Abstract
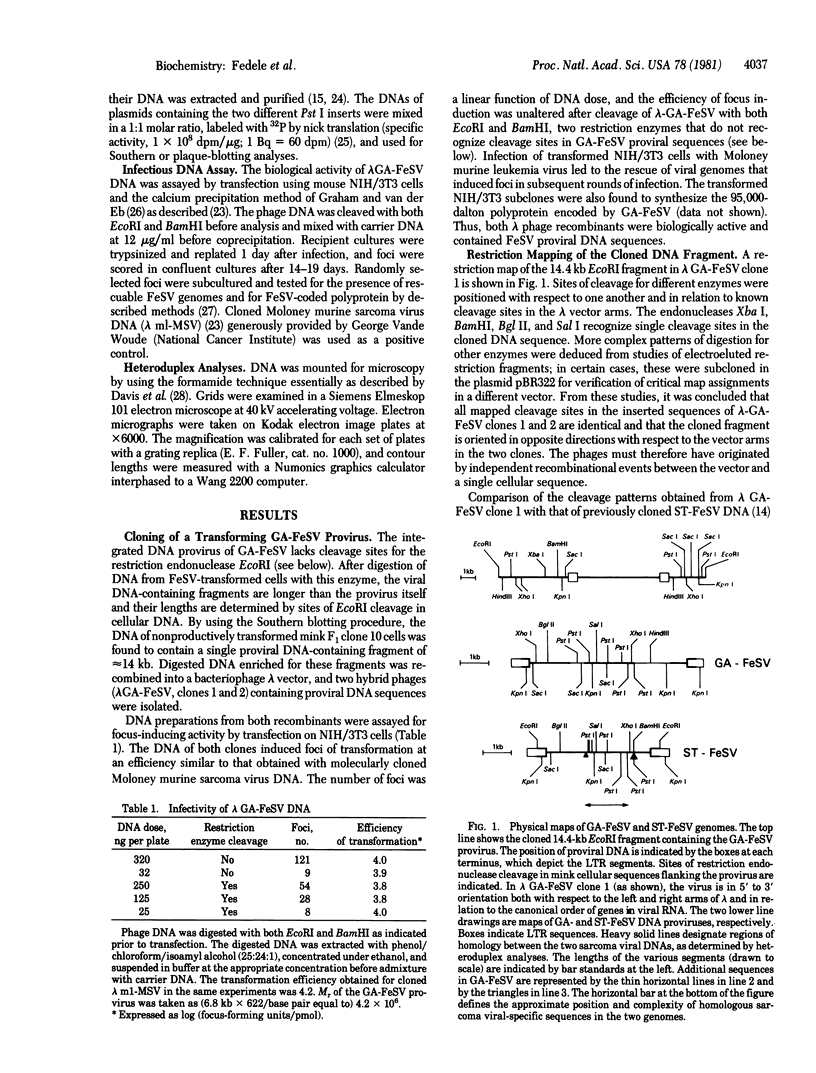
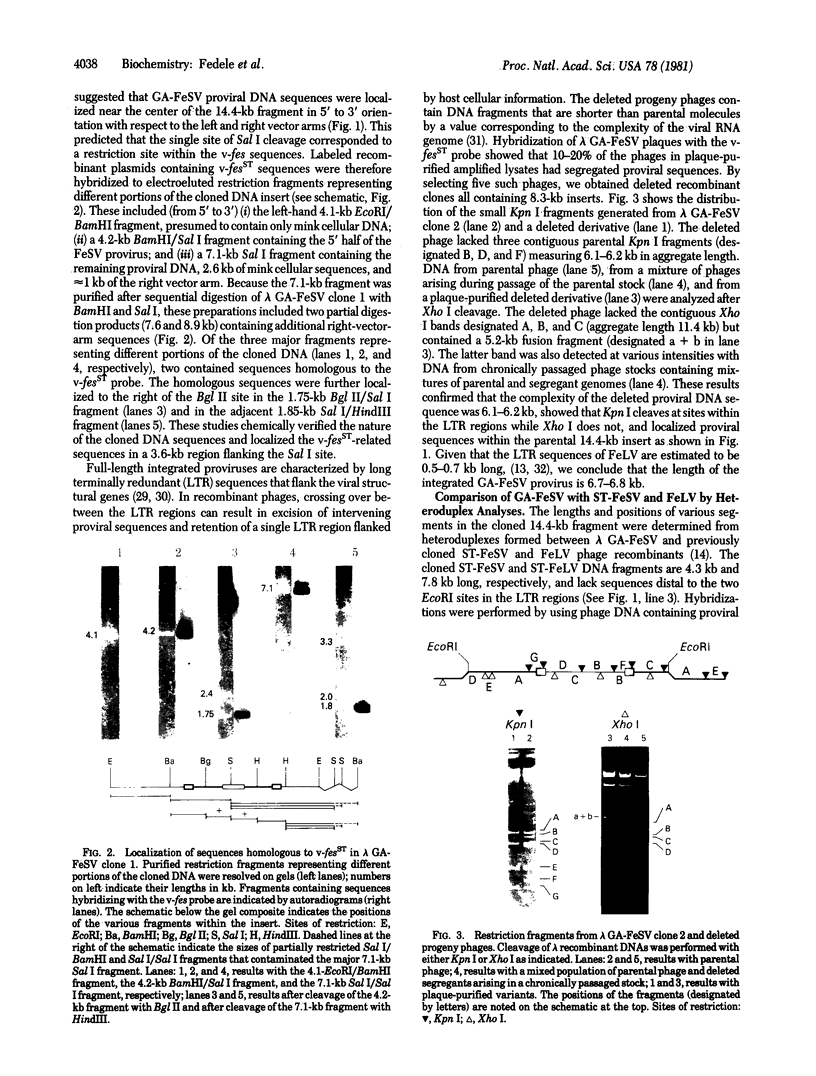
The integrated DNA provirus of the Gardner-Arnstein (GA) strain of feline sarcoma virus (FeSV) was molecularly cloned in a bacteriophage lambda vector. The cloned DNA fragment is 14.4 kilobase pairs long and contains a 6.7-kilobase provirus flanked by cellular sequences derived from nonproductively transformed mink cells. Transfection of mouse NIH/3T3 cells with the cloned DNA fragment induced foci of transformation at efficiencies of 10(4) focus-forming units/pmol of sarcoma virus DNA. Restriction endonuclease mapping and heteroduplex analyses were used to compare the GA-FeSV provirus with that of Snyder-Theilen (ST)-FeSV, a second strain that contains homologous transformation-specific sequences (v-fes). Both viruses have the general structure 5'-gag-fes-env-c region-3', each having retained portions of the feline leukemia virus (FeLV) gag and env genes. In addition to segments shared by the two sarcoma viruses, GA-FeSV contains 1.7 kilobases of extra sequences not found in ST-FeSV. Of these, at least 400-500 base pairs located near the 5' end of v-fes encode a portion of the GA-FeSV polyprotein; the remaining 1.2 kilobases are derived from the FeLV env gene but do not appear to encode any detectable product related to the FeLV envelope glycoprotein. The close homology of the v-fes sequences shows that GA- and ST-FeSV were formed by recombination of FeLV with similar portions of a cat cellular gene (c-fes).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Breitman M. L., Lauver A. V., Long L. K., Vogt P. K. The transformation-specific proteins of avian (Fujinami and PRC-II) and feline (Synder--Theilen and Gardner--Arnstein) sarcoma viruses are immunologically related. Virology. 1981 Apr 30;110(2):411–419. doi: 10.1016/0042-6822(81)90071-4. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Lauver A. V., Devare S. G. Biochemical and immunological characterization of polyproteins coded for by the McDonough, Gardner-Arnstein, and Snyder-Theilen strains of feline sarcoma virus. J Virol. 1980 Jan;33(1):196–207. doi: 10.1128/jvi.33.1.196-207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Donner L., Turek L. P., Ruscetti S. K., Fedele L. A., Sherr C. J. Transformation-defective mutants of feline sarcoma virus which express a product of the viral src gene. J Virol. 1980 Jul;35(1):129–140. doi: 10.1128/jvi.35.1.129-140.