser (2002) | 0 | 0 | 2 | 0 |
| Duggan (2001) | 2 | 0 | 2 | 1 |
| Fitzpatrick (2007) | 0 | 0 | 0 | 0 |
| Fogarty (2007) | 0 | 0 | 2 | 0 |
| Gore-Felton (2003) | 0 | 0 | 0 | 0 |
| Jernewall (2005) | 0 | 0 | 0 | 0 |
| Josephs (2007) | 0 | 0 | 0 | 0 |
| Kaufman (2007) | 0 | 0 | 0 | 0 |
| Kirksey (2002) | 0 | 0 | 1 | 2 |
| Knipples (2000) | 0 | 0 | 0 | 0 |
| Langlois-Klassen (2007) | 0 | 0 | 0 | 0 |
| London (2003) | 0 | 0 | 0 | 0 |
| Mikhail (2004) | 0 | 0 | 0 | 0 |
| Molassiotis (2004) | 0 | 0 | 0 | 0 |
| Nicholas (2007) | 0 | 0 | 1 | 0 |
| Sparber (2000) | 1 | 0 | 2 | 1 |
| Standish (2001) | 1 | 0 | 0 | 0 |
| Suarez (1997) | 0 | 0 | 0 | 0 |
| Suarez (2000) | 0 | 0 | 0 | 0 |
| Sugimoto (2005) | 0 | 0 | 0 | 2 |
| Sukati (2005) | 0 | 0 | 2 | 2 |
| Thomas (2007) | 2 | 0 | 2 | 0 |
| Wanyama (2007) | 0 | 0 | 0 | 0 |
| Wutoh (2001) | 0 | 0 | 0 | 0 |
| Study | Expense | Reliability | Validity | Q score |
|---|---|---|---|---|
| Agnoletto (2003) | 0 | 0 | 0 | 10 |
| Bica (20030 | 0 | 0 | 0 | 7 |
| Burg (2005) | 0 | 0 | 0 | 8 |
| Chang (2003) | 0 | 0 | 0 | 5 |
| Cho (2006) | 0 | 0 | 0 | 13 |
| Chou (2004) | 0 | 0 | 0 | 4 |
| Colebunders (2003) | 2 | 0 | 0 | 8 |
| De Visser (2000) | 0 | 1 | 0 | 9 |
| De Visser (2002) | 2 | 1 | 0 | 11 |
| Duggan (2001) | 0 | 0 | 0 | 9 |
| Fitzpatrick (2007) | 0 | 0 | 0 | 5 |
| Fogarty (2007) | 0 | 1 | 0 | 10 |
| Gore-Felton (2003) | 0 | 0 | 0 | 11 |
| Jernewall (2005) | 0 | 0 | 0 | 8 |
| Josephs (2007) | 0 | 0 | 0 | 3 |
| Kaufman (2007) | 0 | 0 | 0 | 7 |
| Kirksey (2002) | 0 | 0 | 0 | 10 |
| Knipples (2000) | 0 | 0 | 0 | 8 |
| Langlois-Klassen (2007) | 0 | 1 | 0 | 10 |
| London (2003) | 0 | 0 | 0 | 6 |
| Mikhail (2004) | 0 | 0 | 0 | 7 |
| Molassiotis (2004) | 0 | 0 | 0 | 3 |
| Nicholas (2007) | 0 | 0 | 0 | 6 |
| Sparber (2000) | 0 | 0 | 0 | 11 |
| Standish (2001) | 0 | 0 | 0 | 11 |
| Suarez (1997) | 0 | 1 | 0 | 8 |
| Suarez (2000) | 0 | 1 | 0 | 9 |
| Sugimoto (2005) | 0 | 0 | 0 | 8 |
| Sukati (2005) | 0 | 0 | 0 | 7 |
| Thomas (2007) | 1 | 0 | 1 | 12 |
| Wanyama (2007) | 0 | 0 | 0 | 6 |
| Wutoh (2001) | 0 | 0 | 0 | 9 |
Table 3.
