Abstract
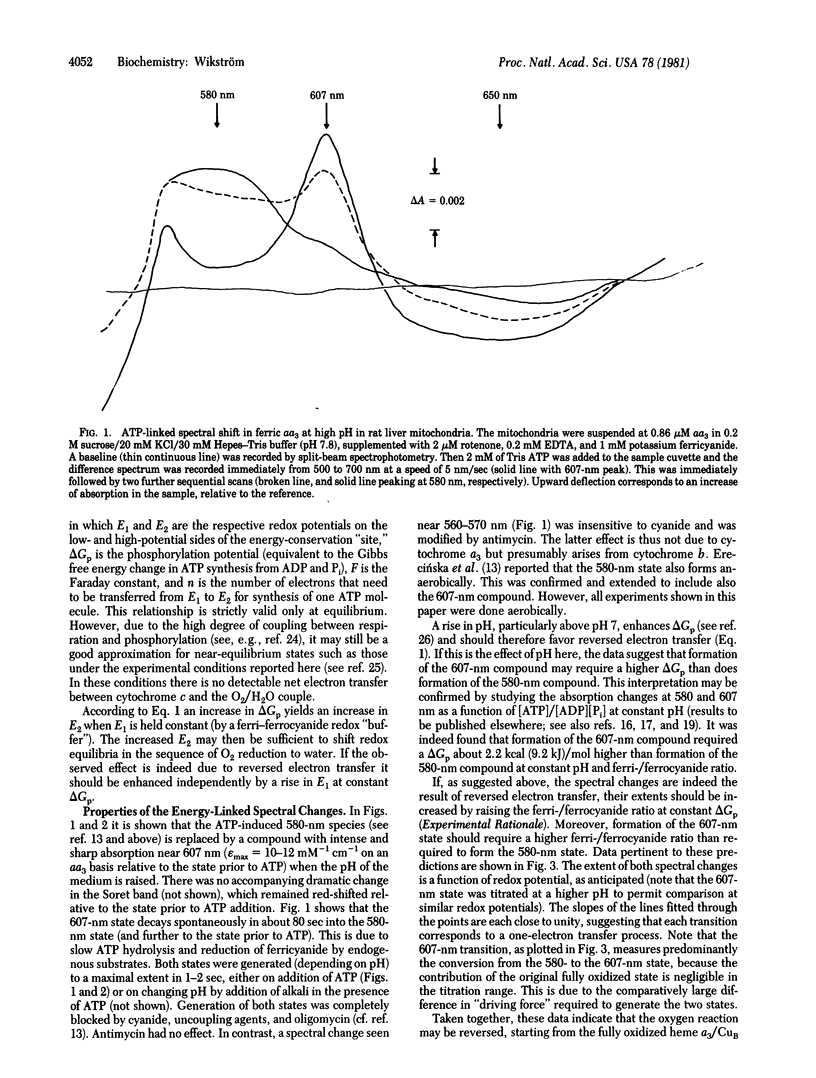
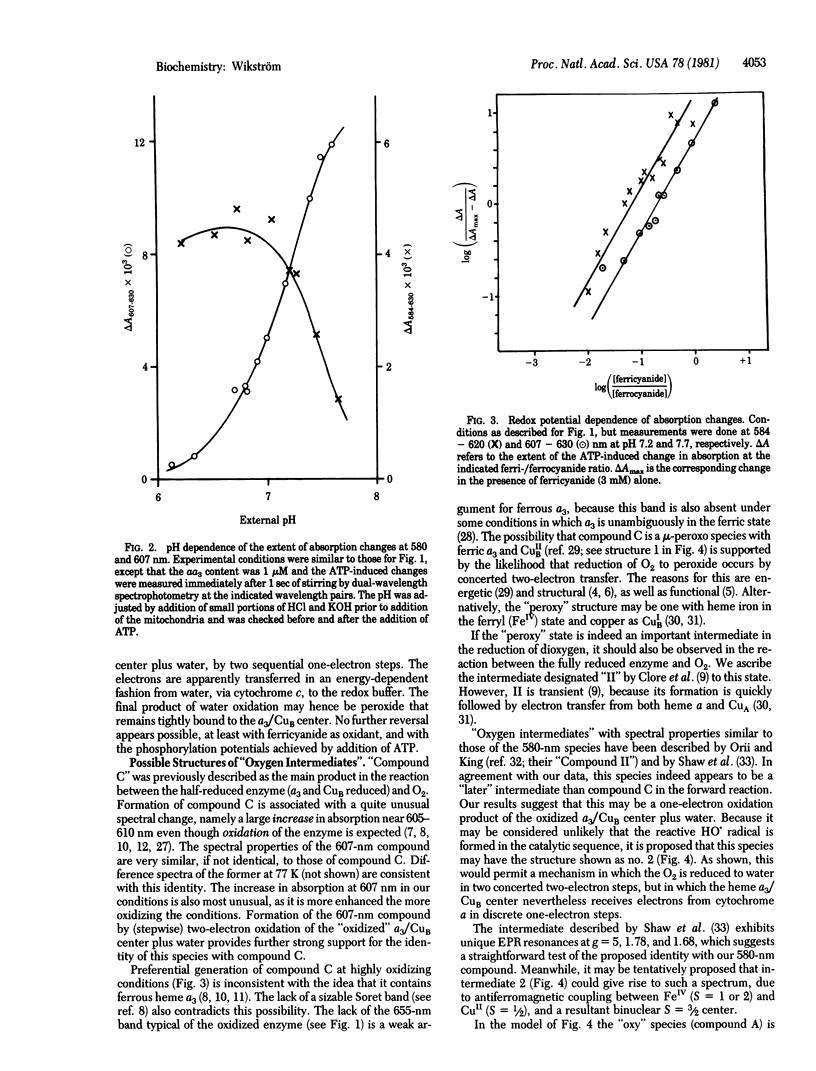
Energization of isolated rat liver mitochondria with ATP under conditions in which cytochrome c is poised in a highly oxidized state shifts the state of cytochrome oxidase (cytochrome c oxidase; ferrocytochrome c:oxygen oxidoreductase, EC 1.9.3.1) from fully oxidized to two new spectroscopically distinguishable states depending on the applied phosphorylation potential and redox potential at cytochrome c. Both new states are spectrally similar or identical to two previously described intermediates in the reaction between reduced enzyme and O2. The data suggest that the energy-dependent transitions are due to reversed electron transfer from water to ferricytochrome c linked to accumulation of intermediates of O2 reduction at the catalytic heme a3/copper center. Titrations with redox potential indicate that each transition is a one-electron step, a finding that would identify the second observed compound as enzyme-bound peroxide or its equivalent. This is consistent with this compound being spectrally identical to "Compound C," previously described as the reaction product between half-reduced oxidase (two electrons) and O2. On the basis of these data a catalytic scheme of O2 reduction is proposed for the heme a3/copper center of cytochrome oxidase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alben J. O., Moh P. P., Fiamingo F. G., Altschuld R. A. Cytochrome oxidase (a3) heme and copper observed by low-temperature Fourier transform infrared spectroscopy of the CO complex. Proc Natl Acad Sci U S A. 1981 Jan;78(1):234–237. doi: 10.1073/pnas.78.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Compound C2, a product of the reaction of oxygen and the mixed-valence state of cytochrome oxidase. Optical evidence for a type-I copper. Biochem J. 1979 Mar 1;177(3):931–941. doi: 10.1042/bj1770931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Clore G. M., Andréasson L. E., Karlsson B., Aasa R., Malmström B. G. Characterization of the intermediates in the reaction of mixed-valence state soluble cytochrome oxidase with oxygen at low temperatures by optical and electron-paramagnetic-resonance spectroscopy. Biochem J. 1980 Jan 1;185(1):155–167. doi: 