Abstract
Various emerging technologies are being developed for patients with heart failure. Well-established preclinical evaluations are necessary to determine their efficacy and safety.
Gene therapy using viral vectors is one of the most promising approaches for treating cardiac diseases. Viral delivery of various different genes by changing the carrier gene has immeasurable therapeutic potential.
In this video, the full process of an animal model of heart failure creation followed by gene transfer is presented using a swine model. First, myocardial infarction is created by occluding the proximal left anterior descending coronary artery. Heart remodeling results in chronic heart failure. Unique to our model is a fairly large scar which truly reflects patients with severe heart failure who require aggressive therapy for positive outcomes. After myocardial infarct creation and development of scar tissue, an intracoronary injection of virus is demonstrated with simultaneous nitroglycerine infusion. Our injection method provides simple and efficient gene transfer with enhanced gene expression. This combination of a myocardial infarct swine model with intracoronary virus delivery has proven to be a consistent and reproducible methodology, which helps not only to test the effect of individual gene, but also compare the efficacy of many genes as therapeutic candidates.
Protocol
1. Myocardial infarct creation
Female Yorkshire pigs (20kg) from Animal Biotech Industries (Doylestown, PA) are pre-medicated using Telazol (tiletamine/zolazepam) (8.0 mg/kg) and Buprenorphine (0.6 mg).
Animals are intubated and then ventilated with 100% oxygen. General anesthesia is maintained with Propofol (5-8mg/kg/hr) throughout the procedure.
The femoral site is prepared with 70% isopropyl alcohol followed by povidone iodine. A percutaneous puncture provides access to the artery and for sheath placement. After sheath insertion, 3000-5000 IU of heparin is administered IV to maintain an activated coagulation time of 250-300 seconds.
A bolus dose of Amiodarone (75 mg) is administered intramuscularly. Intravenous injection of a 1000 ml saline bag with Amiodarone (75 mg), Atropine (2 mg), and Potassium Acetate (20 mEq) is started and maintained using a rate of 150 ml/hr throughout the procedure.
A 7 Fr hockey-stick catheter (Cordis; Miami, FL) is advanced to the left coronary artery and a coronary angiography is performed. A 0.014-inch guide wire (Abbott, Park, IL) is advanced into the left anterior descending coronary artery (LAD). An 8-mm-long, 4.0-mm VOYAGER over-the-wire balloon (Abbott, Park, IL) is advanced and positioned at the proximal LAD, and the coronary balloon is inflated to 3 atm. Occlusion of the LAD is confirmed by angiogram.
With continuous electrocardiogram and hemodynamic monitoring, the occlusion is maintained for 90 minutes. Malignant ventricular arrhythmias may occasionally occur within the first 40 minutes, in which case immediate delivery of direct current shocks from 200 J to 300 J are administered. Usually there are two peak time frames of arrhythmia, one between 2 and 10 minutes and the other between 25 and 35 minutes after the balloon inflation. Diligent monitoring and immediate defibrillation are key to the successful myocardial infarct creation, since there are more than 95% incidence of entering ventricular fibrillation within the time frames indicated. If the immediate and appropriate defibrillation is performed, survival rate is very high. If the blood pressure drops under 80 mmHg, volume load is accomplished with rapid intravenous saline infusion.
The balloon is deflated and all the devices are removed.
The sheath is removed from the femoral artery, anesthesia is withheld, and the animal is monitored during recovery. Intravenous saline with Amiodarone, Atropin and Potassium Acetate infusion is decreased at 50 ml/hr and is given overnight. An intramuscular injections of Nitroglycerline and Furoscemide is administered. Then the animal is allowed to recover and careful ECG monitoring is continued for at least 3 hours.
Aspirin (200mg) PO will be given for five days.
Common complications during and after the myocardial infarct creation procedure are coronary dissection, puncture site hematoma, arrhythmia, and heart failure. A refine technique and thorough monitoring is required to prevent and manage these complications.
2. Virus Injection
The animals are prepared as indicated above in the myocardial infarct creation steps1-3. The same dose of heparin should be given to prevent thrombosis during the virus injection.