Summary of Characteristics of Complementary and Alternative Medicine Measures
| Study characteristic | Number of measures | %a |
|---|---|---|
| Reliability | ||
| Assessed | 6 | 18.8 |
| Validity | ||
| Assessed | 1 | 3.1 |
| Type used | ||
| Closed-ended | 12 | 37.5 |
| Open-ended | 7 | 21.9 |
| Both | 6 | 18.8 |
| Unclear/not assessed | 7 | 21.9 |
| Number used | ||
| ≥5 modalities/≥10 therapies | 22 | 68.8 |
| <5 modalities/<10 therapies | 9 | 28.1 |
| Unclear/not assessed | 1 | 3.1 |
| When useda | ||
| Ever | 7 | 21.9 |
| Currently | 16 | 50.0 |
| Since diagnosis | 7 | 21.9 |
| ANR/RANDb | 1 | 3.1 |
| Unclear/not assessed | 7 | 21.9 |
| Frequency | ||
| Assessed | 1 | 3.1 |
| ANR/RAND | 3 | 9.4 |
| Unclear/not assessed | 28 | 87.5 |
| Dose | ||
| Assessed | 0 | 0 |
| ANR/RAND | 1 | 3.1 |
| Unclear/not assessed | 31 | 96.9 |
| Duration | ||
| Assessed | 0 | 0 |
| ANR/RAND | 2 | 6.3 |
| Unclear/not assessed | 29 | 90.6 |
| How used | ||
| Both | 3 | 9.4 |
| One | 5 | 15.6 |
| No distinction | 18 | 56.3 |
| ANR/RAND | 5 | 15.6 |
| Unclear/not assessed | 1 | 3.1 |
| Study characteristic | Number of measures | % |
|---|---|---|
| Reasons | ||
| Assessed | 6 | 18.8 |
| ANR/RAND | 3 | 9.4 |
| Unclear/Not assessed | 23 | 71.9 |
| Disclosure | ||
| Assessed | 3 | 9.4 |
| ANR/RAND | 2 | 6.3 |
| Unclear/Not assessed | 27 | 84.4 |
| Satisfaction | ||
| Assessed | 0 | 0 |
| ANR/RAND | 0 | 0 |
| Unclear/Not assessed | 32 | 100.0 |
| Perceived efficacy | ||
| Assessed | 8 | 25.0 |
| ANR/RAND | 3 | 9.4 |
| Unclear/Not assessed | 21 | 65.6 |
| Information source | ||
| Assessed | 5 | 15.6 |
| ANR/RAND | 3 | 9.4 |
| Unclear/Not assessed | 24 | 75.0 |
| Expense | ||
| Assessed | 2 | 6.3 |
| ANR/RAND | 1 | 3.1 |
| Unclear/Not assessed | 29 | 90.6 |
Percent sums to greater than 100% because, in some cases, studies assessed more than one construct.
ANR, assessed but not reported; RAND, reported but assessment not described.
With respect to the assessment of the 13 CAM dimensions, most studies (78.2%) assessed the types of CAM modalities being used by study participants; closed-ended question formats were the most common (37.5%). Approximately 75% of studies assessed when participants were using CAM, though the most common time frame of assessment was whether participants were “currently” using CAM (50%). The other CAM dimensions were assessed less often. For example, only 25% of studies made a distinction between “complementary” and “alternative” medicine when asking study participants about CAM use, and 9.4% assessed whether participants were disclosing CAM use to health care providers.
The CAM assessment quality scores ranged from a low of 3 to a high of 13, with a mean of 8.09 (standard deviation=2.52). Articles were classified as “low quality” if their final score was between 0 and 10, of “moderate quality” if their final score was between 11 and 20, and of “high quality” if their final score was between 21 and 31, based on a tertile split. Using these cut points, 26 articles were categorized as low quality, 6 were of moderate quality and none were categorized as high quality.
