Abstract
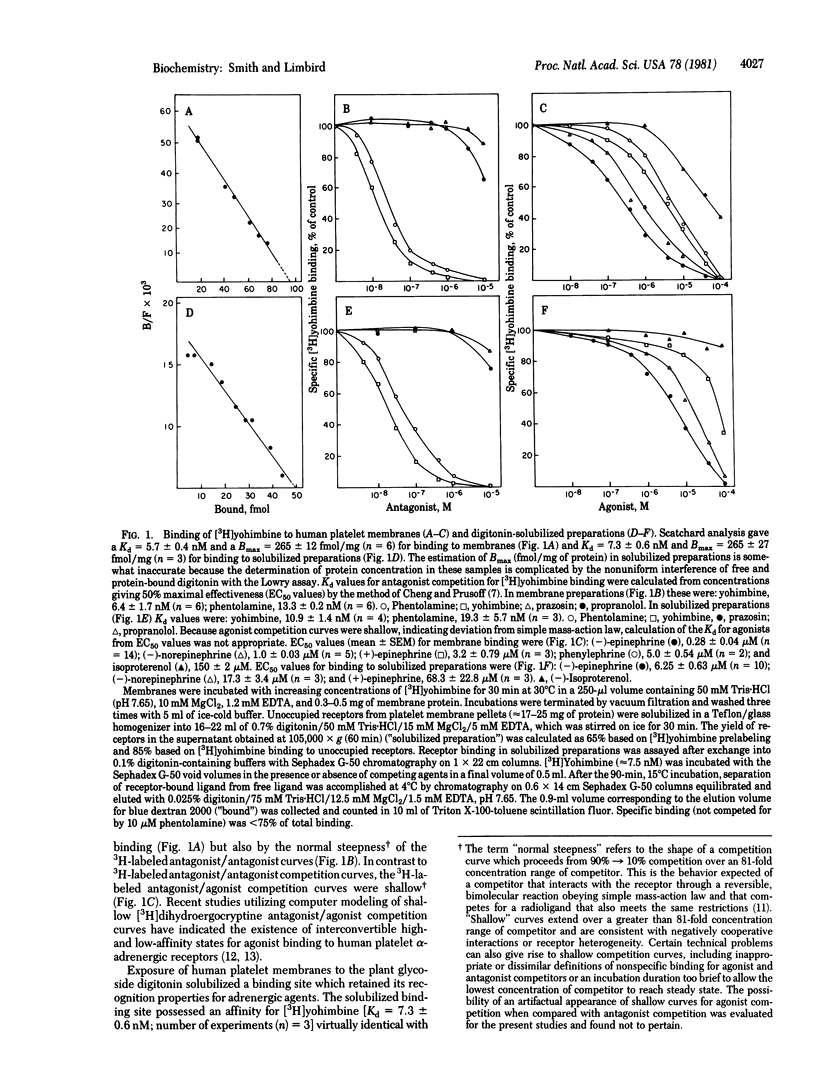
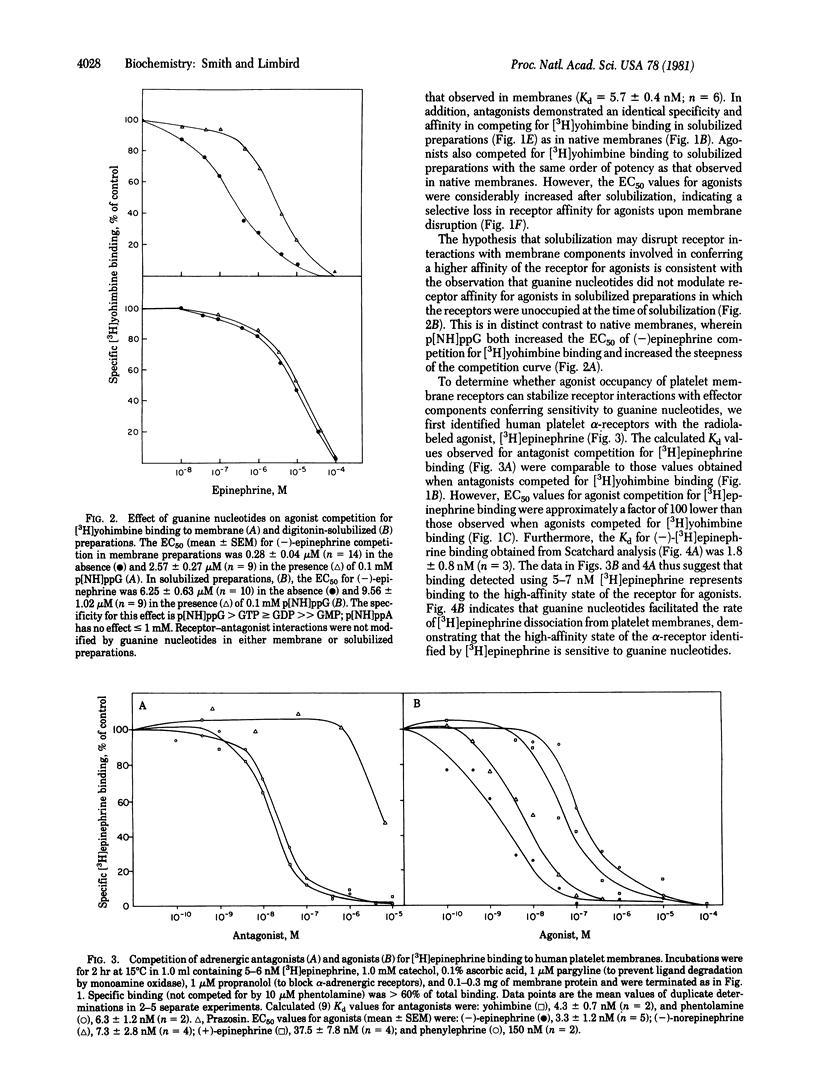
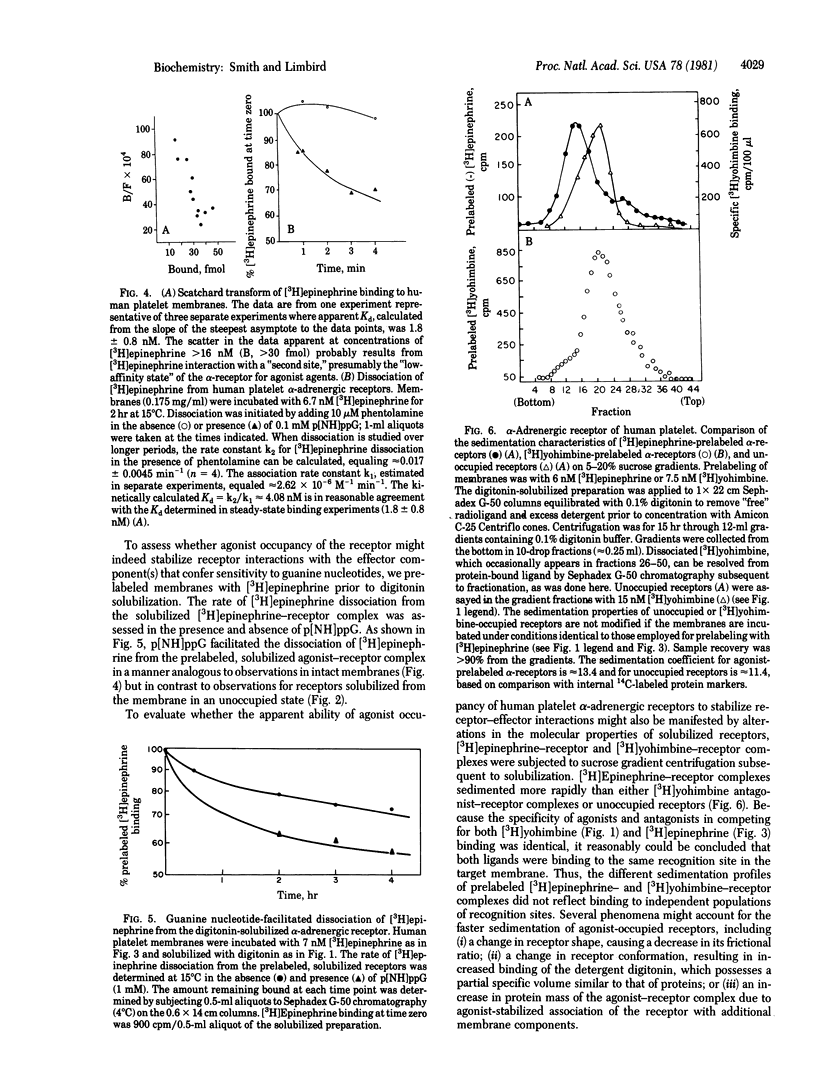
The alpha-adrenergic receptors of human platelet membranes can be directly identified by both a radiolabeled agonist, [3H]epinephrine, and a radiolabeled antagonist, [3H]yohimbine. Digitonin solubilizes a binding component from the membrane that is indistinguishable from the alpha-receptor identified in the native platelet membrane as assessed by (i) order of potency of agonists and antagonists and (ii) affinity of the receptor for [3H]-yohimbine and competing antagonists. However, the solubilized receptor demonstrates a reduced affinity for agonists and a loss of the ability of guanine nucleotides to modulate receptor affinity for agonists. Prelabeling of human platelet membranes with [3H]-epinephrine results in a guanine nucleotide-sensitive agonist-receptor complex that sediments more rapidly in sucrose gradients than do unoccupied or antagonist-occupied receptors. Thus, agonist occupancy of the alpha-receptor prior to membrane solubilization may promote or stabilize receptor interaction with effector components in the membrane, one of which may be the GTP regulatory protein responsible for modulation of receptor affinity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. W., Cooper B., Handin R. I. Characterization of the human platelet alpha-adrenergic receptor. Correlation of [3H]dihydroergocryptine binding with aggregation and adenylate cyclase inhibition. J Clin Invest. 1978 May;61(5):1136–1144. doi: 10.1172/JCI109028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Citri Y., Schramm M. Resolution, reconstitution and kinetics of the primary action of a hormone receptor. Nature. 1980 Sep 25;287(5780):297–300. doi: 10.1038/287297a0. [DOI] [PubMed] [Google Scholar]