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Franchini G., Even J., Sherr C. J., Wong-Staal F. onc sequences (v-fes) of Snyder-Theilen feline sarcoma virus are derived from noncontiguous regions of a cat cellular gene (c-fes). Nature. 1981 Mar 12;290(5802):154–157. doi: 10.1038/290154a0. [DOI] [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- McClements W. L., Enquist L. W., Oskarsson M., Sullivan M., Vande Woude G. F. Frequent site-specific deletion of coliphage lambda murine sarcoma virus recombinants and its use in the identification of a retrovirus integration site. J Virol. 1980 Aug;35(2):488–497. doi: 10.1128/jvi.35.2.488-497.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Davidson N. Sequence organization of feline leukemia virus DNA in infected cells. Nucleic Acids Res. 1980 Aug 11;8(15):3287–3305. doi: 10.1093/nar/8.15.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Turek L. P., Sherr C. J. Three independent isolates of feline sarcoma virus code for three distinct gag-x polyproteins. J Virol. 1980 Jul;35(1):259–264. doi: 10.1128/jvi.35.1.259-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Oskarsson M., Maizel J., Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. doi: 10.1128/jvi.34.1.200-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa T., Hanafusa H., Stephenson J. R. Homology exists among the transforming sequences of avian and feline sarcoma viruses. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6536–6540. doi: 10.1073/pnas.77.11.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliski A. H., Essex M., Meyer C., Todaro G. Feline oncornavirus-associated cell membrane antigen: expression in transformed nonproducer mink cells. Science. 1977 Jun 17;196(4296):1336–1339. doi: 10.1126/science.194310. [DOI] [PubMed] [Google Scholar]
- Snyder S. P., Theilen G. H. Transmissible feline fibrosarcoma. Nature. 1969 Mar 15;221(5185):1074–1075. doi: 10.1038/2211074a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van de Ven W. J., Reynolds F. H., Jr, Stephenson J. R. The nonstructural components of polyproteins encoded by replication-defective mammalian transforming retroviruses are phosphorylated and have associated protein kinase activity. Virology. 1980 Feb;101(1):185–197. doi: 10.1016/0042-6822(80)90495-x. [DOI] [PubMed] [Google Scholar]
- Vande Woude G. F., Oskarsson M., McClements W. L., Enquist L. W., Blair D. G., Fischinger P. J., Maizel J. V., Sullivan M. Characterization of integrated Moloney Sarcoma proviruses and flanking host sequences cloned in bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):735–745. doi: 10.1101/sqb.1980.044.01.079. [DOI] [PubMed] [Google Scholar]