Discussion
In the most recent strategic plan, the NCCAM states that helping health care professionals make informed decisions with their patients about CAM is an important priority.34 For health care providers to be equipped to engage in these dialogues, however, they need access to rigorously conducted, thorough CAM research. Rigorous research should, in addition to many other criteria, employ instruments that have evidence of satisfactory reliability and validity. This evidence not only increases the likelihood that the phenomenon of interest was assessed appropriately but also makes comparing findings across studies and synthesizing research findings possible, a critical process for health care providers and patients as they make decisions about using CAM. The fact that only six articles reported any reliability data and only 1 study reported any validity data highlights a glaring gap in the empirical database and the need for more psychometric evaluation and reporting in the field of CAM assessment.
Though most of the included articles did assess the types of CAM modalities used, many did not investigate CAM use beyond this dimension. Few studies asked study participants about their frequency, dose, and/or duration of CAM use, information which could have important clinical implications. For example, the patient who has been taking 300 mg (dose) of St John's wort once/week (frequency) for 2 months (duration) could be at significantly less risk for HAART drug interactions compared to the patient who has been taking 900 mg/day for 2 years. While it is important to assess what patients are using it is also critically important to assess the dose, frequency, and duration of CAM therapies, particularly for those that are biologically based.
Also notable was the scarcity of studies that examined how CAM was being used (whether the modalities were being used as a complement or alternative to conventional health care). Research suggests that individuals who are using Echinacea, garlic, kava, or St John's wort in addition to their HAART may be at risk for significant drug interactions, including an increase in HIV viral load, a risk of subtherapeutic HAART levels, and hepatotoxicity.35 By contrast, patients who are using CAM therapies instead of conventional medicine may be more likely to develop drug resistance due to inconsistent use of HAART, thereby compromising treatment efficacy.36 Clearly this is an important distinction that should be consistently assessed in CAM research with HIV-positive populations.
Similarly, few studies asked study participants whether they had discussed their CAM use with their HIV health care providers. Given the possibility of drug interactions and/or HAART resistance as a result of CAM use, it is important that patients and providers have candid conversations about whether patients are using CAM and, if so, which therapies they are using. By assessing and reporting the (in)frequency of patient disclosure of CAM use to providers, individuals involved in HIV health care can be more knowledgeable about the importance of initiating these dialogues during patient–provider interactions.
Recommendations for future research
The field of CAM research among HIV-positive populations is still in its nascent stages. However, given the increasing popularity of CAM use among this population, it is imperative that the assessment of CAM be rigorous and thorough so that HIV health care providers can be adequately informed about their patients' CAM-related behaviors, knowledge, and beliefs. More educated providers will ultimately provide better quality of care for the patients.
The first step in this process requires CAM researchers to be more thoughtful in our development and implementation of CAM instruments. Most studies administer a “one size fits all” CAM measure typically consisting of simplistic questions (e.g., “Have you ever used any of the following types of CAM?”) followed by a laundry list of all possible CAM modalities, in spite of the fact that prior literature suggests that the CAM therapies used by study participants often vary by race/ethnicity and stage of disease. For example, while some modalities are considered CAM by most users (e.g., acupuncture), many other modalities are used primarily by only one ethnic group (e.g., the use of green tea and soy products by Asian Americans, the use of a Curandero by Latinos, and the use of prayer or garlic by African Americans37–40). Other evidence indicates that individuals who report more clinic visits and have lower helper T-cell levels and higher HIV viral load may be more likely to use different types of CAM or use CAM more frequently compared to their healthier counterparts.15,41,42 Yet, most studies with HIV-positive populations fail to administer CAM instruments that reflect an understanding of this diversity in CAM use by race/ethnicity or stage of disease. To accurately assess CAM utilization, the questions asked must be tailored to the specific study population.
