Abstract
Purpose
Oral propranolol has become a promising treatment of capillary hemangiomas (CHs) despite concerns of side effects associated with systemic beta-blockers. The objective of this study was to investigate the distribution of propranolol in periocular tissues and in plasma after topical application of propranolol as compared with intravenous and oral administration of propranolol.
Methods
Each rabbit received propranolol as ophthalmic solution (1%) in one eye (1.5 mg dose), intravenous injection (1.5 mg dose), or commercially available propranolol oral solution (5 mg dose). The periocular tissues (e.g., eyelids and extraocular muscles) and blood were collected and assayed for propranolol.
Results
After topical instillation of 1.5 mg propranolol, high amounts of propranolol were rapidly delivered to the eyelids and extraocular muscles (4−32 μg/g at 1 h after dosing). The drug in these tissues was slowly cleared, and significant amounts of the drug (>0.4 μg/g) were still present at 24 h after the topical application. After oral administration of a clinically relevant dose of 5 mg propranolol, the drug concentrations in the periocular tissues were relatively low (<0.4 μg/g) at 1 h after dosing and generally undetectable at 8 h after dosing. After an intravenous injection of 1.5 mg propranolol, the drug concentrations in the eyelids and extraocular muscles were in the range of 0.2−1 μg/g at 1 h after dosing. The plasma concentration of the drug after the intravenous injection was significantly higher than that after topical application of the same dose. The higher drug concentrations in the periocular tissues of the treated eyes as compared with untreated eyes suggest direct penetration of the drug into the periocular tissues from the administration site after topical application.
Conclusions
Topical administration can provide increased concentrations of propranolol in the periocular tissues and, thus, is superior to systemic administration for the treatment of periocular CH.
Introduction
Capillary hemangiomas (CHs) are the most common benign tumors in infants. The CHs can cause serious morbidity and mortality depending on their size and location.1–3 About 7% of CHs involves the periocular regions such as orbit and eyelids.4,5 If untreated or ineffectively treated, complications such as astigmatism, ptosis, eyelid closure, strabismus, amblyopia, and ultimately vision loss may occur in patients with periocular CHs.6,7 The treatment options for periocular CHs include topical, intralesional, or systemic steroids, surgical excision, and α-interferon or vincristine. All these treatments have potential severe side effects and varying response rates to periocular CHs.8
Leaute-Labreze et al. first discovered that propranolol, a non-selective adrenergic beta-blocker, can inhibit growth and cause regression of segmental CHs in 2008.9 Many physicians have since become interested in treating CHs, including periocular CHs, with oral propranolol. Oral propranolol was found to be effective in the treatment of CHs,4,6,10–12 despite potential side effects of systemic propranolol in infants.13,14 Systemic and chronic use of propranolol can cause bradycardia, hypotension, and hypoglycemia. Although oral propranolol has been suggested to be a promising therapy for CHs, no data on the distribution of propranolol in periocular tissues are available, which is important for the design of dosing regimen and development of a more effective and safer drug delivery method in treating periocular CHs.
It is hypothesized that topical drug administration is superior to systemic administration by delivering high concentrations of drugs directly to the target periocular tissues, minimizing systemic exposure, and avoiding undesirable systemic effects. The objectives of this study were to (1) study periocular tissue distribution of propranolol in a rabbit model after a single topical ocular instillation and (2) compare these results with those after oral and intravenous administration. The findings in this study may help physicians optimize the dosing regimen of current oral propranolol treatment in periocular CHs and support the use of topical propranolol as an alternative to oral propranolol for periocular CH therapy.