A 5Fr hockey stick catheter (Cordis; Miami, FL) is advanced to the left coronary artery. After the angiogram, two of 0.014-inch guide wires are advanced, one into the LAD and one into the left circumflex coronary artery (LCX) to fix the position of the catheter.
Arterial blood (-20 ml) is drawn from the catheter. To prevent the ischemia during the infusion, half diluted blood is used for solution. The virus is mixed with 10 ml blood and diluted to 20 ml with saline. The remaining 10 ml is also diluted to 20 ml and used deliver residual virus within the catheter lumen.
Intravenous nitroglycerin (20 μg/min) is started through the ear vein.
The catheter tip is placed at the proximal left main tract. The pressure through the catheter is monitored to ensure that the catheter is not wedging.
The virus mixed blood (15 ml) is injected through the catheter over 15 minutes using an infusion pump into the left coronary artery. After 15 minutes, the syringe is exchanged to the flush, and infusion is continued for 10 minutes.
A wire and a catheter are fixed to right coronary artery the same way as left coronary artery.
The remaining 5 ml of blood mixed with virus is injected into the right coronary artery for 5 minutes followed by 10 minutes of flush.
3. Representative Case
Figure 1 shows the left ventriculography at 1 month after the myocardial infarct creation. The left ventricular wall motion is significantly impaired with an ejection fraction (EF) of 31%. Figure 2 shows the cross sections of the left ventricle at 1 month. A large scar from the base to the apex of the anterior wall is seen. The average EF at 1 month after myocardial infarction in this model is 36% and the average end diastolic volume is 83 ml, whereas the average of those in the same sized animal without a myocardial infarction is 68% and 42 ml, respectively. Figure 3 shows the expression of beta-gal in the left ventricular border zone area 1 month after the injection of adeno-associated virus (2 x 1012 DNAse resitant particles of rAAV1 CMV Lac-Z). An enhanced expression was found in the virus injected area.
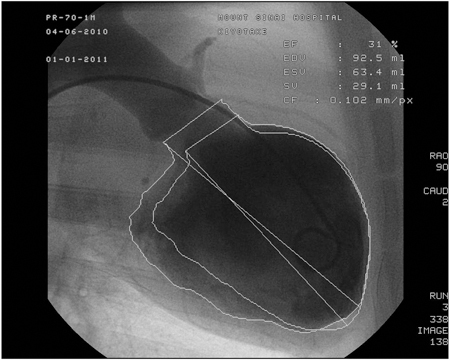 Figure 1. Ejection fraction measured from left ventriculography 1 month after myocardial infarct creation. Left ventricle is dilated to 92.5 ml and anterior wall motion is significantly impaired.
Figure 1. Ejection fraction measured from left ventriculography 1 month after myocardial infarct creation. Left ventricle is dilated to 92.5 ml and anterior wall motion is significantly impaired.
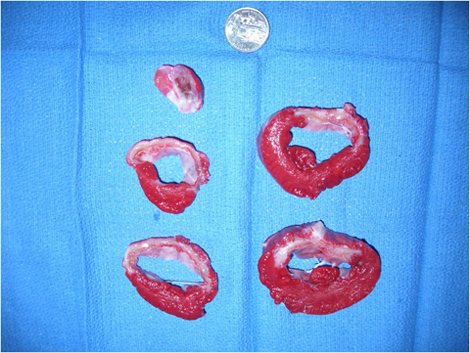 Figure 2. The cross sections of the left ventricle at 1 month after myocardial infarct creation, stained by triphenyl tetrazolium chloride to delineate the scar area. A large scar from the base to the apex of the anterior wall is seen.
Figure 2. The cross sections of the left ventricle at 1 month after myocardial infarct creation, stained by triphenyl tetrazolium chloride to delineate the scar area. A large scar from the base to the apex of the anterior wall is seen.