The second step toward more rigorous CAM measurement involves more thorough assessment of this complex phenomenon in three ways. (1) Because of the inherently subjective nature of CAM,24 questions must assess participants' intentions with respect to their CAM use. For example, green tea may be listed on an instrument as a possible CAM therapy. Two (2) participants may indicate that they drink green tea regularly; however, one does so because she believes it is anticarcinogenic while the other simply likes the taste. The former participant is using CAM, the latter is not. Failure to include an assessment of intention in CAM-related questions may result in measurement inaccuracy. (2) CAM measures should assess multiple dimensions of use. Though investigating the types of CAM used is undoubtedly essential information, so are many other dimensions, including the frequency, dose, and duration of, reasons for, and satisfaction with CAM use as well as the frequency of discussion about CAM use with health care providers. (3) CAM researchers should move beyond providing only dichotomous response options and/or categorizing participants broadly as “users” or “nonusers.” Though this approach makes for more straightforward instruments and data analysis, it does not provide the level of precision needed to generate nuanced research. Instead, CAM instruments should assess degree (intensity) of use.43
The last step toward more sophisticated CAM measurement requires investigators to assess and report the psychometric indices of their CAM instruments. Researchers and health care providers cannot have confidence in the integrity of study findings without evidence that the instruments employed were both reliable and valid. Furthermore, failing to report this information is a missed opportunity to advance the field of CAM research, which relies so heavily on accurate assessment of this complex phenomenon. Developing and implementing instruments that are tailored to the specific study population and are thorough in their assessment of CAM use, the first two steps of this process outlined above, can only increase the likelihood that the instrument will have satisfactory psychometric properties.
Conclusions
The field of CAM research has burgeoned in recent years and is employing now, more than ever, rigorous methods to evaluate complex research questions. Furthering our understanding about and evaluation of these methods can only improve the quality of the research product and, ultimately, health care provider and patient knowledge about CAM.
Footnotes
Within the past 12 months.
Acknowledgments
This project was supported by grant number F31AT004553 from the National Center for Complementary & Alternative Medicine.
Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention. HIV Prevalence Estimates: United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:1073–1076. [PubMed] [Google Scholar]
- 2.Owen-Smith A. Diclemente R. Wingood G. Complementary and alternative medicine use decreases adherence to HAART in HIV-positive women. AIDS Care. 2007;19:589–593. doi: 10.1080/09540120701203279. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Complementary and Alternative Medicine. What is CAM? http://nccam.nih.gov/health/whatiscam/ [Dec 8;2008 ]. http://nccam.nih.gov/health/whatiscam/
- 4.Phillips KD. Skelton WD. Effects of individualized acupuncture on sleep quality in HIV disease. J Assoc Nurses AIDS Care. 2001;12:27–39. doi: 10.1016/S1055-3290(06)60168-4. [DOI] [PubMed] [Google Scholar]
- 5.Shlay JC. Chaloner K. Max MB, et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: A randomized controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. JAMA. 1998;280:1590–1595. doi: 10.1001/jama.280.18.1590. [DOI] [PubMed] [Google Scholar]
- 6.Zhou W. Sun Y. Wu Z. Acupuncture ameliorates AIDS symptoms in 36 cases. J Tradit Chin Med. 2000;20:119–121. [PubMed] [Google Scholar]
- 7.Beal JE. Olson R. Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10:89–97. doi: 10.1016/0885-3924(94)00117-4. [DOI] [PubMed] [Google Scholar]
- 8.Beal JE. Olson R. Lefkowitz L, et al. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage. 1997;14:7–14. doi: 10.1016/S0885-3924(97)00038-9. [DOI] [PubMed] [Google Scholar]
- 9.Gorter R. Seefried M. Volberding P. Dronabinol effects on weight in patients with HIV infection. AIDS. 1992;6:127. doi: 10.1097/00002030-199201000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Shabert JK. Winslow C. Lacey JM. Wilmore DW. Glutamine-antioxidant supplementation increases body cell mass in AIDS patients with weight loss: A randomized, double-blind controlled trial. Nutrition. 1999;15:860–864. doi: 10.1016/s0899-9007(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 11.Struwe M. Kaempfer SH. Geiger CJ, et al. Effect of dronabinol on nutritional status in HIV infection. Ann Pharmacother. 1993;27:827–831. doi: 10.1177/106002809302700701. [DOI] [PubMed] [Google Scholar]