Methods
Materials
Propranolol hydrochloride (purity ≥98%) and timolol maleate (purity ≥98%) were purchased from Sigma-Aldrich (St. Louis, MO) and Spectrum Chemical MFG (Gardena, CA), respectively. Propranolol solutions for topical and intravenous administration (0.5% and 1%) were prepared in pH 7.4 phosphate-buffered saline (PBS). Oral propranolol solution (40 mg in 5 mL) was purchased from Roxane Laboratories (Columbus, OH). Timolol maleate solution (11.7 μg/mL) was prepared in PBS and used in high-performance liquid chromatography (HPLC) assay. PBS (pH 7.4, consisting of 0.01 M phosphate buffer, 0.0027 M potassium chloride, and 0.137 M sodium chloride) was prepared by dissolving PBS tablets (Sigma-Aldrich) in distilled, deionized water. Triethylamine and methanol (HPLC grade) were purchased from Fisher Scientific (Fair Lawn, NJ). All materials were used as received.
Animals
New Zealand white rabbits (male, 2−2.5 kg) were purchased from Myrtle Rabbitry (Thompsons Station, TN). All experiments were conducted in adherence to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and under the approval of the Institutional Animal Care and Use Committee at the University of Cincinnati.
Topical administration
Three drops of 50 μL propranolol solutions (1%) were topically applied ∼30 s apart to the lower cul-de-sac of the right eye of each rabbit with a calibrated pipette. The eyelids were briefly closed to simulate blinking after each topical instillation of propranolol. The contralateral eye was not treated and served as an untreated control. At 1, 2, 4, 8, and 24 h postadministration, blood samples were collected, the animals were euthanized with pentobarbital (200 mg/kg), and periocular tissues were dissected from both treated and untreated eyes. The blood samples were centrifuged at 3,400 g for 10 min to yield plasma samples. The periocular tissue samples were immediately rinsed with PBS and patted dry with Kimwipes®. The plasma and the periocular tissue samples were stored at −20°C until assay.
The periocular tissues analyzed in this study included inner and outer layers of upper and lower eyelids, bulbar conjunctiva, extraocular muscles (labeled superior, inferior, lateral, and medial muscles), and periocular fat. The superior muscles included the superior rectus and the superior oblique. The inferior muscles included the inferior rectus and the inferior oblique. The lateral and medial muscles were the lateral and medial rectus that had been pooled from each eye of the animal in the assay. The inner layers of the eyelids mainly included the palpebral conjunctiva and tarsal plate. The outer layers of the eyelids mainly included the skin and orbicularis muscle.
Oral administration
Commercially available propranolol oral solution of 0.625 mL (5 mg propranolol) was orally administered to rabbits by using a calibrated pipette. The dose was chosen to be similar to the clinical dose given to infants (∼2 mg/kg). Blood samples were withdrawn at 1, 4, and 8 h after dosing, and the rabbits were sacrificed immediately after blood sampling. The periocular tissue and blood samples were collected as described (see “Topical administration” section).
Intravenous administration
Propranolol solution (0.5%) of 0.3 mL (1.5 mg propranolol) was intravenously administered into the marginal ear vein of each rabbit. Blood samples were collected at 1 h after dosing, and the rabbits were sacrificed with pentobarbital overdose. The periocular tissue and blood samples were collected as described (see “Topical administration” section).
Sample extraction
Propranolol was extracted from the plasma by using the method of Dali et al. with minor modifications.15 Briefly, 10 μL of timolol maleate solution (11.7 μg/mL) and 100 μL of 0.25 M sodium hydroxide were added into 500 μL plasma sample placed in a screw-capped glass tube and vortexed for 10 s. Timolol maleate was the internal standard in the HPLC assay. Three milliliters of ethyl acetate were then added into the tube, and the mixture was vortexed for 30 s. The organic phase in the tube was separated by centrifugation at 3,400 g for 10 min, removed from the tube, and dried under a stream of air at 50°C. After drying, the residue was reconstituted in 100 μL of the mobile phase used in the HPLC assay (see “Propranolol assay” section). The reconstituted sample was vortexed for 10 s and centrifuged at 3,400 g for 5 min. An aliquot of the supernatant (25 μL) was then removed for the HPLC assay. The recovery of the extraction method was tested by adding a known amount of propranolol in the plasma and found to be 69%±6% (n=5).