- De Lean A., Stadel J. M., Lefkowitz R. J. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980 Aug 10;255(15):7108–7117. [PubMed] [Google Scholar]
- Eimerl S., Neufeld G., Korner M., Schramm M. Functional implantation of a solubilized beta-adrenergic receptor in the membrane of a cell. Proc Natl Acad Sci U S A. 1980 Feb;77(2):760–764. doi: 10.1073/pnas.77.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. A., Scrutton M. C. Novel alpha2-adrenoreceptors primarily responsible for inducing human platelet aggregation. Nature. 1979 Feb 22;277(5698):659–661. doi: 10.1038/277659a0. [DOI] [PubMed] [Google Scholar]
- Hoffman B. B., Michel T., Kilpatrick D. M., Lefkowitz R. J., Tolbert M. E., Gilman H., Fain J. N. Agonist versus antagonist binding to alpha-adrenergic receptors. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4569–4573. doi: 10.1073/pnas.77.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs K. H. Inhibition of adenylate cyclase by hormones and neurotransmitters. Mol Cell Endocrinol. 1979 Dec;16(3):147–156. doi: 10.1016/0303-7207(79)90023-6. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H. Synthetic alpha-adrenergic agonists are potent alpha-adrenergic blockers in human platelets. Nature. 1978 Aug 24;274(5673):819–820. doi: 10.1038/274819a0. [DOI] [PubMed] [Google Scholar]