10.1042/bj1850155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore G. M., Andréasson L. E., Karlsson B., Aasa R., Malmström B. G. Characterization of the low-temperature intermediates of the reaction of fully reduced soluble cytochrome oxidase with oxygen by electron-paramagnetic-resonance and optical spectroscopy. Biochem J. 1980 Jan 1;185(1):139–154. doi: 10.1042/bj1850139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. The involvement of the fully oxidized state in cytochrome oxidase reaction with oxygen studied with the 655 and nm band as a probe. FEBS Lett. 1977 Dec 15;84(2):296–298. doi: 10.1016/0014-5793(77)80710-2. [DOI] [PubMed] [Google Scholar]
- Devault D. Energy transduction in electron transport. Biochim Biophys Acta. 1971 Jan 12;226(1):193–199. doi: 10.1016/0005-2728(71)90192-7. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Cytochrome c oxidase: a synopsis. Arch Biochem Biophys. 1978 May;188(1):1–14. doi: 10.1016/0003-9861(78)90348-x. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F., Sato N., Nicholls P. The energy dependence of the chemical properties of cytochrome c oxidase. Arch Biochem Biophys. 1972 Jul;151(1):188–193. doi: 10.1016/0003-9861(72)90487-0. [DOI] [PubMed] [Google Scholar]
- Falk K. E., Vänngård T., Angström J. Heme spin-states of cytochrome c oxidase derived from room temperature magnetic susceptibility measurements. FEBS Lett. 1977 Mar 15;75(1):23–27. doi: 10.1016/0014-5793(77)80044-6. [DOI] [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J. G., Owen C. S., Wilson D. F. The invisible copper of cytochrome c oxidase. pH and ATP dependence of its midpoint potential and its role in the oxygen reaction. Arch Biochem Biophys. 1975 Aug;169(2):492–505. doi: 10.1016/0003-9861(75)90192-7. [DOI] [PubMed] [Google Scholar]
- Nicholls P. A new carbon monoxide-induced complex of cytochrome c oxidase. Biochem J. 1978 Dec 1;175(3):1147–1150. doi: 10.1042/bj1751147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii Y., King T. E. New species of the "oxygenated compound" of cytochrome oxidase. FEBS Lett. 1972 Mar 15;21(2):199–202. doi: 10.1016/0014-5793(72)80136-4. [DOI] [PubMed] [Google Scholar]
- Reed C. A., Landrum J. T. Structural models for the heme a3/copper active site of cytochrome c oxidase. FEBS Lett. 1979 Oct 15;106(2):265–267. doi: 10.1016/0014-5793(79)80510-4. [DOI] [PubMed] [Google Scholar]