The periocular tissues were cut into small pieces, incubated with 1 mL of methanol overnight at room temperature, and then sonicated for 30 min. The mixtures were centrifuged at 2,200 g for 5 min, and 500 μL of supernatant were removed. Ten microliters of timolol maleate solution (11.7 μg/mL) was added to the supernatant. The supernatant was then vortexed for 10 s and dried under a stream of air at 50°C. After drying, the residue was reconstituted in 100 μL of the HPLC mobile phase (see “Propranolol assay” section). The reconstituted sample was vortexed for 10 s and centrifuged at 3,400 g for 5 min. An aliquot of the supernatant (25 μL) was removed from the centrifuged sample and analyzed by HPLC. The recovery of the extraction method for the periocular tissues was determined by adding a known amount of propranolol to the periocular tissues. The recovery was found to be 77%±6%, 75%±7%, 97%±10%, 74%±8%, and 79%±20% (n=3) for the inner layers of eyelids, outer layers of eyelids, bulbar conjunctiva, extraocular muscles, and periocular fat, respectively.
Propranolol assay
The HPLC system (Prominence, Shimadzu, Columbia, MD) consisted of CBM-20A system controller, LC-20AT solvent delivery module, SIL-20A autosampler, SPD-20A UV-Vis detector, and a C18 column (Microsorb-MV, 4.6 mm×150 mm; Varian, Inc., Lake Forest, CA). The HPLC assay was performed at room temperature (20°C±2°C). The mobile phase consisted of 500 mL water, 500 mL methanol, and 1.0 mL triethylamine, with its pH adjusted to pH 3.5 using acetic acid. The mobile phase was delivered at a flow rate of 1.0 mL/min. The injection volume was 25 μL, and the detection wavelength was 295 nm. Calibration curves were constructed by using solutions of known concentrations of propranolol in the mobile phase. The calibration curves were found to be linear (r2>0.999) from 10 to 500 ng/mL for the plasma samples and from 0.025 to 75 μg for the periocular tissue samples.
Statistical analysis
In the topical application studies, the average propranolol concentrations in the periocular tissues of treated and untreated eyes were calculated, respectively. The propranolol concentrations of the periocular tissues from both eyes were averaged in the systemic administration studies. All data are presented as means and standard deviations (mean±SD) with n values representing the number of replicates in each set of data. Statistical differences were determined by using Student's t-tests and considered to be significant at a level of P<0.05.
Results
Ocular instillation
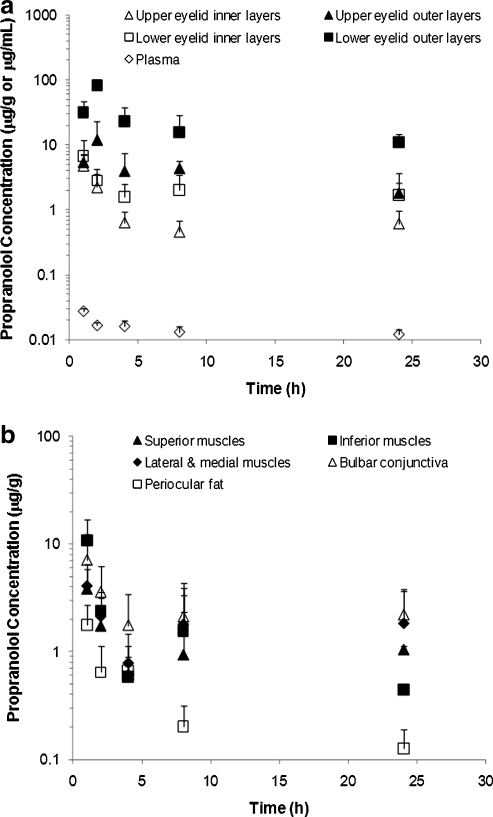
The concentrations of propranolol in the periocular tissues of the treated eyes after topical instillation of 1% propranolol ophthalmic solution at 1.5 mg dose are shown in Fig. 1. The concentrations of propranolol reached their peaks at ∼1−2 h after dosing in all periocular tissues studied. The highest peak concentration was observed in the lower eyelid outer layers (82 μg/g), followed by the upper eyelid outer layers (12 μg/g), inferior muscles (11 μg/g), bulbar conjunctiva (7.1 μg/g), lower eyelid inner layers (6.8 