- 12.Fawzi WW. Msamanga GI. Spiegelman D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–1482. doi: 10.1016/s0140-6736(98)04197-x. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser JD. Campa AM. Ondercin JP, et al. Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretroviral therapy: A prospective, double-blinded, placebo-controlled trial. J Acquir Immune Defic Syndr. 2006;42:523–528. doi: 10.1097/01.qai.0000230529.25083.42. [DOI] [PubMed] [Google Scholar]
- 14.Mikhail IS. DiClemente R. Person S, et al. Association of complementary and alternative medicines with HIV clinical disease among a cohort of women living with HIV/AIDS. J Acquir Immune Defic Syndr. 2004;37:1415–1422. doi: 10.1097/01.qai.0000130549.65946.3d. [DOI] [PubMed] [Google Scholar]
- 15.Fairfield KM. Eisenberg DM. Davis RB, et al. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Arch Intern Med. 1998;158:2257–2264. doi: 10.1001/archinte.158.20.2257. [DOI] [PubMed] [Google Scholar]
- 16.Sparber A. Wootton JC. Bauer L, et al. Use of complementary medicine by adult patients participating in HIV/AIDS clinical trials. J Altern Complement Med. 2000;6:415–422. doi: 10.1089/acm.2000.6.415. [DOI] [PubMed] [Google Scholar]
- 17.de Maat MM. Hoetelmans RM. Math t RA, et al. Drug interaction between St John's wort and nevirapine. AIDS. 2001;15:420–421. doi: 10.1097/00002030-200102160-00019. [DOI] [PubMed] [Google Scholar]
- 18.Piscitelli SC. Burstein AH. Welden N, et al. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis. 2002;34:234–238. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- 19.Slain D. Amsden JR. Khakoo RA, et al. Effect of high-dose vitamin C on the steady-state pharmacokinetics of the protease inhibitor indinavir in healthy volunteers. Pharmacotherapy. 2005;25:165–170. doi: 10.1592/phco.25.2.165.56945. [DOI] [PubMed] [Google Scholar]
- 20.Jernewall N. Zea MC. Reisen CA. Poppen PJ. Complementary and alternative medicine and adherence to care among HIV-positive Latino gay and bisexual men. AIDS Care. 2005;17:601–609. doi: 10.1080/09540120512331314295. [DOI] [PubMed] [Google Scholar]
- 21.Knippels HM. Weiss JJ. Use of alternative medicine in a sample of HIV-positive gay men: An exploratory study of prevalence and user characteristics. AIDS Care. 2000;12:435–446. doi: 10.1080/09540120050123837. [DOI] [PubMed] [Google Scholar]
- 22.Bangsberg DR. Perry S. Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 23.Achilles R. Defining complementary and alternative health care: A Health Canada Publication. www.phac-aspc.gc.ca/publicat/pcahc-pacps/pdf/comp_define.pdf. [Oct 30;2006 ]. www.phac-aspc.gc.ca/publicat/pcahc-pacps/pdf/comp_define.pdf
- 24.Caspi O. Sechrest L. Pitluk HC, et al. On the definition of complementary, alternative, and integrative medicine: Societal mega-stereotypes vs. the patients' perspectives. Altern Ther Health Med. 2003;9:58–62. [PubMed] [Google Scholar]
- 25.Standish LJ. Greene KB. Bain S, et al. Alternative medicine use in HIV-positive men and women: Demographics, utilization patterns and health status. AIDS Care. 2001;13:197–208. doi: 10.1080/095401201300059759. [DOI] [PubMed] [Google Scholar]
- 26.Lengacher CA. Bennett MP. Kipp KE, et al. Design and testing of the use of a complementary and alternative therapies survey in women with breast cancer. Oncol Nurs Forum. 2003;30:811–821. doi: 10.1188/03.ONF.811-821. [DOI] [PubMed] [Google Scholar]
- 27.Pinkerton S. Holtgrave D. Leviton L, et al. Toward a standard sexual behavior data set for HIV prevention evaluation. Am J Health Behav. 1998;22:259–266. [Google Scholar]
- 28.Mallen C. Peat G. Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. J Clin Epidemiol. 2006;59:765–769. doi: 10.1016/j.jclinepi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 29.National Health Service (NHS) Centre for Reviews and Dissemination, University of York. CRD Report #4. 2nd. York, UK: NHS Centre for Reviews and Dissemination, University of York; 2001. Undertaking Systematic Reviews of Research on Effectiveness: CRD's Guidance for Those Carrying Out or Commissioning Reviews. [Google Scholar]
- 30.Gagnon AJ. Tuck J. Barkun L. A systematic review of questionnaires measuring the health of resettling refugee women. Health Care Women Int. 2004;25:111–149. doi: 10.1080/07399330490267503. [DOI] [PubMed] [Google Scholar]
- 31.Noar SM. Cole C. Carlyle K. Condom use measurement in 56 studies of sexual risk behavior: Review and recommendations. Arch Sex Behav. 2006;35:327–345. doi: 10.1007/s10508-006-9028-4. [DOI] [PubMed] [Google Scholar]
- 32.Kitchenham B. Procedures for Performing Systematic Reviews. 2004.