- Rosing J., Slater E. C. The value of G degrees for the hydrolysis of ATP. Biochim Biophys Acta. 1972 May 25;267(2):275–290. doi: 10.1016/0005-2728(72)90116-8. [DOI] [PubMed] [Google Scholar]
- Rosén S., Brändén R., Vänngård T., Malmström B. G. EPR evidence for an active form of cytochrome c oxidase different from the resting enzyme. FEBS Lett. 1977 Feb 15;74(1):25–30. doi: 10.1016/0014-5793(77)80744-8. [DOI] [PubMed] [Google Scholar]
- Shaw R. W., Hansen R. E., Beinert H. Responses of the a3 component of cytochrome c oxidase to substrate and ligand addition. Biochim Biophys Acta. 1978 Oct 11;504(1):187–199. doi: 10.1016/0005-2728(78)90017-8. [DOI] [PubMed] [Google Scholar]
- Shaw R. W., Hansen R. E., Beinert H. The oxygen reactions of reduced cytochrome c oxidase. Position of a form with an unusual EPR signal in the sequence of early intermediates. Biochim Biophys Acta. 1979 Nov 8;548(2):386–396. doi: 10.1016/0005-2728(79)90143-9. [DOI] [PubMed] [Google Scholar]
- Tweedle M. F., Wilson L. J. Electronic state of heme in cytochrome oxidase III. The magnetic susceptibility of beef heart cytochrome oxidase and some of its derivatives from 7-200 K. Direct evidence for an antiferromagnetically coupled Fe (III)/Cu (II) pair. J Biol Chem. 1978 Nov 25;253(22):8065–8071. [PubMed] [Google Scholar]
- Walz D. Thermodynamics of oxidation-reduction reactions and its application to bioenergetics. Biochim Biophys Acta. 1979 Mar 14;505(3-4):279–353. doi: 10.1016/0304-4173(79)90007-7. [DOI] [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Wikström M., Saari H. A spectral shift in cytochrome a induced by calcium ions. Biochim Biophys Acta. 1975 Nov 11;408(2):170–179. doi: 10.1016/0005-2728(75)90009-2. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Brocklehurst E. S. Energy dependent changes in the cytochromes of the mitochondrial respiratory chain. Arch Biochem Biophys. 1973 Sep;158(1):200–212. doi: 10.1016/0003-9861(73)90614-0. [DOI] [PubMed] [Google Scholar]
- van Gelder B. F. On cytochrome c oxidase. I. The extinction coefficients of cytochrome a and cytochrome a3. Biochim Biophys Acta. 1966 Apr 12;118(1):36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]