μg/g), upper eyelid inner layers (4.8 μg/g), lateral and medial muscles (4.1 μg/g), superior muscles (3.9 μg/g), and periocular fat (1.8 μg/g). After reaching the peak concentrations, the drug concentrations in these tissues quickly decreased from 2 to 4 h postdosing and then slowly decreased through 24 h postdosing. For example, the drug concentration in the inner layers of lower eyelids decreased from the peak concentration at 1 h postdosing to about 40% of the peak concentration at 2 h postdosing and then to about 20% of the peak concentration at 4 h postdosing. From 8 to 24 h postdosing, the decrease in drug concentration in these tissues was marginal (<20%). In general, the drug concentrations in these tissues (except the periocular fat) were higher than 0.4 μg/g, and could be as high as 11 μg/g, for example, in the lower eyelid outer layers, at 24 h after dosing. Although relatively high amounts of propranolol were delivered to the periocular tissues of ipsilateral eyes after topical application, significantly lower or insignificant amounts of propranolol were detected in the periocular tissues of the untreated contralateral eyes (e.g., <0.4 μg/g at 1 h postdosing); the concentrations of propranolol in these tissues of the untreated eyes were 1−2 orders of magnitudes less than those of the treated eyes. The drug concentrations in plasma were also significantly lower than the drug concentrations in the periocular tissues of the treated eyes at all time points studied (Fig. 1a). The plasma concentration peaked at 1 h postdosing (27±3 ng/mL) and decreased to 12±2 ng/mL at 24 h postdosing.
FIG. 1.
Concentrations of propranolol in the treated ipsilateral periocular tissues (a) eyelids (e.g., upper eyelid inner layers, upper eyelid outer layers, lower eyelid inner layers, and lower eyelid outer layers) and (b) extraocular muscles (superior muscles, inferior muscles, and lateral and medial muscles), bulbar conjunctiva, and periocular fat, after topical instillation of 1.5 mg propranolol. Drug concentrations in plasma after topical instillation are presented for comparison. Data represent the mean and standard deviation (mean±SD, n=3 −4).
Systemic delivery
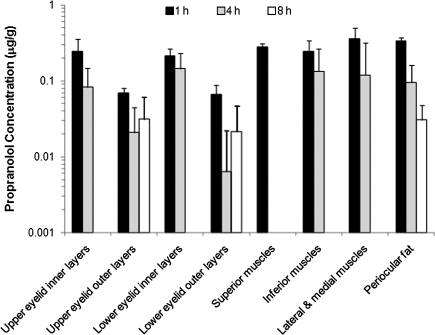
Figure 2 shows the propranolol concentrations in the periocular tissues after oral administration of propranolol at 5 mg dose. Peak concentrations of propranolol were observed at 1 h after dosing in all periocular tissues except bulbar conjunctiva. The drug concentrations in these tissues quickly decreased after reaching the peak concentrations and were generally not detected at 8 h postdosing.
FIG. 2.
Concentrations of propranolol in the periocular tissues after oral administration of a commercially available propranolol oral solution (Roxane) at a dose of 5 mg propranolol. The data represent the average propranolol concentrations in both eyes after administration (mean±SD, n=6). Missing bars indicate that the drug was not detectable in the periocular tissues at 4 and/or 8 h after oral administration. Propranolol was not detectable in the bulbar conjunctiva.