- Kent R. S., De Lean A., Lefkowitz R. J. A quantitative analysis of beta-adrenergic receptor interactions: resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol Pharmacol. 1980 Jan;17(1):14–23. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of catecholamine release. Biochem Pharmacol. 1974 Jul 1;23(13):1793–1800. doi: 10.1016/0006-2952(74)90187-7. [DOI] [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Stadel J. M., Hickey A. R., Lefkowitz R. J. Loss of beta-adrenergic receptor-guanine nucleotide regulatory protein interactions accompanies decline in catecholamine responsiveness of adenylate cyclase in maturing rat erythrocytes. J Biol Chem. 1980 Mar 10;255(5):1854–1861. [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Agonist-induced increase in apparent beta-adrenergic receptor size. Proc Natl Acad Sci U S A. 1978 Jan;75(1):228–232. doi: 10.1073/pnas.75.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M. E., Wiklund R. A., Anderson H. J., Gilman A. G. Binding of (125I)iodohydroxybenzylpindolol to putative beta-adrenergic receptors of rat glioma cells and other cell clones. J Biol Chem. 1976 Mar 10;251(5):1221–1231. [PubMed] [Google Scholar]
- Newman K. D., Williams L. T., Bishopric N. H., Lefkowitz R. J. Identification of alpha-adrenergic receptors in human platelets by [3H]dihydroergocryptine binding. J Clin Invest. 1978 Feb;61(2):395–402. doi: 10.1172/JCI108950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Stadel J. M., DeLean A., Lefkowitz R. J. A high affinity agonist . beta-adrenergic receptor complex is an intermediate for catecholamine stimulation of adenylate cyclase in turkey and frog erythrocyte membranes. J Biol Chem. 1980 Feb 25;255(4):1436–1441. [PubMed] [Google Scholar]
- Tsai B. S., Lefkowitz R. J. Agonist-specific effects of guanine nucleotides on alpha-adrenergic receptors in human platelets. Mol Pharmacol. 1979 Jul;16(1):61–68. [PubMed] [Google Scholar]


