Abstract
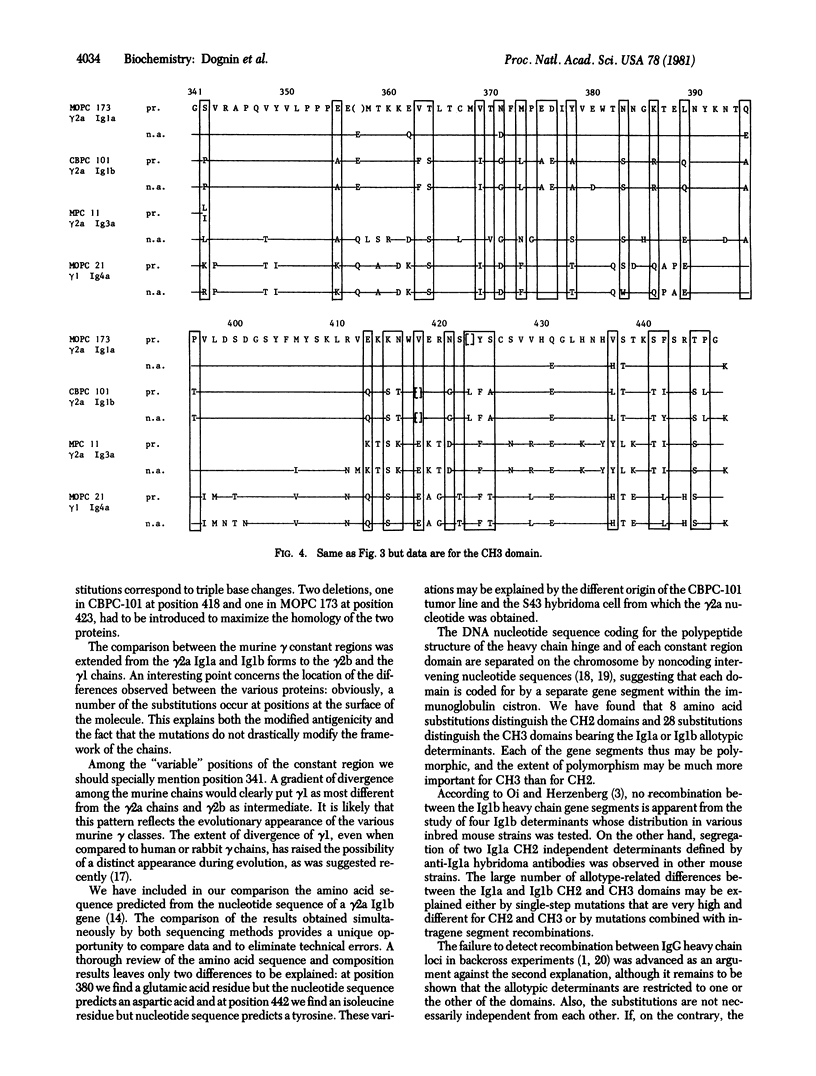
The complete amino acid sequence of the murine gamma 2a heavy chain CBPC-101 Fc region of allotype Ig1b was determined by automated and manual Edman degradation procedures. Both chemical and enzymatic cleavages were used to obtain peptides which were purified by gel filtration followed by high-pressure liquid chromatography. The sequence was in good agreement with that predicted from the nucleotide sequence of a gamma 2a gene of allotype Ig1b except for two positions. Comparison with published data on MOPC 173 gamma 2a heavy chain of allotype Ig1a revealed 8 amino acid substitutions in the CH2 domain (7% differences) and 18 substitutions (27%) in the CH3 domain. Many of the observed interchanges occur at positions at which murine heavy chains of other classes also differ from the gamma 2a chains. Our data suggest that the divergence of the gamma 2a Ig1a gene found in BALB/c mice and of the gamma 2a Ig1b gene found in CB 20 mice must have occurred long ago and that the CH2 domain was much more conserved than the CH3 domain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adetugbo K. Evolution of immunoglobulin subclasses. Primary structure of a murine myeloma gamma1 chain. J Biol Chem. 1978 Sep 10;253(17):6068–6075. [PubMed] [Google Scholar]
- Bosma M. J., Bosma G. C. Congenic mouse strains: the expression of a hidden immunoglobulin allotype in a congenic partner strain of BALB-c mice. J Exp Med. 1974 Mar 1;139(3):512–527. doi: 10.1084/jem.139.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Y. The destruction of serine and threonine thiohydantoins during the sequence determination of peptides by 4-N,N-dimethylaminoazobenzene 4'-isothiocyanate. Biochim Biophys Acta. 1979 May 23;578(1):175–187. doi: 10.1016/0005-2795(79)90125-9. [DOI] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emorine L., Dutka S., Paroutaud P., Strosberg A. D. The structural correlates of the rabbit light chain b allotypes: sequence studies of b5 and b6 chains. Mol Immunol. 1979 Dec;16(12):997–1004. doi: 10.1016/0161-5890(79)90033-6. [DOI] [PubMed] [Google Scholar]
- Farnsworth V., Goodfliesh R., Rodkey S., Hood L. Immunoglobulin allotypes of rabbit kappa chains: polymorphism of a control mechanism regulating closely linked duplicated genes? Proc Natl Acad Sci U S A. 1976 Apr;73(4):1293–1296. doi: 10.1073/pnas.73.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougereau M., Bourgois A., de Preval C., Rocca-Serra J., Schiff C. The complete sequence of the murine monoclonal immunoglobulin MOPC 173 (IgG2a): genetic implications. Ann Immunol (Paris) 1976 Sep-Oct;127(5):607–631. [PubMed] [Google Scholar]
- Francus T., Birshtein B. K. An IgG2a-producing variant of an IgG2b-producing mouse myeloma cell line. Structural studies on the Fc region of parent and variant heavy chains. Biochemistry. 1978 Oct 3;17(20):4324–4331. doi: 10.1021/bi00613a033. [DOI] [PubMed] [Google Scholar]