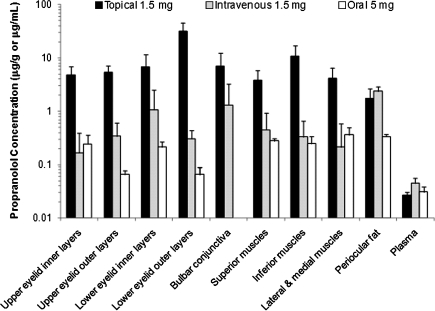
The drug concentrations in the periocular tissues at 1 h after topical (1.5 mg), intravenous (1.5 mg), and oral (5 mg) administration of propranolol are compared in Fig. 3. After intravenous and oral administration, the drug concentrations in the periocular tissues (except the bulbar conjunctiva and periocular fat) were in the range of 0.2–1.1 μg/g and 0.05–0.4 μg/g, respectively, which were ∼6–500-fold lower as compared with those after topical application. The drug was not detectable in the bulbar conjunctiva after oral administration, and drug concentration in the bulbar conjunctiva after intravenous injection was lower than that after topical application. The periocular fat showed the highest drug concentration among the periocular tissues studied (2.4±0.5 μg/g) after intravenous injection, which was comparable to that after topical application (1.8±0.9 μg/g) and higher than that after oral propranolol (0.34±0.03 μg/g). The plasma concentration of propranolol at 1 h after intravenous injection of 1.5 mg of propranolol (45±11 ng/mL) was significantly higher (P<0.05) than the level observed after topical administration of the same dose (27±3 ng/mL) or oral administration of 5 mg of propranolol (31±8 ng/mL). However, the plasma concentrations after topical and oral administration of propranolol at the respective doses were not significantly different (P>0.05).
FIG. 3.
Comparison of propranolol concentrations in the periocular tissues and plasma at 1 h after topical (1.5 mg dose), intravenous (1.5 mg dose), and oral (5 mg dose) administration of propranolol. The data represent the average propranolol concentrations in the treated eye after topical application and in both eyes after systemic administration (mean±SD, n=3 −8). Missing bar indicates that the drug was not detectable in the bulbar conjunctiva at 1 h after oral administration.
Discussion
Topical delivery
The CH is the most common periocular tumor in childhood that may involve the eyelids, the orbit, or both. They can be small or extensive and superficial or deep.16 Superficial CHs are near the surface of the skin and have a bright red appearance. Deep CHs generally grow in the deeper layers of the skin and are manifested as compressible masses in the periocular regions. Tumor growth in the eyelids, orbit, and extraocular muscles can cause severe ocular complications such as strabismus, amblyopia, and vision loss.16,17 Particularly, when the visual development is affected due to tumor growth in these periocular regions, patients need to be treated immediately.18 Oral propranolol is more clinically acceptable for periocular CHs than corticosteroid treatments due to the well-known side effects of corticosteroid treatment and effectiveness of propranolol therapy.19 Currently, propranolol is only administered systemically despite the potential side effects associated with systemic propranolol. There is no generally accepted protocol for CH therapy using oral propranolol, and the dosing regimen is usually based on the clinical experience of physicians.
Topical application has been shown to be a preferred route of administration for treating diseases affecting anterior tissues of the eye as compared with systemic delivery. The feasibility and effectiveness of delivering therapeutic levels of drugs to periocular tissues after topical application have not been well established. The literature of drug delivery to periocular tissues is very limited. As shown in Fig. 1, the results in the present study show that topical application can deliver relatively high concentrations of propranolol (on the order of micrograms per gram tissue) to the periocular tissues including the eyelids and extraocular muscles. The peak concentrations of the drug in the periocular tissues studied occurred at ∼1−2 h after topical application, indicating that the drug was rapidly taken up by the tissues from the administration site. Significant levels of propranolol (>0.4 μg/g) were still detectable in the periocular tissues (except the periocular fat) at 24 h postdosing, thus suggesting slow clearance of the drug from these tissues. Propranolol preferentially distributed into the outer layers of lower eyelid after topical instillation. It was also observed that the drug concentrations were higher in the lower eyelid tissues and inferior muscles than in the upper eyelid tissues and superior muscles. This was likely related to the instillation of the topical propranolol solution in the lower cul-de-sac during drug administration. Together, the results in this study demonstrate the feasibility and effectiveness of delivering significant amounts of propranolol to the periocular tissues after topical instillation.