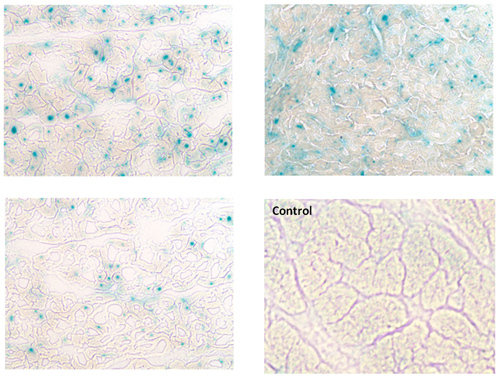 Figure 3. Beta-gal expression induced by adeno-associated virus (2 x 1012 DNAse resistant particles of rAAV1 CMV Lac-Z). An enhanced expression is found at the border zone area between the infarction and the normal tissue, 1 month after the intracoronary virus injection. Right lower picture shows the negative control, where only normal saline was injected in the coronary arteries using the same protocol.
Figure 3. Beta-gal expression induced by adeno-associated virus (2 x 1012 DNAse resistant particles of rAAV1 CMV Lac-Z). An enhanced expression is found at the border zone area between the infarction and the normal tissue, 1 month after the intracoronary virus injection. Right lower picture shows the negative control, where only normal saline was injected in the coronary arteries using the same protocol.
Discussion
Among the various causes of heart failure, ischemia is by far the most frequent in etiology. To overcome this serious disease, various therapeutic approaches are being developed. Careful evaluations of therapies are required before these promising therapies are can be applied to the clinics. Therefore, establishment of an ischemic heart failure model that represents the clinical conditions of patients is critical to development of heart failure therapies. Our swine myocardial infarct model meets these translational requirements.
To induce heart failure by creating a myocardial infarct, the infarct area needs to be large. Our model has a larger scar compared to the previous studies which also used an endovascular myocardial infarct model1. We have found that administering Amiodarone and maintaining blood pressure over 80 mmHg during the coronary occlusion is essential to increase both survival rate and the infarct area. While Isoflurane is an effective anesthetic for this procedure1-2, we found that with Propofol, higher blood pressure can be maintained. In addition, Isoflurane is reported to have more cardio-protective effects compared to Propofol3, and this could lead to a decreased size of the infarction.
Cardiac gene therapy is now one of the most promising approaches to heart failures, and there are variety of strategies to transfer the gene. Above all, intracoronary injection of viral vectors carrying a gene, is a simple and less invasive technique. Various attempts are made to increase gene expression. Our lab has found that infusing nitroglycerin during the virus injection increases the gene expression (I. Karakikes, unpublished).
Gene transfer of SERCA2a using adeno-associated viral vector has resulted in positive outcomes in humans4. Further improvements to cardiac gene therapy are expected with additional carrier genes as well as vector refinements. Our delivery method provides simple and efficient gene transfer with enhanced gene expression, and can be readily translated the clinic.
Disclosures
All authors have no commercial associations that might pose as a conflict of interest in connection with the manuscript.
Acknowledgments
This work is supported by Leducq Foundation through the Caerus network (RJH), by NIH R01 HL093183, HL088434, HL071763, HL080498, HL083156, and P20HL100396 (RJH). DL was supported by the German Research Foundation. We would like to thank Lauren Leonardson for her technical assistance in this project.
References
- Perez de Prado A. Closed-chest experimental porcine model of acute myocardial infarction-reperfusion. J Pharmacol Toxicol Methods. 2009;60:301–306. doi: 10.1016/j.vascn.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Angeli FS. Left ventricular remodeling after myocardial infarction: characterization of a swine model on beta-blocker therapy. Comp Med. 2009;59:272–279. [PMC free article] [PubMed] [Google Scholar]
- Urdaneta F, Lobato EB, Kirby DS&, Sidi A. Treating myocardial stunning randomly, with either propofol or isoflurane following transient coronary occlusion and reperfusion in pigs. Ann Card Anaesth. 2009;12:113–121. doi: 10.4103/0971-9784.51362. [DOI] [PubMed] [Google Scholar]
- Jaski BE. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