- 33.Pai M. McCulloch M. Gorman JD, et al. Systematic reviews and meta-analyses: An illustrated, step-by-step guide. Natl Med J India. 2004;17:86–95. [PubMed] [Google Scholar]
- 34.National Center for Complementary and Alternative Medicine. Expanding Horizons of Health Care: Strategic Plan, 2005–2009. http://nccam.nih.gov/about/plans/2005/strategicplan.pdf. [Jan 5;2009 ]. http://nccam.nih.gov/about/plans/2005/strategicplan.pdf
- 35.Ladenheim D. Horn O. Werneke U, et al. Potential health risks of complementary alternative medicines in HIV patients. HIV Med. 2008;9:653–659. doi: 10.1111/j.1468-1293.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 36.Klimas N. Koneru AO. Fletcher MA. Overview of HIV. Psychosom Med. 2008;70:523–530. doi: 10.1097/PSY.0b013e31817ae69f. [DOI] [PubMed] [Google Scholar]
- 37.Hsiao AF. Wong MD. Goldstein MS, et al. Variation in complementary and alternative medicine (CAM) use across racial/ethnic groups and the development of ethnic-specific measures of CAM use. J Altern Complement Med. 2006;12:281–290. doi: 10.1089/acm.2006.12.281. [DOI] [PubMed] [Google Scholar]
- 38.Kakai H. Maskarinec G. Shumay DM, et al. Ethnic differences in choices of health information by cancer patients using complementary and alternative medicine: An exploratory study with correspondence analysis. Soc Sci Med. 2003;56:851–862. doi: 10.1016/s0277-9536(02)00086-2. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin LA. Braun KL. Asian and Pacific Islander cultural values: Considerations for health care decision making. Health Soc Work. 1998;23:116–126. doi: 10.1093/hsw/23.2.116. [DOI] [PubMed] [Google Scholar]
- 40.Murguia A. Peterson RA. Zea MC. Use and implications of ethnomedical health care approaches among Central American immigrants. Health Soc Work. 2003;28:43–51. doi: 10.1093/hsw/28.1.43. [DOI] [PubMed] [Google Scholar]
- 41.Dwyer JT. Salvato-Schille AM. Coulston A, et al. The use of unconventional remedies among HIV-positive men living in California. J Assoc Nurses AIDS Care. 1995;6:17–28. doi: 10.1016/s1055-3290(05)80026-3. [DOI] [PubMed] [Google Scholar]
- 42.Furler MD. Einarson TR. Walmsley S, et al. Use of complementary and alternative medicine by HIV-infected outpatients in Ontario, Canada. AIDS Patient Care STDS. 2003;17:155–168. doi: 10.1089/108729103321619764. [DOI] [PubMed] [Google Scholar]
- 43.Kristoffersen AE. Fonnebo V. Norheim AJ. Use of complementary and alternative medicine among patients: Classification criteria determine level of use. J Altern Complement Med. 2008;14:911–919. doi: 10.1089/acm.2008.0127. [DOI] [PubMed] [Google Scholar]