To study the penetration mechanisms after topical application, the propranolol concentrations in the untreated eyes were compared with those in the treated eyes. The lower drug concentrations detected in the untreated eyes as compared with those in the treated eyes suggest that the drug penetrated directly into the periocular tissues from the administration site and that redistribution of the drug to the tissues from systemic circulation was insignificant.
Systemic delivery
Clinically, propranolol is often orally administered at a dose of 2 mg/kg/d in a divided dose. The pharmacologically optimal dosing interval for propranolol is every 6 h, but patient compliance is improved if the medication is given every 8–12 h.8,20,21 In the present study, a clinically relevant oral dose of 5 mg (∼2 mg/kg) was chosen but was administered in a single-dosing regimen to ensure measurable propranolol concentrations in the periocular tissues. It was found that propranolol was rapidly absorbed and distributed to the periocular tissues reaching their peak concentrations at 1 h after oral administration. The peak drug concentrations (<0.4 μg/g) in the periocular tissues after 5 mg oral dose were significantly lower than those after topical application at a lower dose of 1.5 mg propranolol. After reaching the peak concentrations, drug concentrations in the tissues quickly decreased and were not detectable in all periocular tissues at 8 h postadministration in oral administration except in the outer layers of eyelids and the periocular fat. This shows that oral administration at a clinical dose delivered only small amounts of propranolol into these periocular tissues compared with topical application at a lower dose.
It is known that propranolol is subjected to extensive first-pass metabolism after oral administration. In adults, only about 25% of the dose reaches systemic circulation. In rare cases, intravenous propranolol has been used to treat deep and extensive periocular CHs.11 In the present study, the same dose of propranolol as topical application (1.5 mg propranolol) was intravenously administered to rabbits for comparison. After intravenous injection, the periocular fat showed the highest drug concentration among the periocular tissues studied (Fig. 3). This trend was also observed after oral administration (Fig. 2) and is likely attributable to the high lipophilicity of propranolol [log Koct (octanol/water partition coefficient)=3.5]. Another notable observation was that drug concentration in the plasma after intravenous injection was significantly higher than after topical application. These results suggest that intravenous injection was less effective than topical application in delivering the drug to the target periocular tissues but produced higher peak plasma concentration, which may increase the likelihood of systemic side effects.
Clinical implications
Although oral propranolol is considered a safe and well-tolerated medication even in children,19 systemic side effects of propranolol in the treatment of CHs have been reported, and careful monitoring of patients with CHs during propranolol therapy is generally required.13 The results in Fig. 1 show that topical application of 1.5 mg propranolol could produce and sustain relatively high concentrations of propranolol in the periocular tissues in the animal model. Particularly, drug concentrations in the 0.4−90 μg/g range were observed in eyelids and extraocular muscles, tissues in which or tissues adjacent to areas where CHs often grow. Although the molecular mechanisms of propranolol and the therapeutic concentrations of propranolol in the treatment of CHs are not known, the half maximal inhibitory concentration (IC50) for propranolol in inhibiting tubulogenesis in a cell culture model has been reported.22 Considering the IC50 of ∼1.7 μM (0.4 μg/g), therapeutic concentrations of propranolol could be achieved in the target periocular tissues following topical delivery. The results in the present study suggest that topical propranolol could be a promising alternative to oral propranolol in the treatment of periocular CHs. Topically instilled propranolol could distribute into the target tissues of the eye at pharmacologically active concentrations with lower systemic exposure. Side effects caused by systemic long-term administration of propranolol in the treatment of periocular CHs might be avoided by topical instillation. To our knowledge, this is the first systematic study of periocular tissue distribution of propranolol. It should be noted that the effectiveness of topical delivery needs to be further investigated in human clinical study, as propranolol distribution in humans can be different from that in animals and propranolol distribution to CH tissues may be different from that to normal tissues. In addition, propranolol-associated cytotoxicity to the ocular tissues has been observed in cell culture models.23 Although the concentrations of propranolol in the periocular tissues in the present study were below the level considered to be toxic using the average half maximal inhibitory concentration of 0.4 mM (103 μg/g) determined in cell proliferation assay,23 local toxicity of ophthalmic propranolol needs to be further investigated.