- Guyer R. L., Koshland M. E., Knopf P. M. Immunoglobulin binding by mouse intestinal epithelial cell receptors. J Immunol. 1976 Aug;117(2):587–593. [PubMed] [Google Scholar]
- HERZENBERG L. A. A CHROMOSOME REGION FOR GAMMA 2A AND BETA 2A GLOBULIN H CHAIN ISOANTIGENS IN THE MOUSE. Cold Spring Harb Symp Quant Biol. 1964;29:455–462. doi: 10.1101/sqb.1964.029.01.048. [DOI] [PubMed] [Google Scholar]
- Honjo T., Obata M., Yamawaki-Katoaka Y., Kataoka T., Kawakami T., Takahashi N., Mano Y. Cloning and complete nucleotide sequence of mouse immunoglobulin gamma 1 chain gene. Cell. 1979 Oct;18(2):559–568. doi: 10.1016/0092-8674(79)90072-2. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Lieberman R., Potter M. Close linkage in genes controlling gamma-A and gamma-G heavy chain structure in BALB/c mice. J Mol Biol. 1966 Jul;18(3):516–528. doi: 10.1016/s0022-2836(66)80040-2. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Mandy W. J. Serological and chemical studies of latent allotypes in the rabbit. Ann Immunol (Paris) 1979 Mar-Apr;130(2):115=31–115=31. [PubMed] [Google Scholar]
- Oi V. T., Herzenberg L. A. Localization of murine Ig-1b and Ig-1a (IgG 2a) allotypic determinants detected with monoclonal antibodies. Mol Immunol. 1979 Dec;16(12):1005–1017. doi: 10.1016/0161-5890(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Bothwell A. L., Mueller-Hill B., Baltimore D. Multiple differences between the nucleic acid sequences of the IgG2aa and IgG2ab alleles of the mouse. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4495–4499. doi: 10.1073/pnas.78.7.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorav J. L., Auffray C., Rougeon F. Structure of the constant and 3' untranslated regions of the murine Balb/c gamma 2a heavy chain messenger RNA. Nucleic Acids Res. 1980 Jul 25;8(14):3143–3155. doi: 10.1093/nar/8.14.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosberg A. D., Emorine L., Zeeuws R. The structural polymorphism of the rabbit and other lagomorph allotypes constitute evidence for a control mechanism regulating the expression of closely linked duplicated genes. Ann Immunol (Paris) 1979 Mar-Apr;130(2):157–166. [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Marcu K. B., Newell N., Richards J., Blattner F. R. Sequence of the cloned gene for the constant region of murine gamma 2b immunoglobulin heavy chain. Science. 1979 Dec 14;206(4424):1303–1306. doi: 10.1126/science.117549. [DOI] [PubMed] [Google Scholar]
- Yarmush M. L., Sogn J. A., Kindt T. J. Latent allotypes: a window to a genetic enigma. Ann Immunol (Paris) 1979 Mar-Apr;130(2):143–156. [PubMed] [Google Scholar]
- Zeeuws R., Strosberg A. D. Proceedings: Extensive sequence differences in rabbit light chains of allotype b4 and b9. Arch Int Physiol Biochim. 1975 Feb;83(1):205–206. [PubMed] [Google Scholar]
- Zeeuws R., Strosberg A. D. The use of methanol in high-performance liquid chromatography of phenylthiohydantoin-amino acids. FEBS Lett. 1978 Jan 1;85(1):68–72. doi: 10.1016/0014-5793(78)81250-2. [DOI] [PubMed] [Google Scholar]