Conclusions
Distribution of propranolol in periocular tissues including eyelids and extraocular muscles has been studied and compared after topical, intravenous, and oral administration. The feasibility of topical delivery of propranolol to the periocular tissues has been demonstrated. Topical instillation produced higher concentrations of the drug in the periocular tissues and lower concentration of the drug in plasma as compared with intravenous injection of the same dose of 1.5 mg propranolol. The drug concentrations in the eyelids and extraocular muscles were generally maintained at higher than 0.4 μg/g for 24 h after the topical application in the present study. Conversely, oral administration of a clinically relevant dose of 5 mg propranolol produced peak concentrations in the target periocular tissues of at least an order of magnitude lower than those after topical application of 1.5 mg propranolol. Topical delivery of propranolol can provide higher drug concentrations in local periocular tissues than oral and intravenous administration and, thus, may increase therapeutic efficacy in the treatment of periocular CHs. Topical propranolol could be a promising alternative to oral propranolol in the treatment of periocular CHs.
Acknowledgments
This research was supported by Faculty Research Grant of the University Research Council at the University of Cincinnati, and in part by NIH grant EY015181 and unrestricted grants from Research to Prevent Blindness and the Quest for Vision Fund to the Department of Ophthalmology at the University of Cincinnati.
Author Disclosure Statement
There is no financial interest or a conflict of interest for all the authors.
References
- 1.Atherton D.J. Infantile haemangiomas. Early Hum. Dev. 2006;82:789–795. doi: 10.1016/j.earlhumdev.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Rademaker M. A revolution in the management of infantile haemangiomas. J. R. Coll. Physicians Edinb. 2010;40:128–129. doi: 10.4997/JRCPE.2010.222. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz R.A. Sidor M.I. Musumeci M.L. Lin R.L. Micali G. Infantile haemangiomas: a challenge in paediatric dermatology. J. Eur. Acad. Dermatol. Venereol. 2010;24:631–638. doi: 10.1111/j.1468-3083.2010.03650.x. [DOI] [PubMed] [Google Scholar]
- 4.Li Y.C. McCahon E. Rowe N.A. Martin P.A. Wilcsek G.A. Martin F.J. Successful treatment of infantile haemangiomas of the orbit with propranolol. Clin. Exp. Ophthalmol. 2010;38:554–559. doi: 10.1111/j.1442-9071.2010.02327.x. [DOI] [PubMed] [Google Scholar]
- 5.Haggstrom A.N. Drolet B.A. Baselga E. Chamlin S.L. Garzon M.C. Horii K.A. Lucky A.W. Mancini A.J. Metry D.W. Newell B. Nopper A.J. Frieden I.J. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 6.Fabian I.D. Ben-Zion I. Samuel C. Spierer A. Reduction in astigmatism using propranolol as first-line therapy for periocular capillary hemangioma. Am. J. Ophthalmol. 2011;151:53–58. doi: 10.1016/j.ajo.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz S.R. Blei F. Ceisler E. Steele M. Furlan L. Kodsi S. Risk factors for amblyopia in children with capillary hemangiomas of the eyelids and orbit. J. AAPOS. 2006;10:262–268. doi: 10.1016/j.jaapos.2006.01.210. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann A.P. Wiegand S. Werner J.A. Eivazi B. Propranolol therapy for infantile haemangiomas: review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2010;74:338–342. doi: 10.1016/j.ijporl.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Leaute-Labreze C. Dumas de la Roque E. Hubiche T. Boralevi F. Thambo J.B. Taieb A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 10.Mishra A. Holmes W.J. Gorst C. Liew S.H. Role of propranolol in the management of periocular hemangiomas. Plast. Reconstr. Surg. 2010;126:671. doi: 10.1097/PRS.0b013e3181de1a32. [DOI] [PubMed] [Google Scholar]
- 11.Fay A. Nguyen J. Jakobiec F.A. Meyer-Junghaenel L. Waner M. Propranolol for isolated orbital infantile hemangioma. Arch. Ophthalmol. 2010;128:256–258. doi: 10.1001/archophthalmol.2009.375. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen J. Fay A. Pharmacologic therapy for periocular infantile hemangiomas: a review of the literature. Semin. Ophthalmol. 2009;24:178–184. doi: 10.1080/08820530902805602. [DOI] [PubMed] [Google Scholar]
- 13.Lawley L.P. Siegfried E. Todd J.L. Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr. Dermatol. 2009;26:610–614. doi: 10.1111/j.1525-1470.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- 14.Schiestl C. Neuhaus K. Zoller S. Subotic U. Forster-Kuebler I. Michels R. Balmer C. Weibel L. Efficacy and safety of propranolol as first-line treatment for infantile hemangiomas. Eur. J. Pediatr. 2011;170:493–501. doi: 10.1007/s00431-010-1324-2. [DOI] [PubMed] [Google Scholar]
- 15.Dali M.M. Moench P.A. Mathias N.R. Stetsko P.I. Heran C.L. Smith R.L. A rabbit model for sublingual drug delivery: comparison with human pharmacokinetic studies of propranolol, verapamil and captopril. J. Pharm. Sci. 2006;95:37–44. doi: 10.1002/jps.20312. [DOI] [PubMed] [Google Scholar]
- 16.Yap E.Y. Bartley G.B. Hohberger G.G. Periocular capillary hemangioma: a review for pediatricians and family physicians. Mayo Clin. Proc. 1998;73:753–759. doi: 10.4065/73.8.753. [DOI] [PubMed] [Google Scholar]
- 17.Durairaj V.D. Treatment of deep orbital hemangiomas of infancy: an overview. Arch. Facial Plast. Surg. 2006;8:217–220. doi: 10.1001/archfaci.8.3.217. [DOI] [PubMed] [Google Scholar]
- 18.Frank R.C. Cowan B.J. Harrop A.R. Astle W.F. McPhalen D.F. Visual development in infants: visual complications of periocular haemangiomas. J. Plast. Reconstr. Aesthet. Surg. 2010;63:1–8. doi: 10.1016/j.bjps.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 19.Claerhout I. Buijsrogge M. Delbeke P. Walraedt S. De Schepper S. De Moerloose B. De Groote K. Decock C. The use of propranolol in the treatment of periocular infantile haemangiomas: a review. Br. J. Ophthalmol. doi: 10.1136/bjo.2010.192245. [DOI] [PubMed] [Google Scholar]
- 20.Holmes W.J. Mishra A. Gorst C. Liew S.H. Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J. Plast. Reconstr. Aesthet. Surg. 2011;64:445–451. doi: 10.1016/j.bjps.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Manunza F. Syed S. Laguda B. Linward J. Kennedy H. Gholam K. Glover M. Giardini A. Harper J.I. Propranolol for complicated infantile haemangiomas: a case series of 30 infants. Br. J. Dermatol. 2010;162:466–468. doi: 10.1111/j.1365-2133.2009.09597.x. [DOI] [PubMed] [Google Scholar]
- 22.Annabi B. Lachambre M.P. Plouffe K. Moumdjian R. Beliveau R. Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol. Res. 2009;60:438–445. doi: 10.1016/j.phrs.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Cheong H.I. Johnson J. Cormier M. Hosseini K. In vitro cytotoxicity of eight beta-blockers in human corneal epithelial and retinal pigment epithelial cell lines: comparison with epidermal keratinocytes and dermal fibroblasts. Toxicol. In Vitro. 2008;22:1070–1076. doi: 10.1016/j.tiv.2008.01.013. [DOI] [PubMed] [Google Scholar]